Deposition Technologies of High-Efficiency CIGS Solar Cells: Development of Two-Step and Co-Evaporation Processes
Abstract
:1. Introduction
2. Experimental Details
2.1. CIGS Film Preparation and Device Fabrication
2.2. Film and Device Characterization
3. Results and Discussion
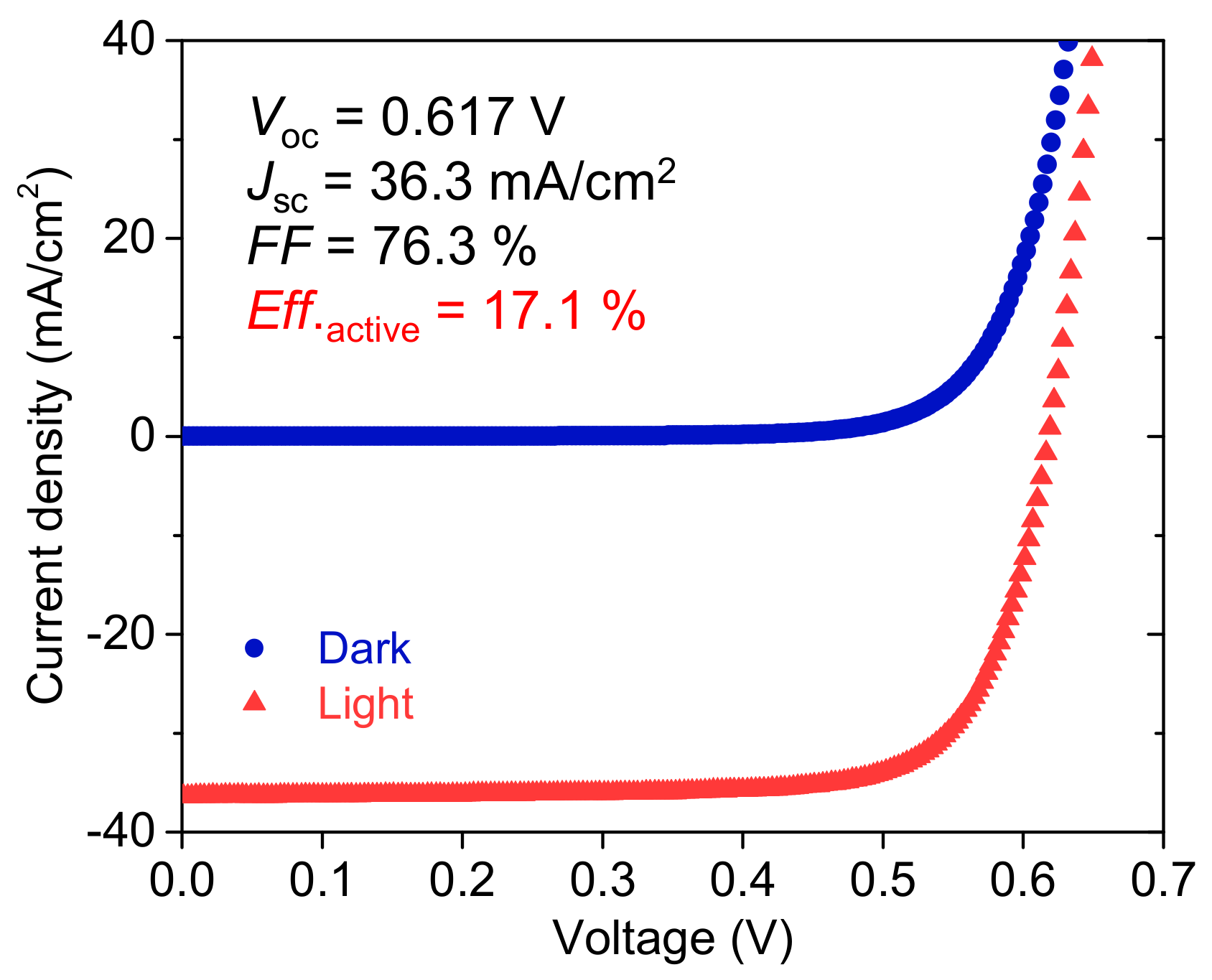
3.1. Two-Step Process
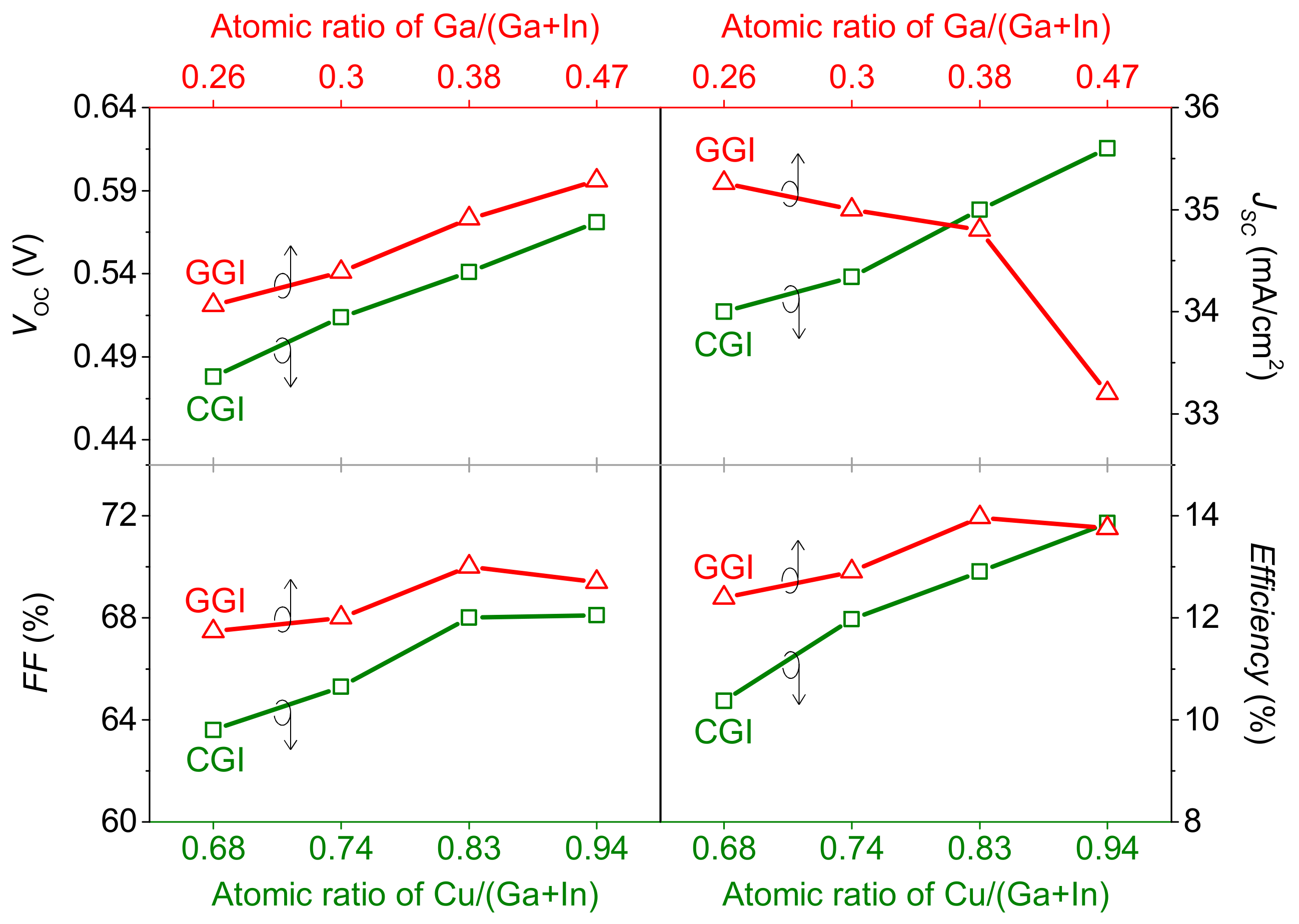
3.2. Effects of Ga Content on the Co-Evaporated CIGS Solar Cells
3.3. Characteristics of CIGS Solar Cells Prepared by Various Deposition Processes
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niki, S.; Contreras, M.; Repins, I.; Powalla, M.; Kushiya, K.; Ishizuka, S.; Matsubara, K. CIGS absorbers and processes. Prog. Photovolt. Res. Appl. 2010, 18, 453–466. [Google Scholar] [CrossRef]
- Repins, I.; Contreras, M.A.; Egaas, B.; DeHart, C.; Scharf, J.; Perkins, C.L.; To, B.; Noufi, R. 19.9%-efficient ZnO/CdS/CuInGaSe2 solar cell with 81.2% fill factor. Prog. Photovolt. Res. Appl. 2008, 16, 235–239. [Google Scholar] [CrossRef]
- Chirilǎ, A.; Reinhard, P.; Pianezzi, F.; Bloesch, P.; Uhl, A.R.; Fella, C.; Kranz, L.; Keller, D.; Gretener, C.; Hagendorfer, H.; et al. Potassium-induced surface modification of Cu(In,Ga)Se2 thin films for high-efficiency solar cells. Nat. Mater. 2013, 12, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Hariskos, D.; Wuerz, R.; Kiowski, O.; Bauer, A.; Friedlmeier, T.M.; Powalla, M. Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7%. Phys. Status Solidi-R. 2015, 9, 28–31. [Google Scholar] [CrossRef]
- Jackson, P.; Wuerz, R.; Hariskos, D.; Lotter, E.; Witte, W.; Powalla, M. Effects of heavy alkali elements in Cu(In,Ga)Se2 solar cells with efficiencies up to 22.6%. Phys. Status Solidi-R. 2016, 10, 583–586. [Google Scholar] [CrossRef]
