Metal–Organic Framework Hybrid Materials and Their Applications
Abstract
:1. Introduction
2. Covalent Modifications
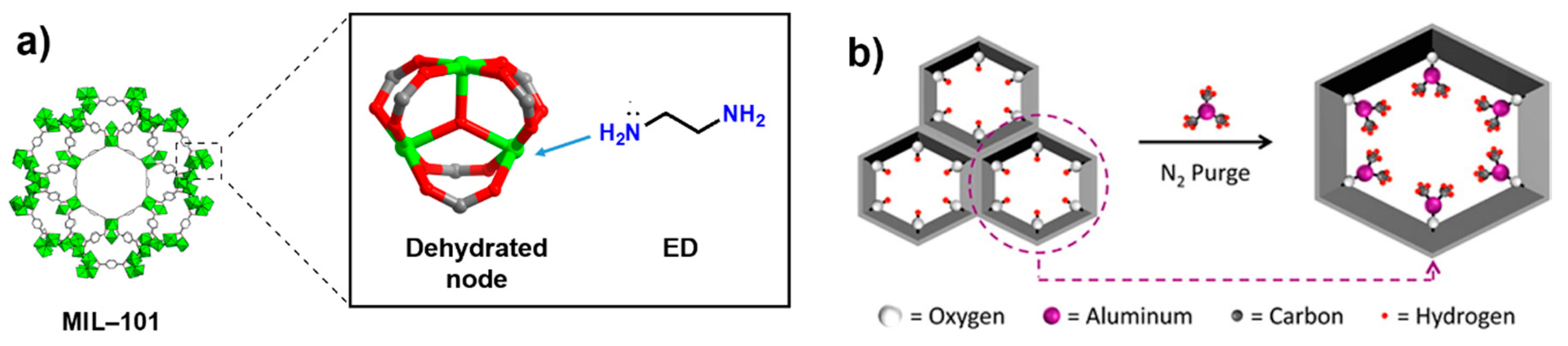
2.1. Covalent Modifications on the Metal Nodes
2.2. Covalent Modifications on the Ligands
3. Noncovalent Interactions
3.1. Encapsulation
3.2. Layer-by-Layer Deposition
3.3. In Situ Growth
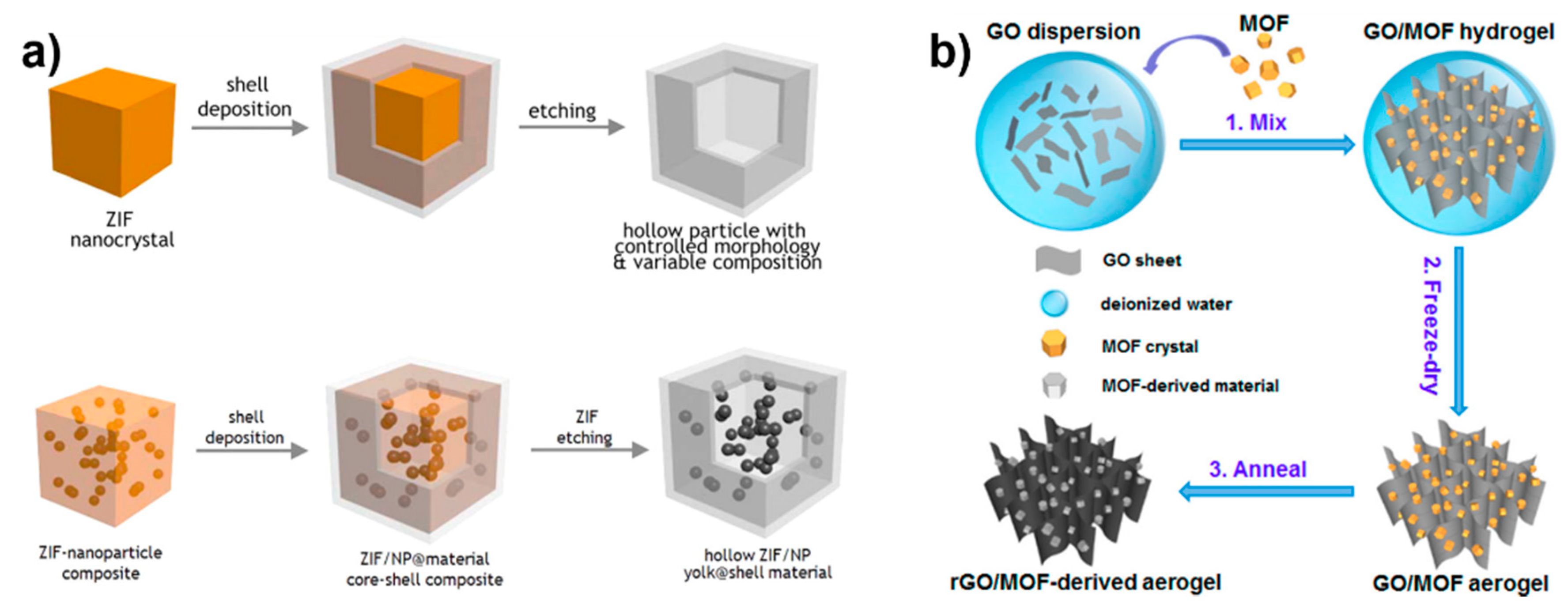
4. Using MOFs as Sacrificial Templates or Precursors
4.1. MOFs as Sacrificial Templates
4.2. MOFs as Sacrificial Precursors
5. Conclusions and Future Outlook
Funding
Conflicts of Interest
References
- Batten, S.; Champness, N.; Chen, X.-M.; García-Martínez, J.; Kitagawa, S.; Öhrström, L.; O Keeffe, M.; Suh, M.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013)*. Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, P.Z.; Li, A.; Wiggin, S.B.; Tao, A.; Maloney, A.G.P.; Wood, P.A.; Ward, S.C.; Fairen-Jimenez, D. Development of a Cambridge Structural Database Subset: A Collection of Metal–Organic Frameworks for Past, Present, and Future. Chem. Mater. 2017, 29, 2618–2625. [Google Scholar] [CrossRef]
- Nguyen, J.G.; Cohen, S.M. Moisture-Resistant and Superhydrophobic Metal−Organic Frameworks Obtained via Postsynthetic Modification. J. Am. Chem. Soc. 2010, 132, 4560–4561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang Seung, J.; Park Chong, R. Preparation of Highly Moisture-Resistant Black-Colored Metal Organic Frameworks. Adv. Mater. 2012, 24, 4010–4013. [Google Scholar] [CrossRef] [PubMed]
- Küsgens, P.; Siegle, S.; Kaskel, S. Crystal Growth of the Metal—Organic Framework Cu3(BTC)2 on the Surface of Pulp Fibers. Adv. Eng. Mater. 2009, 11, 93–95. [Google Scholar] [CrossRef]
- Aijaz, A.; Xu, Q. Catalysis with Metal Nanoparticles Immobilized within the Pores of Metal–Organic Frameworks. J. Phys. Chem. Lett. 2014, 5, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tan, C.; Sindoro, M.; Zhang, H. Hybrid micro-/nano-structures derived from metal–organic frameworks: Preparation and applications in energy storage and conversion. Chem. Soc. Rev. 2017, 46, 2660–2677. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Preuss, K.; Titirici, M.-M.; Rodríguez-Reinoso, F. Nanoporous Materials for the Onboard Storage of Natural Gas. Chem. Rev. 2017, 117, 1796–1825. [Google Scholar] [CrossRef] [PubMed]
- Herm, Z.R.; Bloch, E.D.; Long, J.R. Hydrocarbon Separations in Metal–Organic Frameworks. Chem. Mater. 2014, 26, 323–338. [Google Scholar] [CrossRef]
- Rojas, S.; Carmona, F.J.; Maldonado, C.R.; Horcajada, P.; Hidalgo, T.; Serre, C.; Navarro, J.A.R.; Barea, E. Nanoscaled Zinc Pyrazolate Metal–Organic Frameworks as Drug-Delivery Systems. Inorg. Chem. 2016, 55, 2650–2663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Howarth Ashlee, J.; Hupp Joseph, T.; Farha Omar, K. Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal–Organic Framework. Angew. Chem. Int. Ed. 2015, 54, 9001–9005. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Dong, L.-Z.; Li, S.-L.; Xu, G.; Liu, J.; Zhang, F.-M.; Lu, L.-S.; Lan, Y.-Q. Synergistic Conductivity Effect in a Proton Sources-Coupled Metal–Organic Framework. ACS Energy Lett. 2017, 2, 2313–2318. [Google Scholar] [CrossRef]
- Huang, W.; Li, S.; Cao, X.; Hou, C.; Zhang, Z.; Feng, J.; Ci, L.; Si, P.; Chi, Q. Metal–Organic Framework Derived Iron Sulfide–Carbon Core–Shell Nanorods as a Conversion-Type Battery Material. ACS Sustain. Chem. Eng. 2017, 5, 5039–5048. [Google Scholar] [CrossRef]
- Buru, C.T.; Li, P.; Mehdi, B.L.; Dohnalkova, A.; Platero-Prats, A.E.; Browning, N.D.; Chapman, K.W.; Hupp, J.T.; Farha, O.K. Adsorption of a Catalytically Accessible Polyoxometalate in a Mesoporous Channel-type Metal–Organic Framework. Chem. Mater. 2017, 29, 5174–5181. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, Z.-G.; Fu, W.-Q.; Wang, F.; Zhang, J. A Confined Fabrication of Perovskite Quantum Dots in Oriented MOF Thin Film. ACS Appl. Mater. Interfaces 2016, 8, 28737–28742. [Google Scholar] [CrossRef] [PubMed]
- He, C.-T.; Tian, J.-Y.; Liu, S.-Y.; Ouyang, G.; Zhang, J.-P.; Chen, X.-M. A porous coordination framework for highly sensitive and selective solid-phase microextraction of non-polar volatile organic compounds. Chem. Sci. 2013, 4, 351–356. [Google Scholar] [CrossRef]
