Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of the Fe3O4 Nanoparticles
2.2.2. Phase Transfer of Fe3O4 Nanoparticles to Aqueous Media
2.2.3. Preparation of PDMAEMA-Coated Fe3O4 Magnetic Nanoparticles
2.2.4. Characterization of the Samples
3. Results and Discussion
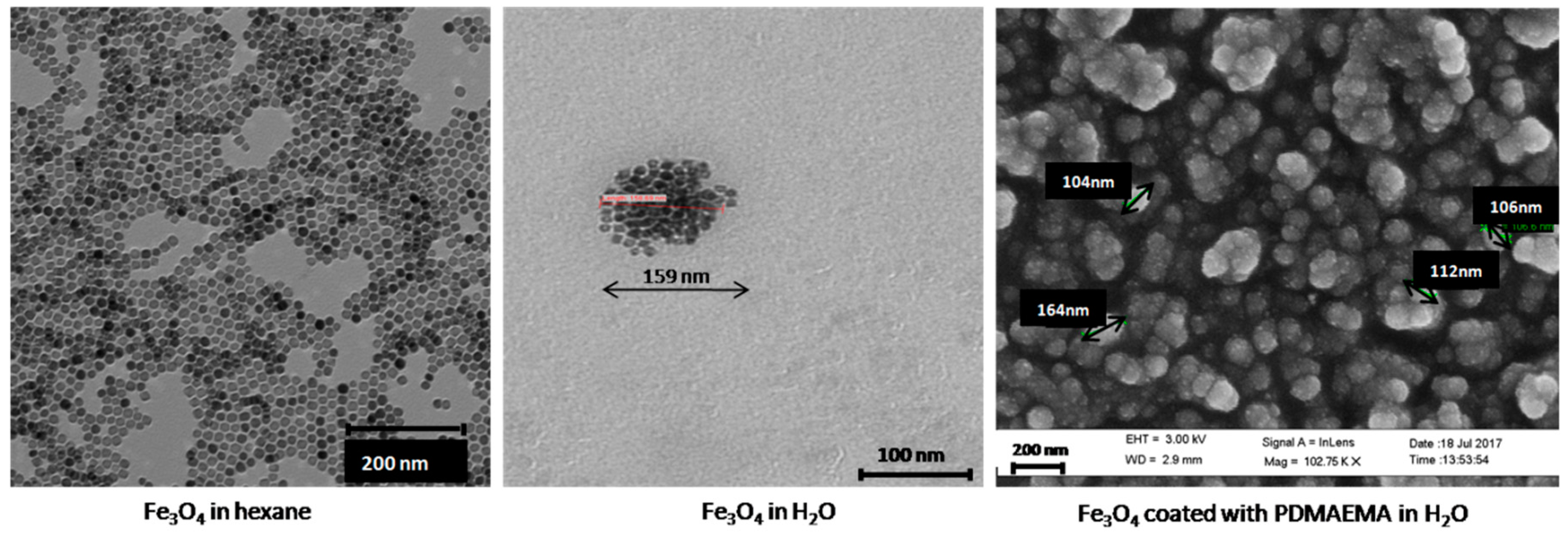