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (version 52). Prog. Photovolt. Res. Appl. 2018, 26, 427–436. [Google Scholar] [CrossRef]
- Brown, G.; Stone, P.; Woodruff, J.; Cardozo, B.; Jackrel, D. Device characteristics of a 17.1% efficient solar cell deposited by a non-vacuum printing method on flexible foil. In Proceedings of the 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 3230–3233. [Google Scholar]
- Aksu, S.; Pethe, S.; Kleiman-Shwarsctein, A.; Kundu, S.; Pinarbasi, M. Recent advances in electroplating based CIGS solar cell fabrication. In Proceedings of the 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 3092–3097. [Google Scholar]
- Todorov, T.K.; Gunawan, O.; Gokmen, T.; Mitzi, D.B. Solution-processed Cu(In,Ga)(S,Se)2 absorber yielding a 15.2% efficient solar cell. Prog. Photovolt. Res. Appl. 2013, 21, 82–87. [Google Scholar] [CrossRef]
- Mazzer, M.; Rampino, S.; Gombia, E.; Bronzoni, M.; Bissoli, F.; Pattini, F.; Calicchio, M.; Kingma, A.; Annoni, F.; Calestani, D; et al. Progress on low-temperature pulsed electron deposition of CuInGaSe2 solar cells. Energies 2016, 9, 207. [Google Scholar] [CrossRef]
- Kato, T. Cu(In,Ga)(Se,S)2 solar cell research in Solar Frontier: Progress and current status. Jpn. J. Appl. Phys. 2017, 56. [Google Scholar] [CrossRef]
- Herrmann, D.; Kratzert, P.; Weeke, S.; Zimmer, M.; Djordjevic-Reiss, J.; Hunger, R.; Lindberg, P.; Wallin, E.; Lundberg, O.; Stolt, L. CIGS module manufacturing with high deposition rates and efficiencies. In Proceedings of the 40th IEEE Photovoltaic Specialist Conference, Denver, CO, USA, 8–13 June 2014; pp. 2775–2777. [Google Scholar]