- Wang, Z.; Cohen, S.M. Postsynthetic Covalent Modification of a Neutral Metal–Organic Framework. J. Am. Chem. Soc. 2007, 129, 12368–12369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, G.; Cui, C.; Liu, Y.; Li, S.; Yan, W.; Xing, C.; Chi Yonggui, R.; Yang, Y.; Huo, F. A Family of Metal-Organic Frameworks Exhibiting Size-Selective Catalysis with Encapsulated Noble-Metal Nanoparticles. Adv. Mater. 2014, 26, 4056–4060. [Google Scholar] [CrossRef] [PubMed]
- Anbia, M.; Hoseini, V. Development of MWCNT@MIL-101 hybrid composite with enhanced adsorption capacity for carbon dioxide. Chem. Eng. J. 2012, 191, 326–330. [Google Scholar] [CrossRef]
- Lu, G.; Farha Omar, K.; Zhang, W.; Huo, F.; Hupp Joseph, T. Engineering ZIF-8 Thin Films for Hybrid MOF-Based Devices. Adv. Mater. 2012, 24, 3970–3974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ji, S.; Wang, N.; Wang, L.; Zhang, G.; Li, J.R. Coordination-driven in situ self-assembly strategy for the preparation of metal–organic framework hybrid membranes. Angew. Chem. Int. Ed. 2014, 53, 9775–9779. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Hu, X.; Li, Z.; Qie, L.; Hu, C.; Zeng, R.; Jiang, Y.; Huang, Y. MOF-Derived Porous ZnO/ZnFe2O4/C Octahedra with Hollow Interiors for High-Rate Lithium-Ion Batteries. Adv. Mater. 2014, 26, 6622–6628. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, W.; Lv, L.-P.; Kong, S.; Wang, Y. Microwave-Assisted Morphology Evolution of Fe-Based Metal–Organic Frameworks and Their Derived Fe2O3 Nanostructures for Li-Ion Storage. ACS Nano 2017, 11, 4198–4205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.K.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks—A progress report. Chem. Soc. Rev. 2011, 40, 498–519. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Bae, J.; Lee, E.J.; Jeong, N.C. A Chemical Role for Trichloromethane: Room-Temperature Removal of Coordinated Solvents from Open Metal Sites in the Copper-Based Metal–Organic Frameworks. Inorg. Chem. 2018, 57, 5225–5231. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Choi, J.S.; Hwang, S.; Yun, W.S.; Song, D.; Lee, J.; Jeong, N.C. Multiple Coordination Exchanges for Room-Temperature Activation of Open-Metal Sites in Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 24743–24752. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Yun, W.S.; Kim, M.-B.; Kim, J.Y.; Bae, Y.-S.; Lee, J.; Jeong, N.C. A Chemical Route to Activation of Open Metal Sites in the Copper-Based Metal–Organic Framework Materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.C.; Samanta, B.; Lee, C.Y.; Farha, O.K.; Hupp, J.T. Coordination-Chemistry Control of Proton Conductivity in the Iconic Metal–Organic Framework Material HKUST-1. J. Am. Chem. Soc. 2012, 134, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kuang, Q.; Wang, Q.; Xie, Z. Engineering a High Energy Surface of Anatase TiO2 Crystals Towards Enhanced Performance for Energy Conversion and Environmental Applications. RSC Adv. 2015, 5, 20396–20409. [Google Scholar] [CrossRef]
- Choi, S.; Watanabe, T.; Bae, T.-H.; Sholl, D.S.; Jones, C.W. Modification of the Mg/DOBDC MOF with Amines to Enhance CO2 Adsorption from Ultradilute Gases. J. Phys. Chem. Lett. 2012, 3, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Schweitzer, N.M.; League, A.B.; Bernales, V.; Peters, A.W.; Getsoian, A.B.; Wang, T.C.; Miller, J.T.; Vjunov, A.; Fulton, J.L.; et al. Sintering-Resistant Single-Site Nickel Catalyst Supported by Metal–Organic Framework. J. Am. Chem. Soc. 2016, 138, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Rimoldi, M.; Platero-Prats Ana, E.; Chapman Karena, W.; Hupp Joseph, T.; Farha Omar, K. Stabilizing a Vanadium Oxide Catalyst by Supporting on a Metal–Organic Framework. ChemCatChem 2017, 10, 1772–1777. [Google Scholar] [CrossRef]
- Li, Z.; Peters, A.W.; Liu, J.; Zhang, X.; Schweitzer, N.M.; Hupp, J.T.; Farha, O.K. Size effect of the active sites in UiO-66-supported nickel catalysts synthesized via atomic layer deposition for ethylene hydrogenation. Inorg. Chem. Front. 2017, 4, 820–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondloch, J.E.; Bury, W.; Fairen-Jimenez, D.; Kwon, S.; DeMarco, E.J.; Weston, M.H.; Sarjeant, A.A.; Nguyen, S.T.; Stair, P.C.; Snurr, R.Q.; et al. Vapor-Phase Metalation by Atomic Layer Deposition in a Metal–Organic Framework. J. Am. Chem. Soc. 2013, 135, 10294–10297. [Google Scholar] [CrossRef] [PubMed]