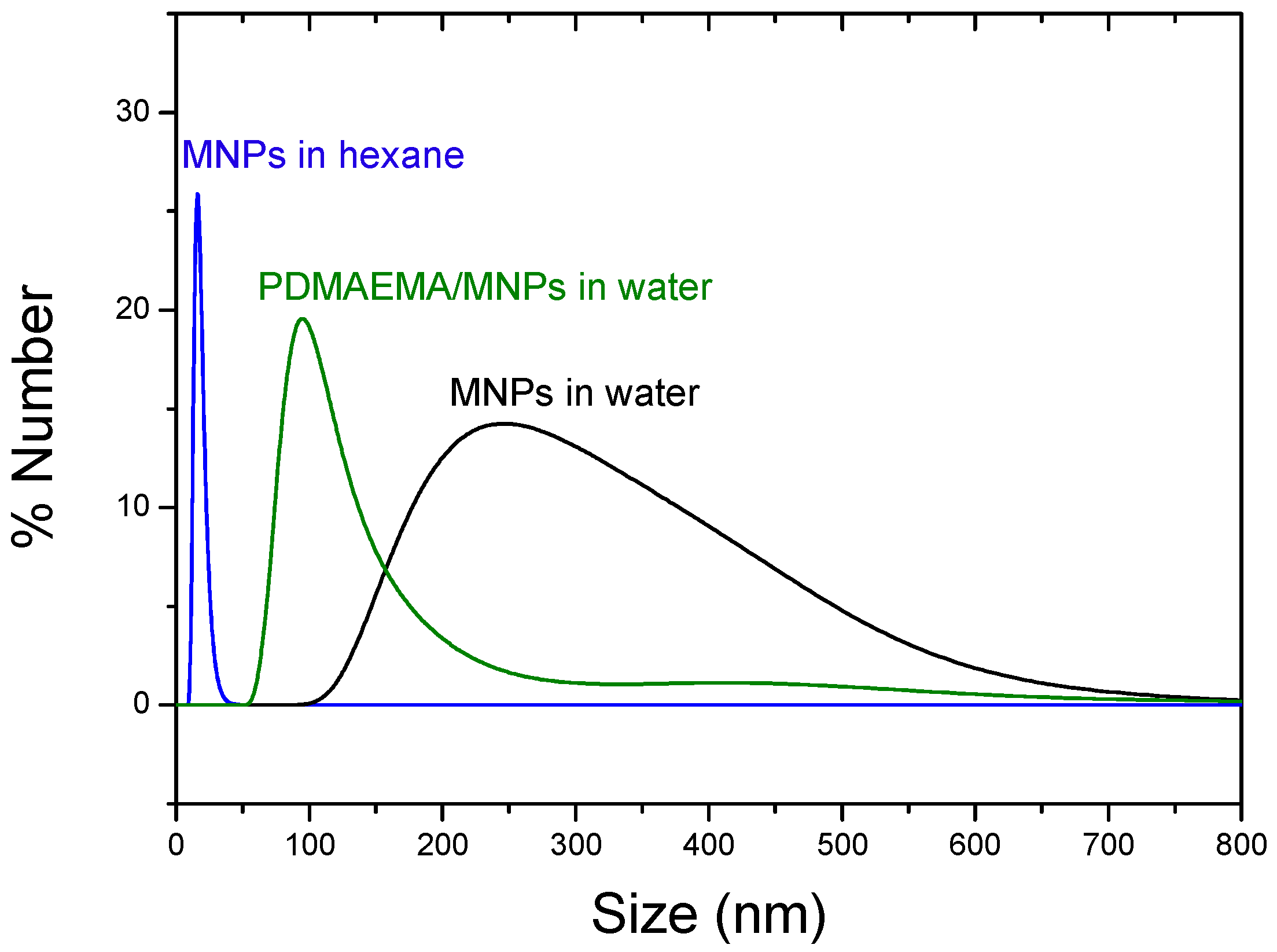
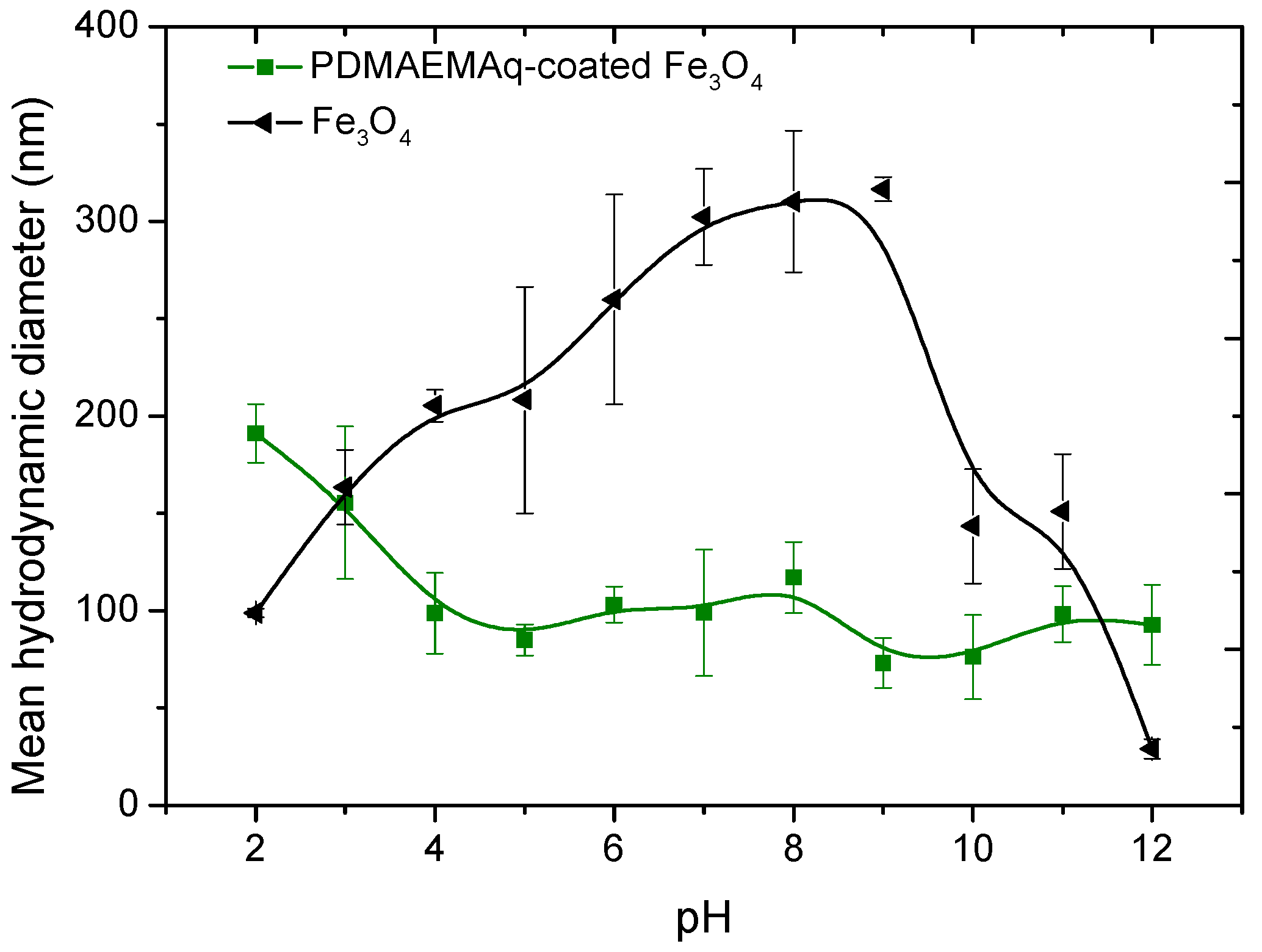
3.1. Morphology and Particle