- Dalibor, T.; Eraerds, P.; Grave, M.; Algasinger, M.; Visbeck, S.; Niesen, T.; Palm, J. Advanced PVD buffers on the road to GW-scale CIGSSe production. In Proceedings of the 43rd IEEE Photovoltaic Specialists Conference, Portland, OR, USA, 5–10 June 2016; pp. 1433–1437. [Google Scholar]
- Mickelsen, R.A.; Chen, W.S. Development of a 9.4% efficient thin-film CuInSe2/CdS solar cell. In Proceedings of the 15th IEEE Photovoltaic Specialists Conference, Kissimmee, FL, USA, 12–15 May 1981; pp. 800–804. [Google Scholar]
- Mickelsen, R.A.; Chen, W.S. Polycrystalline thin-film CuInSe2 solar cells. In Proceedings of the 16th IEEE Photovoltaic Specialists Conference, San Diego, CA, USA, 27–30 September 1982; pp. 781–785. [Google Scholar]
- Gabor, A.M.; Tuttle, J.R.; Albin, D.S.; Contreras, M.A.; Noufi, R.; Hermann, A.M. High-efficiency CuInxGa1−xSe2 solar cells made from (Inx,Ga1−x)2Se3 precursor films. Appl. Phys. Lett. 1994, 65, 198–200. [Google Scholar] [CrossRef]
- Hegedus, S.S.; Shafarman, W.N. Thin-film solar cells: Device measurements and analysis. Prog. Photovolt. Res. Appl. 2004, 12, 155–176. [Google Scholar] [CrossRef]
- Sites, J.R.; Mauk, P.H. Diode quality factor determination for thin-film solar cells. Sol. Cells 1989, 27, 411–417. [Google Scholar] [CrossRef]
- Lundberg, O.; Edoff, M.; Stolt, L. The effect of Ga-grading in CIGS thin film solar cells. Thin Solid Films 2005, 480–481, 520–525. [Google Scholar] [CrossRef]
- Heath, J.T.; Cohen, J.D.; Shafarman, W.N.; Liao, D.X.; Rockett, A.A. Effect of Ga content on defect states in CuIn1−xGaxSe2 photovoltaic devices. Appl. Phys. Lett. 2002, 80, 4540–4542. [Google Scholar] [CrossRef]