- Gallington, L.C.; Kim, I.S.; Liu, W.-G.; Yakovenko, A.A.; Platero-Prats, A.E.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K.; Truhlar, D.G.; et al. Regioselective Atomic Layer Deposition in Metal–Organic Frameworks Directed by Dispersion Interactions. J. Am. Chem. Soc. 2016, 138, 13513–13516. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Borycz, J.; Platero-Prats, A.E.; Tussupbayev, S.; Wang, T.C.; Farha, O.K.; Hupp, J.T.; Gagliardi, L.; Chapman, K.W.; Cramer, C.J.; et al. Targeted Single-Site MOF Node Modification: Trivalent Metal Loading via Atomic Layer Deposition. Chem. Mater. 2015, 27, 4772–4778. [Google Scholar] [CrossRef]
- Peters, A.W.; Li, Z.; Farha, O.K.; Hupp, J.T. Atomically Precise Growth of Catalytically Active Cobalt Sulfide on Flat Surfaces and within a Metal–Organic Framework via Atomic Layer Deposition. ACS Nano 2015, 9, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, M.; Bernales, V.; Borycz, J.; Vjunov, A.; Gallington, L.C.; Platero-Prats, A.E.; Kim, I.S.; Fulton, J.L.; Martinson, A.B.F.; Lercher, J.A.; et al. Atomic Layer Deposition in a Metal–Organic Framework: Synthesis, Characterization, and Performance of a Solid Acid. Chem. Mater. 2017, 29, 1058–1068. [Google Scholar] [CrossRef]
- Kung, C.-W.; Mondloch, J.E.; Wang, T.C.; Bury, W.; Hoffeditz, W.; Klahr, B.M.; Klet, R.C.; Pellin, M.J.; Farha, O.K.; Hupp, J.T. Metal–Organic Framework Thin Films as Platforms for Atomic Layer Deposition of Cobalt Ions To Enable Electrocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 28223–28230. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal−Organic Frameworks for Imaging and Drug Delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Das, S.; Xing, G.; Ben, T.; Valtchev, V.; Qiu, S. Fabrication of COF-MOF Composite Membranes and Their Highly Selective Separation of H2/CO2. J. Am. Chem. Soc. 2016, 138, 7673–7680. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2009, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H.-C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-M.; Dong, H.; Zhang, X.; Sun, X.-J.; Liu, M.; Yang, D.-D.; Liu, X.; Wei, J.-Z. Postsynthetic Modification of ZIF-90 for Potential Targeted Codelivery of Two Anticancer Drugs. ACS Appl. Mater. Interfaces 2017, 9, 27332–27337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-G.; Dong, Z.-Y.; Cheng, H.; Wan, S.-S.; Chen, W.-H.; Zou, M.-Z.; Huo, J.-W.; Deng, H.-X.; Zhang, X.-Z. A multifunctional metal–organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale 2015, 7, 16061–16070. [Google Scholar] [CrossRef] [PubMed]
- Abánades Lázaro, I.; Haddad, S.; Sacca, S.; Orellana-Tavra, C.; Fairen-Jimenez, D.; Forgan, R.S. Selective Surface PEGylation of UiO-66 Nanoparticles for Enhanced Stability, Cell Uptake, and pH-Responsive Drug Delivery. Chem 2017, 2, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically Active Graphene–Porphyrin MOF Composite for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.S.; Enthaler, S.; Junge, K.; Beller, M. Iron-catalyzed enantioselective hydrosilylation of ketones. Angew. Chem. Int. Ed. 2008, 47, 2497–2501. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Feng, K.; Tang, B.; Wu, P. Construction of well interconnected metal-organic framework structure for effectively promoting proton conductivity of proton exchange membrane. J. Membr. Sci. 2017, 533, 160–170. [Google Scholar] [CrossRef]
- Maina, J.W.; Schütz, J.A.; Grundy, L.; Des Ligneris, E.; Yi, Z.; Kong, L.; Pozo-Gonzalo, C.; Ionescu, M.; Dumée, L.F. Inorganic Nanoparticles/Metal Organic Framework Hybrid Membrane Reactors for Efficient Photocatalytic Conversion of CO2. ACS Appl. Mater. Interfaces 2017, 9, 35010–35017. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Deng, K.; He, L.; Liu, Y.; Li, G.; Zhao, H.; Tang, Z. Core–Shell Palladium Nanoparticle@Metal–Organic Frameworks as Multifunctional Catalysts for Cascade Reactions. J. Am. Chem. Soc. 2014, 136, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Hu, J.; Zhou, L.; Li, S.; Hu, X.; Huang, H. Removal of Dibenzothiophene with Composite Adsorbent MOF-5/Cu(I). Energy Fuels 2013, 27, 816–821. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Huang, J.; Chen, L.; Ma, L.; Huang, X. Conversion of Cellulose and Cellobiose into Sorbitol Catalyzed by Ruthenium Supported on a Polyoxometalate/Metal–Organic Framework Hybrid. ChemSusChem 2013, 6, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Buru, C.T.; Platero-Prats, A.E.; Chica, D.G.; Kanatzidis, M.G.; Chapman, K.W.; Farha, O.K. Thermally induced migration of a polyoxometalate within a metal–organic framework and its catalytic effects. J. Mater. Chem. A 2018, 6, 7389–7394. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Gao, M.-R.; Liu, C.-H.; Li, S.-L.; Jiang, H.-L.; Lan, Y.-Q.; Han, M.; Yu, S.-H. Porous Molybdenum-Based Hybrid Catalysts for Highly Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2015, 54, 12928–12932. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhang, Z.; Xu, X.; Gong, Q.; Li, J.; Wu, C.-D. A Multifunctional Organic–Inorganic Hybrid Structure Based on MnIII–Porphyrin and Polyoxometalate as a Highly Effective Dye Scavenger and Heterogenous Catalyst. J. Am. Chem. Soc. 2012, 134, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xu, S.; Guo, C.; Chen, Y.; Wang, L. Embedding Nanocluster in MOF via Crystalline Ion-Triggered Growth Strategy for Improved Emission and Selective Sensing. ACS Appl. Mater. Interfaces 2018, 10, 16059–16065. [Google Scholar] [CrossRef] [PubMed]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef] [PubMed]
- Masih, D.; Chernikova, V.; Shekhah, O.; Eddaoudi, M.; Mohammed, O.F. Zeolite-like Metal–Organic Framework (MOF) Encaged Pt(II)-Porphyrin for Anion-Selective Sensing. ACS Appl. Mater. Interfaces 2018, 10, 11399–11405. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gao, G.; Zheng, L.; Chi, Y.; Chen, G. Encapsulation of Strongly Fluorescent Carbon Quantum Dots in Metal–Organic Frameworks for Enhancing Chemical Sensing. Anal. Chem. 2014, 86, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lin, B.; Hu, X.; Wei, Y.; Zhang, C.; An, B.; Wang, C.; Lin, W. Warm-White-Light-Emitting Diode Based on a Dye-Loaded Metal–Organic Framework for Fast White-Light Communication. ACS Appl. Mater. Interfaces 2017, 9, 35253–35259. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Shin, C.Y.; Yoon, S.J.; Lee, H.Y.; Lee, W.; Shrestha, N.K.; Lee, J.K.; Han, S.-H. Enhanced photovoltaic performance of Cu-based metal-organic frameworks sensitized solar cell by addition of carbon nanotubes. Sci. Rep. 2014, 4, 3930. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-Light-Promoted Photocatalytic Hydrogen Production by Using an Amino-Functionalized Ti(IV) Metal–Organic Framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Agostoni, V.; Horcajada, P.; Noiray, M.; Malanga, M.; Aykaç, A.; Jicsinszky, L.; Vargas-Berenguel, A.; Semiramoth, N.; Daoud-Mahammed, S.; Nicolas, V.; et al. A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci. Rep. 2015, 5, 7925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Tang, B.; Wu, P. Rational Design of S-UiO-66@GO Hybrid Nanosheets for Proton Exchange Membranes with Significantly Enhanced Transport Performance. ACS Appl. Mater. Interfaces 2017, 9, 26077–26087. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.-H.; Lee Jong, S.; Qiu, W.; Koros William, J.; Jones Christopher, W.; Nair, S. A High-Performance Gas-Separation Membrane Containing Submicrometer-Sized Metal–Organic Framework Crystals. Angew. Chem. Int. Ed. 2010, 49, 9863–9866. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Mixed-matrix membranes containing MOF-5 for gas separations. J. Membr. Sci. 2009, 328, 165–173. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; He, S.; Zhang, J.; Xu, X.; Yang, Y.; Nosheen, F.; Saleem, F.; He, W.; Wang, X. Hierarchical Zn/Ni-MOF-2 nanosheet-assembled hollow nanocubes for multicomponent catalytic reactions. Angew. Chem. Int. Ed. 2014, 53, 12517–12521. [Google Scholar] [CrossRef]
- Li, R.; Hu, J.; Deng, M.; Wang, H.; Wang, X.; Hu, Y.; Jiang, H.-L.; Jiang, J.; Zhang, Q.; Xie, Y.; et al. Integration of an Inorganic Semiconductor with a Metal–Organic Framework: A Platform for Enhanced Gaseous Photocatalytic Reactions. Adv. Mater. 2014, 26, 4783–4788. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Shinde, D.V.; Yoon, S.J.; Cho, K.N.; Lee, W.; Shrestha, N.K.; Han, S.-H. Cu-Based Metal–Organic Frameworks for Photovoltaic Application. J. Phys. Chem. C 2014, 118, 16328–16334. [Google Scholar] [CrossRef]
- Grätzel, M. Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J. Photochem. Photobiol. Chem. 2004, 164, 3–14. [Google Scholar] [CrossRef]