Size Distribution of the Coated Nanoparticles by TEM and DLS
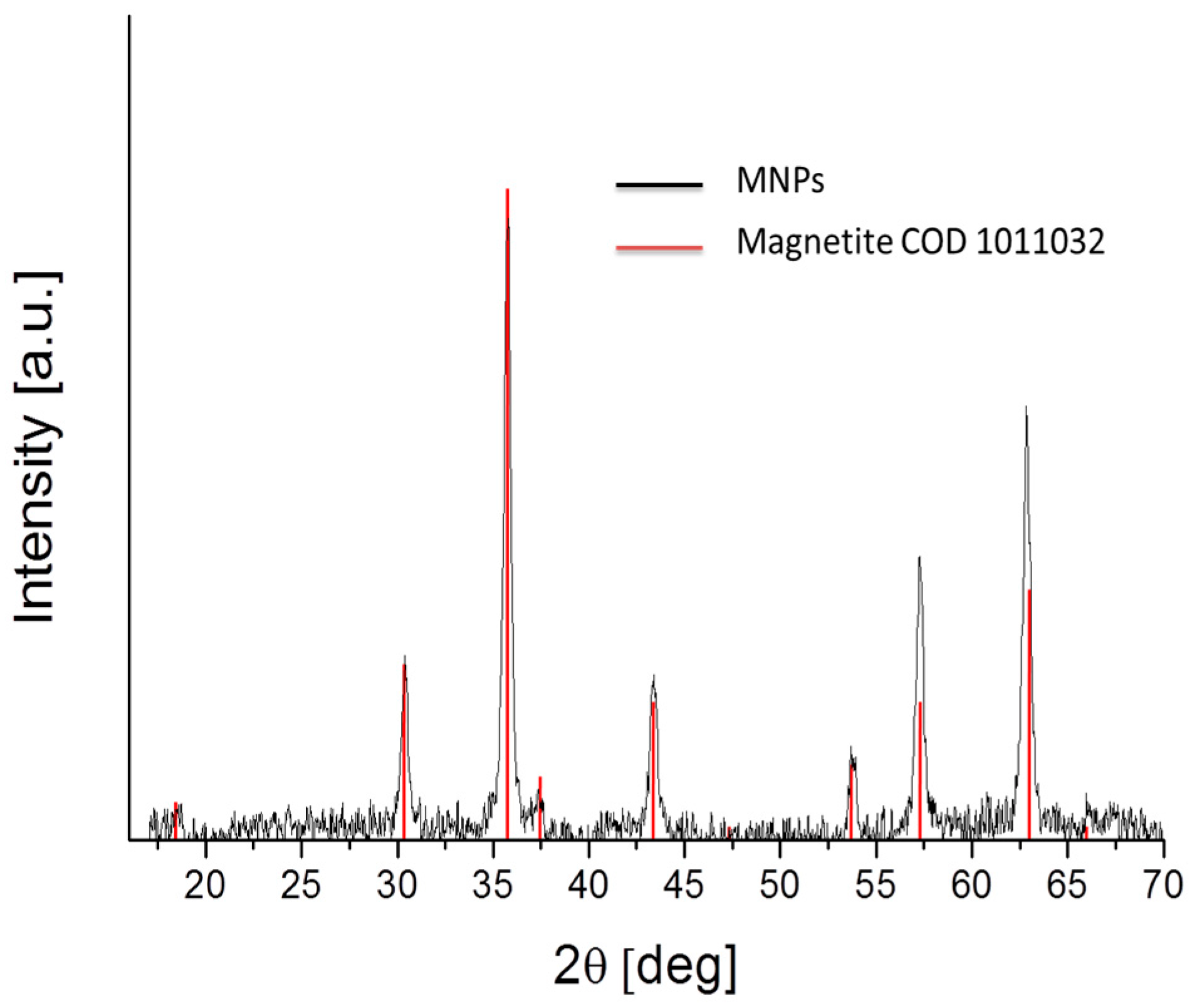