- Pudov, A.O.; Sites, J.R.; Contreras, M.A.; Nakada, T.; Schock, H.-W. CIGS J-V distortion in the absence of blue photons. Thin Solid Films 2005, 480–481, 273–278. [Google Scholar] [CrossRef]
- Cao, Q.; Gunawan, O.; Copel, M.; Reuter, K.B.; Chey, S.J.; Deline, V.R.; Mitzi, D.B. Defects in Cu(In,Ga)Se2 chalcopyrite semiconductors: A comparative study of material properties, defect states, and photovoltaic performance. Adv. Energy Mater. 2011, 1, 845–853. [Google Scholar] [CrossRef]
- Kamada, R.; Shafarman, W.N.; Birkmire, R.W. Cu(In,Ga)Se2 film formation from selenization of mixed metal/metal-selenide precursors. Sol. Energy Mater. Sol. Cells 2010, 94, 451–456. [Google Scholar] [CrossRef]
- Hanket, G.M.; Kamada, R.; Kim, W.K.; Shafarman, W.N. Effect of reaction temperature on Cu(InGa)(SeS)2 formation by a sequential H2Se/H2S precursor reaction process. In Proceedings of the 33rd IEEE Photovoltaic Specialists Conference, San Diego, CA, USA, 11–16 May 2008; pp. 1–5. [Google Scholar]
- Delsol, T.; Samantilleke, A.P.; Chaure, N.B.; Gardiner, P.H.; Simmonds, M.; Dharmadasa, I.M. Experimental study of graded bandgap Cu(InGa)(SeS)2 thin films grown on glass/molybdenum substrates by selenization and sulphidation. Sol. Energy Mater. Sol. Cells 2004, 82, 587–599. [Google Scholar] [CrossRef]
- Marudachalam, M.; Birkmire, R.W.; Hichri, H.; Schultz, J.M.; Swartzlander, A.; Al-Jassim, M.M. Phases, morphology, and diffusion in CulnxGa1−xSe2 thin films. J. Appl. Phys. 1997, 82, 2896–2905. [Google Scholar] [CrossRef]