- Ji, H.; Hwang, S.; Kim, K.; Kim, C.; Jeong, N.C. Direct in Situ Conversion of Metals into Metal–Organic Frameworks: A Strategy for the Rapid Growth of MOF Films on Metal Substrates. ACS Appl. Mater. Interfaces 2016, 8, 32414–32420. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, E.J.; Choi, J.S.; Jeong, N.C. Diffusion Control in the in Situ Synthesis of Iconic Metal-Organic Frameworks within an Ionic Polymer Matrix. ACS Appl. Mater. Interfaces 2018, 10, 3793–3800. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Y.; Gu, Z.Y.; Jiang, D.Q.; Li, Y.; Wang, H.F.; Yan, X.P. In situ hydrothermal growth of metal-organic framework 199 films on stainless steel fibers for solid-phase microextraction of gaseous benzene homologues. Anal. Chem. 2009, 81, 9771–9777. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, S.; Wang, Y.; Li, L.; Su, C.; Liu, H.; Zhu, F.; Jiang, R.; Ouyang, G. In situ growth of IRMOF-3 combined with ionic liquids to prepare solid-phase microextraction fibers. Anal. Chim. Acta 2014, 829, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-W.; Sharma, R.; Meduri, P.; Arey, B.W.; Schaef, H.T.; Lutkenhaus, J.L.; Lemmon, J.P.; Thallapally, P.K.; Nandasiri, M.I.; McGrail, B.P.; et al. In Situ One-Step Synthesis of Hierarchical Nitrogen-Doped Porous Carbon for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 7214–7222. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, B.; Zhou, L.; Xia, Z.; Feng, N.; Ding, J.; Wang, L.; Wan, H.; Guan, G. Synthesis of Hierarchically Structured Hybrid Materials by Controlled Self-Assembly of Metal–Organic Framework with Mesoporous Silica for CO2 Adsorption. ACS Appl. Mater. Interfaces 2017, 9, 23060–23071. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Hill, A.J.; Nairn, K.M.; Jasieniak, J.; Mardel, J.I.; Bastow, T.J.; Mayo, S.C.; Gimona, M.; Gomez, D.; Whitfield, H.J.; et al. A new method to position and functionalize metal-organic framework crystals. Nat. Commun. 2011, 2, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dybtsev, D.N.; Ponomareva, V.G.; Aliev, S.B.; Chupakhin, A.P.; Gallyamov, M.R.; Moroz, N.K.; Kolesov, B.A.; Kovalenko, K.A.; Shutova, E.S.; Fedin, V.P. High Proton Conductivity and Spectroscopic Investigations of Metal–Organic Framework Materials Impregnated by Strong Acids. ACS Appl. Mater. Interfaces 2014, 6, 5161–5167. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilp, W.; Hu, M.; Wang, H.; Huang, H.-S.; Fujita, T.; Wu, K.C.W.; Chen, L.-C.; Yamauchi, Y.; Ariga, K. Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes. Chem. Commun. 2012, 48, 7259–7261. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kruger, P.E.; Telfer, S.G. Metal–Organic Framework Nanocrystals as Sacrificial Templates for Hollow and Exceptionally Porous Titania and Composite Materials. Inorg. Chem. 2015, 54, 9483–9490. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, W.; Li, P.; Ye, S.; Ye, C.; Ye, H.; Lu, T.; Zheng, A.; Zhu, J.; Xu, L.; et al. Facile Fabrication of Three-Dimensional Graphene and Metal–Organic Framework Composites and Their Derivatives for Flexible All-Solid-State Supercapacitors. Chem. Mater. 2017, 29, 6058–6065. [Google Scholar] [CrossRef]
- Malonzo, C.D.; Shaker, S.M.; Ren, L.; Prinslow, S.D.; Platero-Prats, A.E.; Gallington, L.C.; Borycz, J.; Thompson, A.B.; Wang, T.C.; Farha, O.K.; et al. Thermal Stabilization of Metal–Organic Framework-Derived Single-Site Catalytic Clusters through Nanocasting. J. Am. Chem. Soc. 2016, 138, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, C.; Gao, L.; Li, Q.; Song, Y.; Xu, F.; Wang, T.; Wang, L. Metal–Organic Framework-Derived Copper Nanoparticle@Carbon Nanocomposites as Peroxidase Mimics for Colorimetric Sensing of Ascorbic Acid. Chem. Eur. J. 2014, 20, 16377–16383. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Hong, D.-Y.; Chang, J.-S.; Jhung, S.H.; Seo, Y.-K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Férey, G. Amine Grafting on Coordinatively Unsaturated Metal Centers of MOFs: Consequences for Catalysis and Metal Encapsulation. Angew. Chem. Int. Ed. 2008, 47, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Platero-Prats, A.E.; Li, Z.; Gallington, L.C.; Peters, A.W.; Hupp, J.T.; Farha, O.K.; Chapman, K.W. Addressing the characterisation challenge to understand catalysis in MOFs: The case of nanoscale Cu supported in NU-1000. Faraday Discuss. 2017, 201, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Lownsbury, J.M.; Santos-López, I.A.; Zhang, W.; Campbell, C.T.; Yu, H.S.; Liu, W.-G.; Cramer, C.J.; Truhlar, D.G.; Wang, T.; Hupp, J.T.; et al. Calcium Vapor Adsorption on the Metal–Organic Framework NU-1000: Structure and Energetics. J. Phys. Chem. C 2016, 120, 16850–16862. [Google Scholar] [CrossRef]