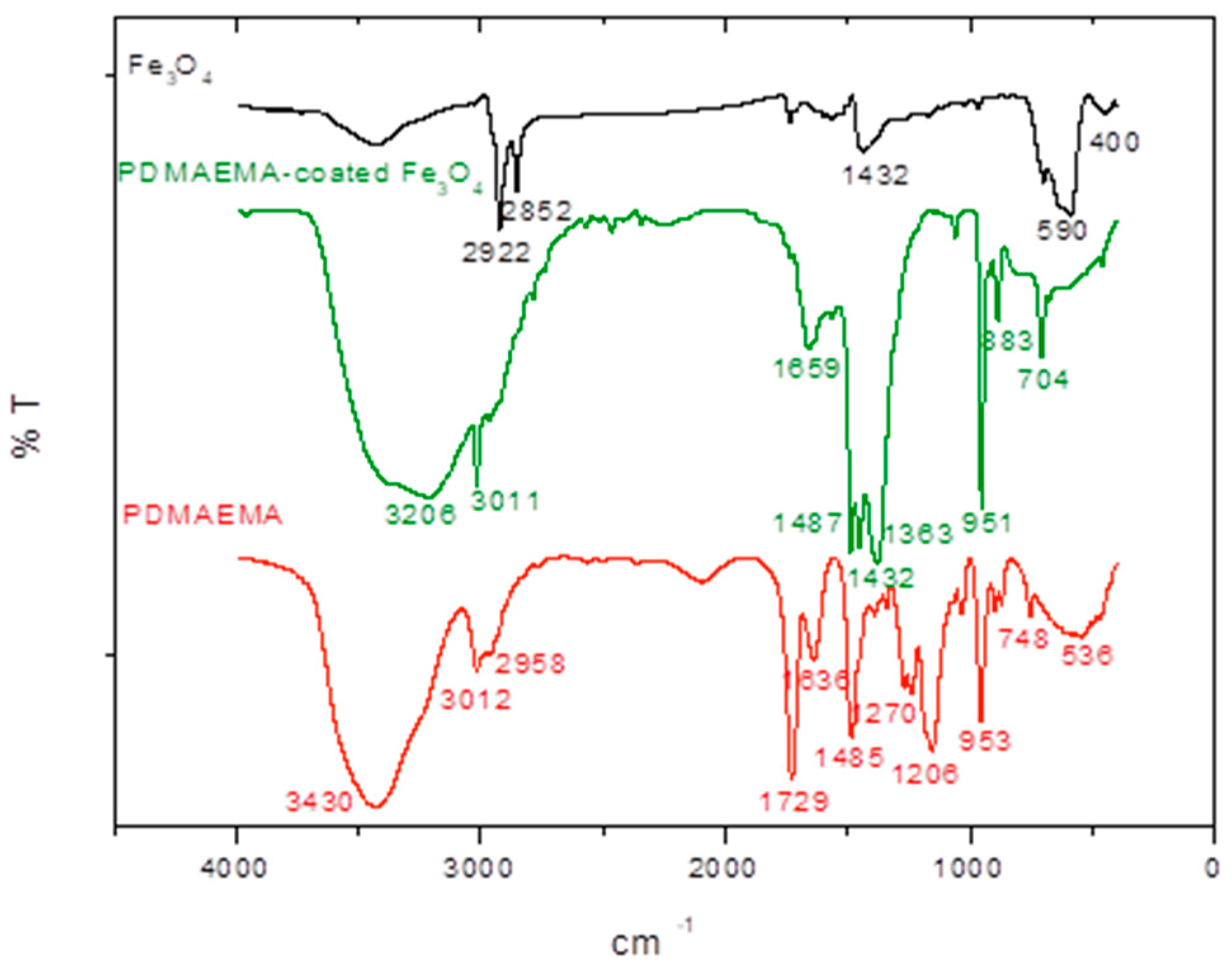
3.2. Physicochemical Characterization
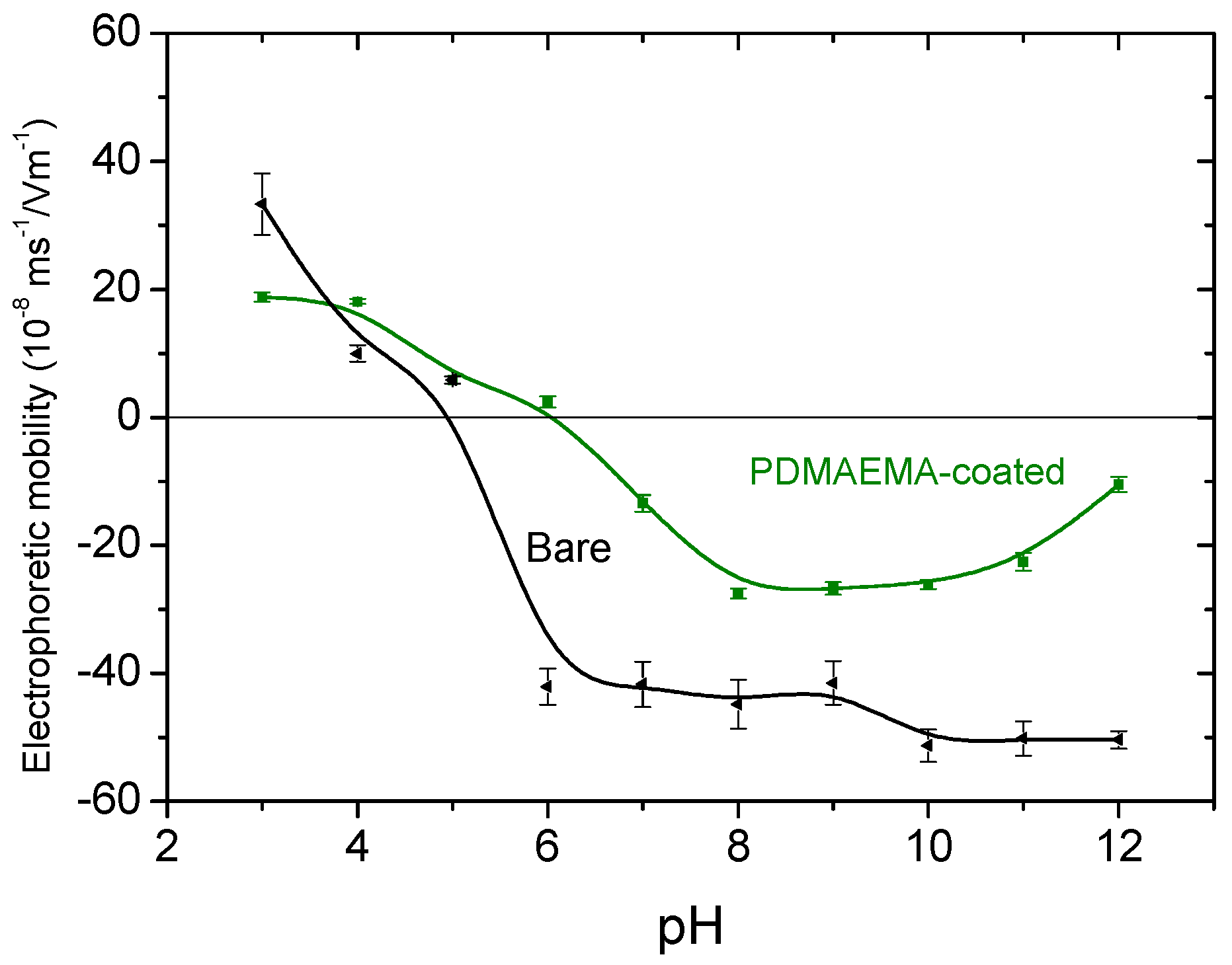