- Haalboom, T.; Gödecke, T.; Ernst, F.; Rühle, M.; Herberholz, R.; Schock, H.-W.; Beilharz, C.; Benz, K. Phase relations and microstructure in bulk materials and thin films of the ternary system Cu-In-Se. In Proceedings of the 11th ternary and multinary compounds, Salford, UK, 8–12 September 1997; IOP Publishing: Bristol, UK, 1998; pp. 249–252. [Google Scholar]
- Huang, C.-H.; Lin, C.-P.; Jan, Y.-L. Characteristics of CIGS photovoltaic devices co-evaporated with various Se flux rates at low temperatures. Semicond. Sci. Tech. 2016, 31, 085004. [Google Scholar] [CrossRef]
- Kroemer, H. Quasi-electric and quasi-magnetic fields in nonuniform semiconductors. RCA Rev. 1957, 18, 332–342. [Google Scholar]
- Kim, K.; Park, H.; Hanket, G.M.; Kim, W.K.; Shafarman, W.N. Composition and bandgap control in Cu(In,Ga)Se2-based absorbers formed by reaction of metal precursors. Prog. Photovolt. Res. Appl. 2015, 23, 765–772. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, D.H.; Huh, K.S.; Jung, S.J.; Lee, J.W.; Lee, H.C.; Baek, D.H.; Kim, B.J.; Kim, D.S.; Nam, J.G.; et al. Influence of surface properties on the performance of Cu(In,Ga)(Se,S)2 thin-film solar cells using kelvin probe force microscopy. RSC Adv. 2015, 5, 40719–40725. [Google Scholar] [CrossRef]
- Chantana, J.; Kato, T.; Sugimoto, H.; Minemoto, T. Investigation of correlation between open-circuit voltage deficit and carrier recombination rates in Cu(In,Ga)(S,Se)2-based thin-film solar cells. Appl. Phys. Lett. 2018, 112, 151601. [Google Scholar] [CrossRef]
- Mansfield, L.M.; Garris, R.L.; Counts, K.D.; Sites, J.R.; Thompson, C.P.; Shafarman, W.N.; Ramanathan, K. Comparison of CIGS solar cells made with different structures and fabrication techniques. IEEE J. Photovolt. 2017, 7, 286–293. [Google Scholar] [CrossRef]