- Rimoldi, M.; Gallington Leighanne, C.; Chapman Karena, W.; MacRenaris, K.; Hupp Joseph, T.; Farha Omar, K. Catalytically Active Silicon Oxide Nanoclusters Stabilized in a Metal–Organic Framework. Chem. Eur. J. 2017, 23, 8532–8536. [Google Scholar] [CrossRef] [PubMed]
- Beyzavi, M.H.; Klet, R.C.; Tussupbayev, S.; Borycz, J.; Vermeulen, N.A.; Cramer, C.J.; Stoddart, J.F.; Hupp, J.T.; Farha, O.K. A Hafnium-Based Metal–Organic Framework as an Efficient and Multifunctional Catalyst for Facile CO2 Fixation and Regioselective and Enantioretentive Epoxide Activation. J. Am. Chem. Soc. 2014, 136, 15861–15864. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Li, Z.; Zheng, J.; Platero-Prats Ana, E.; Mavrandonakis, A.; Pellizzeri, S.; Ferrandon, M.; Vjunov, A.; Gallington Leighanne, C.; Webber Thomas, E.; et al. Sinter-Resistant Platinum Catalyst Supported by Metal–Organic Framework. Angew. Chem. Int. Ed. 2017, 57, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Nauert, S.L.; Buru, C.T.; Rimoldi, M.; Choi, H.; Schweitzer, N.M.; Hupp, J.T.; Farha, O.K.; Notestein, J.M. Pushing the Limits on Metal–Organic Frameworks as a Catalyst Support: NU-1000 Supported Tungsten Catalysts for o-Xylene Isomerization and Disproportionation. J. Am. Chem. Soc. 2018, 140, 8535–8543. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.K.; Cohen, S.M. Engineering a metal-organic framework catalyst by using postsynthetic modification. Angew. Chem. Int. Ed. 2009, 48, 7424–7427. [Google Scholar] [CrossRef] [PubMed]
- Toyao, T.; Miyahara, K.; Fujiwaki, M.; Kim, T.-H.; Dohshi, S.; Horiuchi, Y.; Matsuoka, M. Immobilization of Cu Complex into Zr-Based MOF with Bipyridine Units for Heterogeneous Selective Oxidation. J. Phys. Chem. C 2015, 119, 8131–8137. [Google Scholar] [CrossRef]
- Ingleson, M.J.; Barrio, J.P.; Guilbaud, J.B.; Khimyak, Y.Z.; Rosseinsky, M.J. Framework functionalisation triggers metal complex binding. Chem. Commun. 2008, 23, 2680–2682. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Albero, J.; Xu, L.; García, H.; Li, Z. Construction of a Stable Ru–Re Hybrid System Based on Multifunctional MOF-253 for Efficient Photocatalytic CO2 Reduction. Inorg. Chem. 2018, 57, 8276–8286. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, H.; Li, Y. One-pot synthesis of Pd@MOF composites without the addition of stabilizing agents. Chem. Commun. 2014, 50, 14752–14755. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; He, C.; Zhao, M.; Qi, B.; Niu, J.; Duan, C. Engineering Chiral Polyoxometalate Hybrid Metal–Organic Frameworks for Asymmetric Dihydroxylation of Olefins. J. Am. Chem. Soc. 2013, 135, 10186–10189. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, A.L.; Kim, K.-H.; Deep, A. A novel CdTe/Eu-MOF photoanode for application in quantum dot-sensitized solar cell to improve power conversion efficiency. J. Ind. Eng. Chem. 2017, 53, 77–81. [Google Scholar] [CrossRef]
- Liu, J.-J.; Shan, Y.-B.; Fan, C.-R.; Lin, M.-J.; Huang, C.-C.; Dai, W.-X. Encapsulating Naphthalene in an Electron-Deficient MOF to Enhance Fluorescence for Organic Amines Sensing. Inorg. Chem. 2016, 55, 3680–3684. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Pan, F.; Liu, Y.; Huang, S.; Li, Y.; Yong, J.; Li, Y.; Kirillov, A.M.; Wu, D. An Efficient Blue-Emissive Metal–Organic Framework (MOF) for Lanthanide-Encapsulated Multicolor and Stimuli-Responsive Luminescence. Inorg. Chem. 2017, 56, 6362–6370. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene Oxide and Copper-Centered Metal Organic Framework Composite as a Tri-Functional Catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Yang, S.J.; Hyun Cho, J.; Nahm, K.-S.; Park, C. Enhanced hydrogen storage capacity of Pt-loaded CNT@MOF-5 hybrid composites. Int. J. Hydrog. Energy 2010, 35, 13062–13067. [Google Scholar] [CrossRef]
- Jiang, H.; Feng, Y.; Chen, M.; Wang, Y. Synthesis and hydrogen-storage performance of interpenetrated MOF-5/MWCNTs hybrid composite with high mesoporosity. Int. J. Hydrog. Energy 2013, 38, 10950–10955. [Google Scholar] [CrossRef]