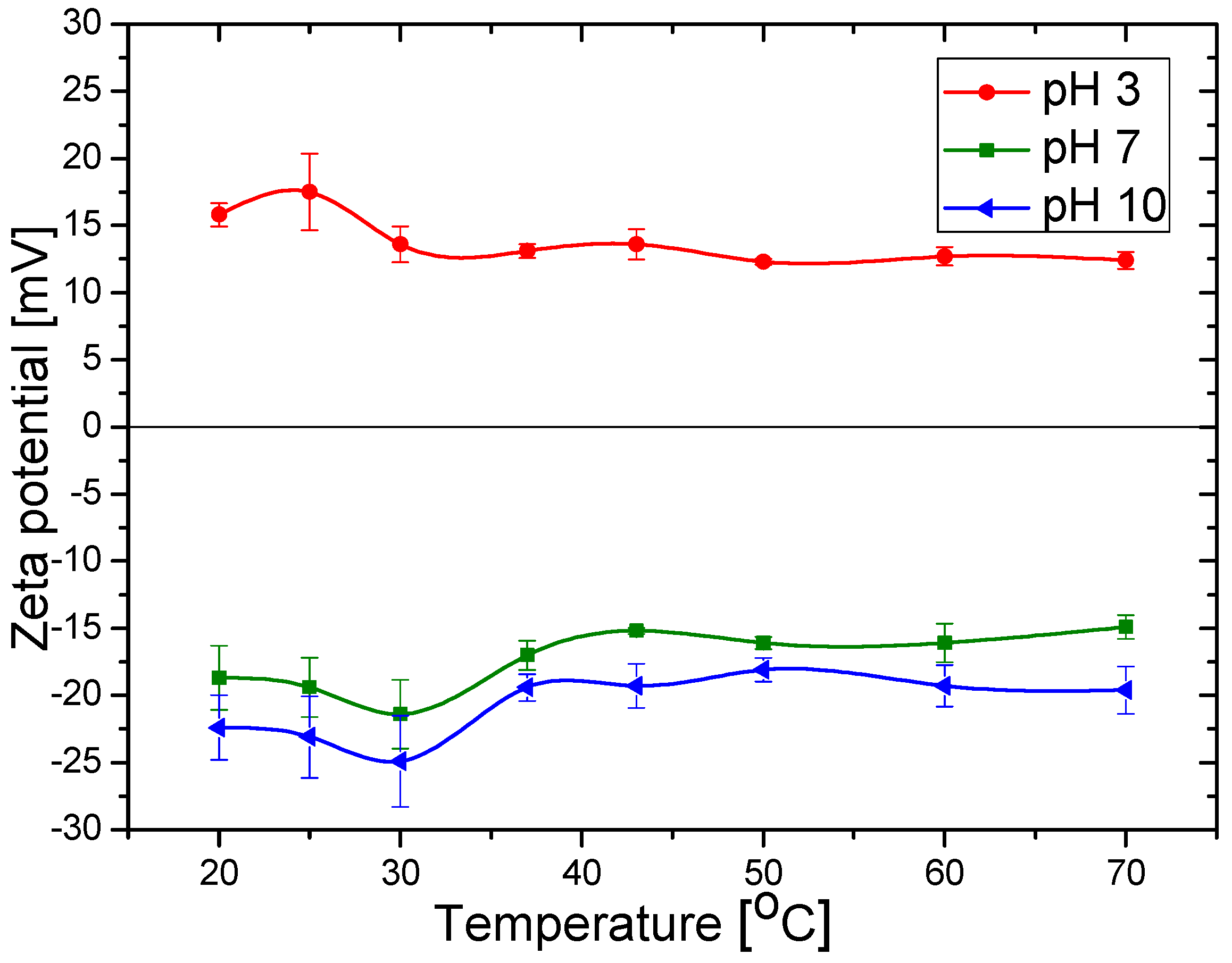
3.3. Zeta Potential of the Magnetite Particles