- Hages, C.J.; Carter, N.J.; Agrawal, R. Generalized quantum efficiency analysis for non-ideal solar cells: Case of Cu2ZnSnSe4. J. Appl. Phys. 2016, 119, 014505. [Google Scholar] [CrossRef]
- Contreras, M.A.; Tuttle, J.; Gabor, A.; Tennant, A.; Ramanathan, K.; Asher, S.; Franz, A.; Keane, J.; Wang, L.; Scofield, J.; et al. High efficiency Cu(In,Ga)Se2-based solar cells: Processing of novel absorber structures. In Proceedings of the 1st World Conference on Photovoltaic Energy Conversion, Waikoloa, HI, USA, 5–9 December 1994; pp. 68–75. [Google Scholar]
- Topič, M.; Smole, F.; Furlan, J. Band-gap engineering in CdS/Cu(In,Ga)Se2 solar cells. J. Appl. Phys. 1996, 79, 8537–8540. [Google Scholar] [CrossRef]
- Gabor, A.M.; Tuttle, J.R.; Bode, M.H.; Franz, A.; Tennant, A.L.; Contreras, M.A.; Noufi, R.; Jensen, D.G.; Hermann, A.M. Band-gap engineering in Cu(In,Ga)Se2 thin films grown from (In,Ga)2Se3 precursors. Sol. Energy Mater. Sol. Cells 1996, 41–42, 247–260. [Google Scholar] [CrossRef]
- Contreras, M.A.; Egaas, B.; Ramanathan, K.; Hiltner, J.; Swartzlander, A.; Hasoon, F.; Noufi, R. Progress toward 20% efficiency in Cu(In,Ga)Se2 polycrystalline thin-film solar cells. Prog. Photovolt. Res. Appl. 1999, 7, 311–316. [Google Scholar] [CrossRef]
- Contreras, M.A.; Romero, M.J.; Noufi, R. Characterization of Cu(In,Ga)Se2 materials used in record performance solar cells. Thin Solid Films 2006, 511–512, 51–54. [Google Scholar] [CrossRef]
- Phillips, J.E.; Birkmire, R.W.; McCandless, B.E.; Meyers, P.V.; Shafarman, W.N. Polycrystalline heterojunction solar cells: A device perspective. Phys. Status Solidi B 1996, 194, 31–39. [Google Scholar] [CrossRef]