- Yang, S.J.; Choi, J.Y.; Chae, H.K.; Cho, J.H.; Nahm, K.S.; Park, C.R. Preparation and Enhanced Hydrostability and Hydrogen Storage Capacity of CNT@MOF-5 Hybrid Composite. Chem. Mater. 2009, 21, 1893–1897. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, X.; Zhang, J.; Yan, X.; Liu, Y.; Yuan, A. Enhanced room-temperature hydrogen storage capacity in Pt-loaded graphene oxide/HKUST-1 composites. Int. J. Hydrog. Energy 2014, 39, 2160–2167. [Google Scholar] [CrossRef]
- Xiang, Z.; Hu, Z.; Cao, D.; Yang, W.; Lu, J.; Han, B.; Wang, W. Metal–Organic Frameworks with Incorporated Carbon Nanotubes: Improving Carbon Dioxide and Methane Storage Capacities by Lithium Doping. Angew. Chem. Int. Ed. 2010, 50, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Qiu, F.; Zaia, E.W.; Wang, Z.; Kunz, M.; Guo, J.; Brady, M.; Mi, B.; Urban, J.J. Dual-Channel, Molecular-Sieving Core/Shell ZIF@MOF Architectures as Engineered Fillers in Hybrid Membranes for Highly Selective CO2 Separation. Nano Lett. 2017, 17, 6752–6758. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lun, Z.; Xia, G.; Zheng, F.; He, M.; Chen, Q. Non-precious alloy encapsulated in nitrogen-doped graphene layers derived from MOFs as an active and durable hydrogen evolution reaction catalyst. Energy Environ. Sci. 2015, 8, 3563–3571. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-Organic Framework as a Template for Porous Carbon Synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, Q.; Lu, Z.; Liu, J.; Chen, R.; Li, R.; Song, D.; Jing, X.; Liu, P.; Wang, J. Rational Design of Sandwiched Ni–Co Layered Double Hydroxides Hollow Nanocages/Graphene Derived from Metal–Organic Framework for Sustainable Energy Storage. ACS Sustain. Chem. Eng. 2017, 5, 9923–9934. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, B.; Sun, Y.; Han, P.; Wang, J.; Ding, X.; Zhang, X.; Yang, H. MoO2@Cu@C Composites Prepared by Using Polyoxometalates@Metal-Organic Frameworks as Template for All-Solid-State Flexible Supercapacitor. Electrochim. Acta 2016, 188, 490–498. [Google Scholar] [CrossRef]
- Shen, J.Q.; Liao, P.Q.; Zhou, D.D.; He, C.T.; Wu, J.X.; Zhang, W.X.; Zhang, J.P.; Chen, X.M. Modular and Stepwise Synthesis of a Hybrid Metal–Organic Framework for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2017, 139, 1778–1781. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal-organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Zhou, X.-L.; Zhang, Y.-H.; Zhou, Z.; Bu, X.-H. MOF-Derived Porous Co3O4 Hollow Tetrahedra with Excellent Performance as Anode Materials for Lithium-Ion Batteries. Inorg. Chem. 2015, 54, 8159–8161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, S.; Yang, J.; Bai, T.; Ren, Y.; Tian, H. Metal–Organic Frameworks Derived Okra-like SnO2 Encapsulated in Nitrogen-Doped Graphene for Lithium Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 14309–14318. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.-Y.; Liu, Y.-C.; Jiang, Y.-F.; Yu, C.-Y.; Yue, M.-L.; Li, Z.-X. Benzoate Acid-Dependent Lattice Dimension of Co-MOFs and MOF-Derived CoS2@CNTs with Tunable Pore Diameters for Supercapacitors. Inorg. Chem. 2017, 56, 6184–6196. [Google Scholar] [CrossRef] [PubMed]




| Hybrid Method | MOF Used | Hybridized with | Applications | Ref | |
|---|---|---|---|---|---|
| Covalent Modification | on metal nodes | Cr(III) MIL-101 | Ethylene diamine | Catalysis | [90] |
| Mg/DOBDC | Ethylene diamine | Gas storage | [32] | ||
| NU-1000 | ALD in MOFs: Ni, ZnOx, AlOx, InOx, CoOx, CoSx, Cu2O, Ca(OH)2, SiO2, HfO, WOx, PtO | Catalysis | [37,39,40,41,91,92,93,94,95,96] | ||
| UiO-66 | ALD in MOFs: Ni; 4-(azidomethyl)benzoic acid, then PEG alkyne | Catalysis; drug delivery | [35,49] | ||
| Zn2(BDC)2(DABCO) and ZIF-8 | COF-300 (SiO2 and polyaniline disks) | Gas separation | [44] | ||
| MIL-88A and MIL-100 | CH3-O-PEG-NH2 | Drug delivery | [45] | ||
| on ligands | UMCM-1-NH2 | [Fe(acac)3] and [Cu(acac)2] | Catalysis | [97] | |
| Zr-MOF-bpy | CuBr2 | Catalysis | [98] | ||
| (Fe−P)n MOF | pyridine functionalized rGO sheets | Catalysis | [50] | ||
| IRMOF-3 | vanadium clusters, Folate | Catalysis, drug delivery | [47,99] | ||
| MOF-253 | Rhenium, Rubidium | Catalysis | [100] | ||
| MIL-101(Fe)-NH2 | Ethoxysuccinato-cisplatin, then thin silica coating | Drug delivery | [42] | ||
| ZIF-90 | Doxorubicin | Drug delivery | [47] | ||
| MIL-101(Fe)-N3 | Bicyclonyne-functionalized β-CD, then PEG-Ad | Drug delivery | [41] | ||