- Scheer, R.; Schock, H.-W. Thin Film Heterostructures. In Chalcogenide Photovoltaics: Physics, Technologies, and Thin Film Devices; Wiley-VCH: Weinheim, Germany, 2011; p. 38. ISBN 978-352-731-459-1. [Google Scholar]
- Contreras, M.A.; Ramanathan, K.; AbuShama, J.; Hasoon, F.; Young, D.L.; Egaas, B.; Noufi, R. Diode characteristics in state-of-the-art ZnO/CdS/Cu(In1−xGax)Se2 solar cells. Prog. Photovolt. Res. Appl. 2005, 13, 209–216. [Google Scholar] [CrossRef]
- Rau, U.; Jasenek, A.; Schock, H.W.; Engelhardt, F.; Meyer, T. Electronic loss mechanisms in chalcopyrite based heterojunction solar cells. Thin Solid Films 2000, 361, 298–302. [Google Scholar] [CrossRef]
- Hanna, G.; Jasenek, A.; Rau, U.; Schock, H.W. Influence of the Ga-content on the bulk defect densities of Cu(In,Ga)Se2. Thin Solid Films 2001, 387, 71–73. [Google Scholar] [CrossRef]
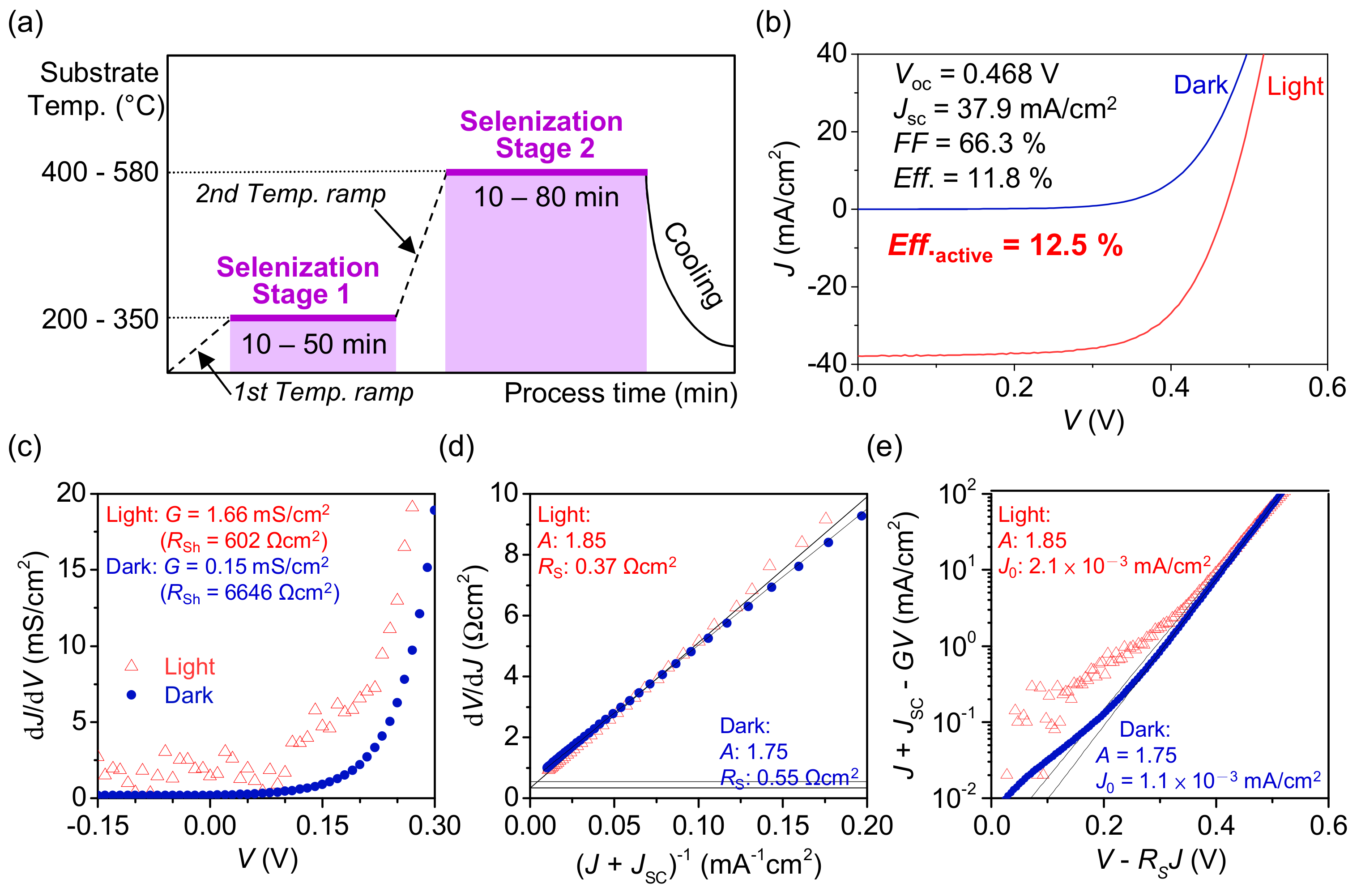
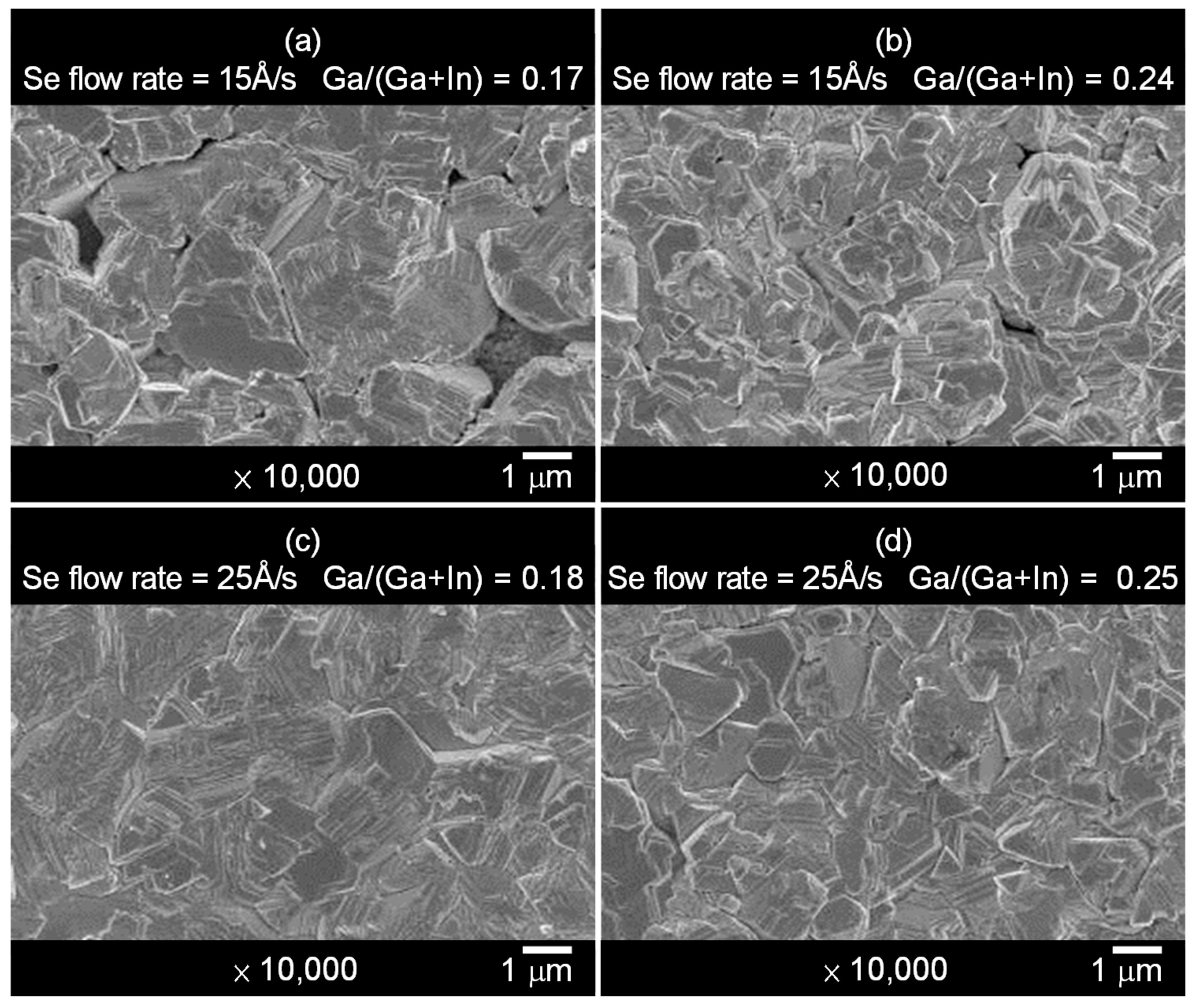
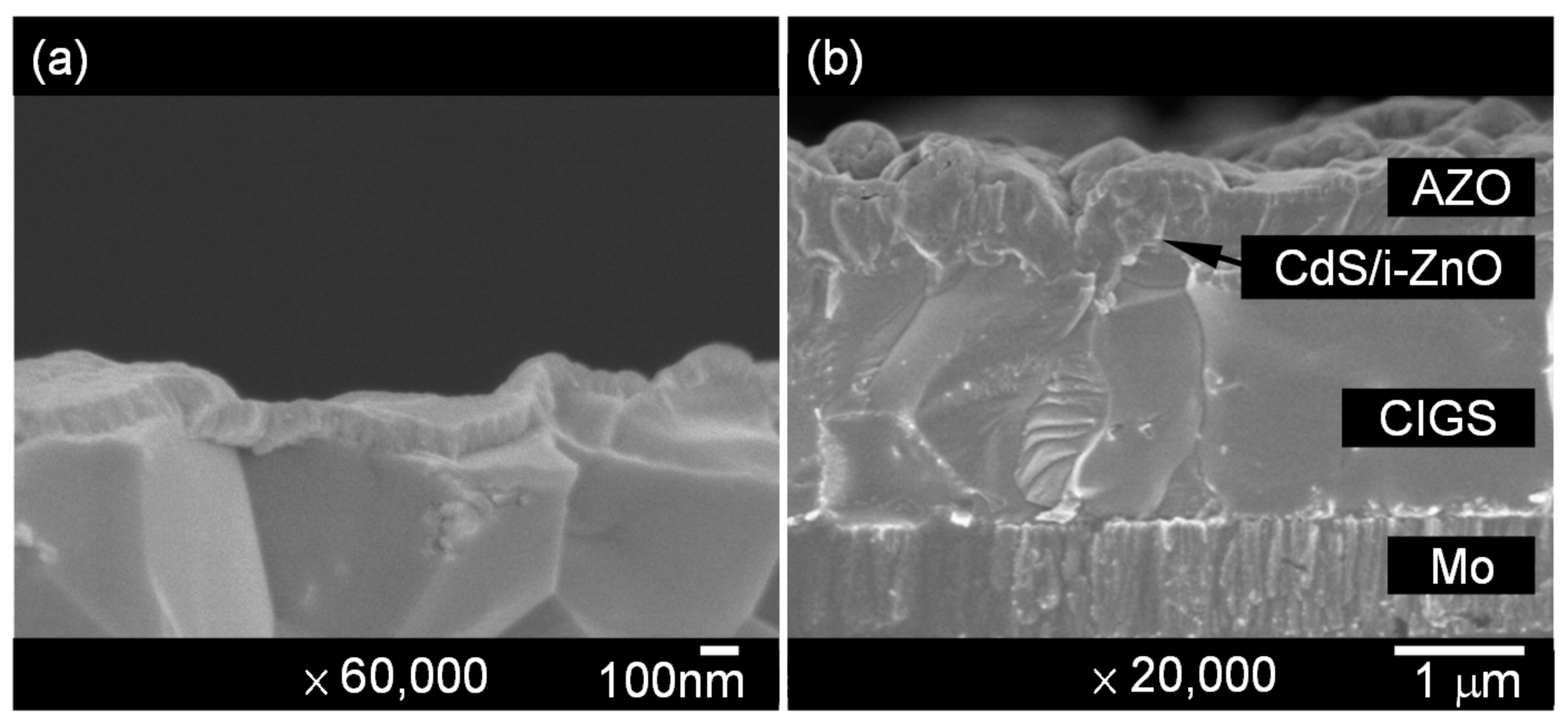





| Sample No. | Se Flow Rate | Composition (at %) | Cu/(Ga + In) | Ga/(Ga + In) | VOC (V) | JSC (mA/cm2) | FF (%) | η (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | In | Ga | Se | ||||||||
| (a) | 15 Å/s | 21.9 | 23.1 | 4.8 | 50.2 | 0.79 | 0.17 | 0.432 | 39.0 | 62.2 | 10.5 |
| (b) | 15 Å/s | 22.8 | 21.0 | 6.7 | 49.5 | 0.82 | 0.24 | 0.451 | 38.6 | 64.5 | 11.2 |
| (c) | 25 Å/s | 21.4 | 22.6 | 5.1 | 50.9 | 0.77 | 0.18 | 0.452 | 40.1 | 64.7 | 11.7 |
| (d) | 25 Å/s | 22.2 | 19.7 | 6.6 | 51.5 | 0.84 | 0.25 | 0.468 | 40.1 | 66.3 | 12.5 |
| Ga Flux Rate | VOC (V) | JSC (mA/cm2) | FF (%) | η (%) | RSh (Ωcm2) | RSh (Ωcm2) | A | J0 (mA/cm2) |
|---|---|---|---|---|---|---|---|---|
| 0.40 Å/s | 0.593 | 35.5 | 71.4 | 14.9 | 0.18 | 1304 | 1.64 | 2.2 × 10−5 |
| 0.45 Å/s | 0.597 | 35.4 | 72.1 | 15.2 | 0.08 | 1326 | 1.61 | 1.5 × 10−5 |
| 0.50 Å/s | 0.604 | 35.2 | 72.6 | 15.4 | 0.09 | 1345 | 1.61 | 1.3 × 10−5 |
| 0.55 Å/s | 0.598 | 35.1 | 71.8 | 15.1 | 0.09 | 1352 | 1.63 | 1.9 × 10−5 |
| Process | VOC (V) | JSC (mA/cm2) | FF (%) | η (%) | RS (Ωcm2) | RSh (Ωcm2) | A | J0 (mA/cm2) | GGI | Eg (eV) | Eg,EQE (eV) | Eg/q-VOC (V) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Two-step | 0.468 | 40.1 | 66.3 | 12.5 | 0.37 | 602 | 1.85 | 2.1 × 10−3 | 0.25 | 1.14 | 1.03 | 0.672 |
| Single-stage | 0.564 | 35.4 | 72.2 | 14.4 | 0.06 | 1269 | 1.64 | 5.0 × 10−5 | 0.28 | 1.16 | 1.15 | 0.596 |
| Bi-layer | 0.531 | 35.1 | 70.6 | 13.2 | 0.11 | 1190 | 1.69 | 1.7 × 10−4 | 0.24 | 1.14 | 1.15 | 0.609 |
| Three-stage | 0.624 | 36.3 | 74.6 | 16.9 | 0.06 | 1572 | 1.60 | 9.1 × 10−6 | 0.30 | 1.17 | 1.14 | 0.546 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Chuang, W.-J.; Lin, C.-P.; Jan, Y.-L.; Shih, Y.-C. Deposition Technologies of High-Efficiency CIGS Solar Cells: Development of Two-Step and Co-Evaporation Processes. Crystals 2018, 8, 296. https://doi.org/10.3390/cryst8070296
Huang C-H, Chuang W-J, Lin C-P, Jan Y-L, Shih Y-C. Deposition Technologies of High-Efficiency CIGS Solar Cells: Development of Two-Step and Co-Evaporation Processes. Crystals. 2018; 8(7):296. https://doi.org/10.3390/cryst8070296
Chicago/Turabian StyleHuang, Chia-Hua, Wen-Jie Chuang, Chun-Ping Lin, Yueh-Lin Jan, and Yu-Chiu Shih. 2018. "Deposition Technologies of High-Efficiency CIGS Solar Cells: Development of Two-Step and Co-Evaporation Processes" Crystals 8, no. 7: 296. https://doi.org/10.3390/cryst8070296