| Non-covalent Interactions | Encapsulation | UiO-66 | Pt NPs, Pd NPs | Catalysis | [19,101] |
| IRMOF-3 | Pd NPs | Catalysis | [55] | ||
| [Cd(DMF)2MnIII(DMF)2TPyP]n3n+ | POMs | Catalysis | [60] | ||
| NU-1000 | POMs | Catalysis | [15,58] | ||
| MIL-100(Cr) | POM-MIL-100(Cr) with Ru NPs | Catalysis | [58] | ||
| Ni-PYI | Ni NP-supported POMs | Catalysis | [102] | ||
| HKUST-1 | quantum dots (QDs) | Photovoltaics | [16] | ||
| ZIF-8 | Branched poly-(ethylenimine)-capped carbon QDs; ZnO; Au, Ag, NaYF4, Pt and CdTe NPs | Sensing, catalysis | [54,61,62,64] | ||
| Rho-ZMOF | Pt-metalated porphyrin | Sensing | [63] | ||
| Eu-MOF | CdTe QDs | Photovoltaics | [103] | ||
| MIL-101 | H2SO4, Toluenesulfonic, triflic acids | Proton conductivity | [13,84] | ||
| Zn-NPD | Naphthalene | Sensing | [104] | ||
| Al-DBA | Rhodamine B | Lighting | [65] | ||
| Zn-DPP | Co-doped Tb3+ and Eu3+ | Lighting | [105] | ||
| Layer-by-Layer Deposition | ZIF-8 | TiO2/Cu−TiO2 and GO | Catalysis | [53] | |
| Ti-MOF | Pt NPs | Catalysis | [67] | ||
| MIL-100(Fe) | Phosphated β-CD, then PEG-Ad | Drug Delivery | [68] | ||
| HKUST-1 | TiO2 and multi-walled carbon nanotubes | Photovoltaics | [66] | ||
| UiO-66 | GO | Proton conductivity | [52] | ||
| UiO-66-SO3H | GO nanosheets | Proton conductivity | [69] | ||
| MOF-5 | Cu(I) | Desulfurization of flue gas | [56] | ||
| In situ Growth | Cu-TED-BDC | GO sheets | Catalysis | [106] | |
| ZIF-8 | Polysulfone, Matrimid | Gas separation | [71] | ||
| MOF-5 | Multi-Walled Carbon Nanotubes, Pt-loaded Multi-Walled Carbon Nanotubes, Polymeric Ionic Liquids, Matrimid, Pluronic F127 | Gas storage, gas separation, PAHs extraction, sensing | [72,80,83,107,108,109] | ||
| HKUST-1 | Pt-loaded GO, SBA-15, Li-Doped Carbon Nanotubes, Matrimid | Gas storage, gas separation | [71,82,110,111] | ||
| UiO-66-NH2 | Polysulfone | Gas separation | [112] | ||
| ZIF-90 | Ultim, Matrimid, 6FDA-DAM | Gas separation | [70] | ||
| MIL-53(Al) | Matrimid | Gas separation | [71] | ||
| MOF-74(Zn) | Alginate | Molecular sieves | [78] | ||
| Using MOF as a | Template | Fe3[Co(CN)6]2 | Graphene | Catalysis | [113] |
| MIL-88 (Fe) | carbon-film-coated iron sulfide nanorods (C@Fe7S8) | Li ion battery | [14] | ||
| MOF-5 | Nitrogen-doped porous carbon; Carbon | Supercapacitors; electrodes; catalyst support | [81,114] | ||
| ZIF-67 | Ni-Co layered double hydroxides on graphene nanosheets, Ti(OBu)4 | energy storage, catalyst support | [86,115] | ||
| ZIF-8 | Nanoporous carbons, Ti(OBu)4 | Electrode materials, fuel cells, supercapacitors, catalyst support | [85,86,89] | ||
| HKUST-1 | Mo-POMs | Energy storage, electronics, sensing and catalysis | [85,116] | ||
| Precursor | HKUST-1 | Decomposition of the organic MOF ligand into a Cu-supported carbon matrix | catalysis | [89,117] | |
| NU-1000 | Nanocasting with silica | catalysis | [88] | ||
| Co-NDC | Carbonization of Co-NDC grown on Cu sheets | Catalysis | [118] | ||
| MOF-5 | Zn/Ni-MOF-5 to MOF-2 nanosheets followed by deposition of Pd clusters | catalysis | [73] | ||
| MIL-53 (Fe) | porous Fe2O3 nanostructures | Li ion battery | [24,73] | ||
| [Co3L2(TPT)2xG]n | Porous Co3O4 hollow tetrahedra | Li ion battery | [119] | ||
| MOF-200 | Nitrogen-doped graphene wrapped okra-like SnO2 composites | Li ion battery | [120] | ||
| [Co(BIB)(o-BDC)]∞, [Co2(BIB)2(m-BDC)2]∞, and {[Co(BIB)(p-BDC)(H2O)](H2O)0.5}∞ | Porous Co@carbon nanotube composites (CO@CNTs) and after in situ gas-sulfurization (CoS2@CNTs) | Supercapacitor | [121] | ||
| Fe-MOF, Ni-MOF, ZIF-8, MOF-5, Sn-MOF, Co-MOF | Graphene oxide composite aerogels: rGO/Fe2O3 and rGO/NiO/Ni | Supercapacitor | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa, J.D.; Bennett, T.F.; Nelms, K.J.; Liu, B.M.; Tovar, R.C.; Liu, Y. Metal–Organic Framework Hybrid Materials and Their Applications. Crystals 2018, 8, 325. https://doi.org/10.3390/cryst8080325
Sosa JD, Bennett TF, Nelms KJ, Liu BM, Tovar RC, Liu Y. Metal–Organic Framework Hybrid Materials and Their Applications. Crystals. 2018; 8(8):325. https://doi.org/10.3390/cryst8080325
Chicago/Turabian StyleSosa, Joshua D., Timothy F. Bennett, Katherine J. Nelms, Brandon M. Liu, Roberto C. Tovar, and Yangyang Liu. 2018. "Metal–Organic Framework Hybrid Materials and Their Applications" Crystals 8, no. 8: 325. https://doi.org/10.3390/cryst8080325