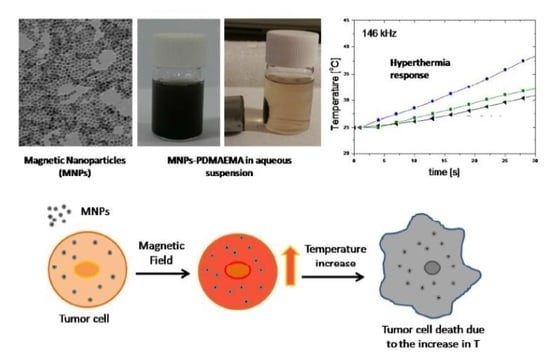
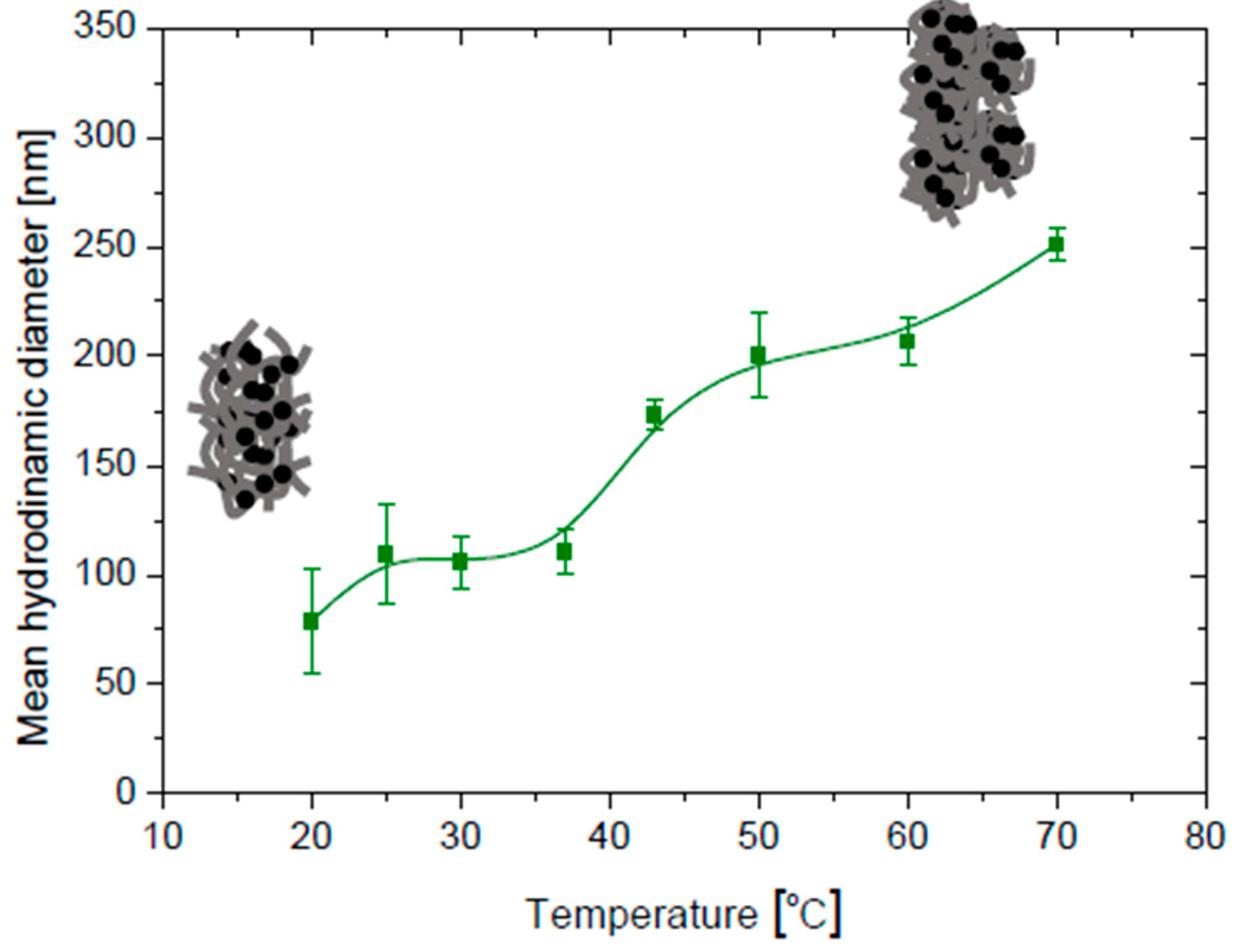
3.4. Temperature Effects
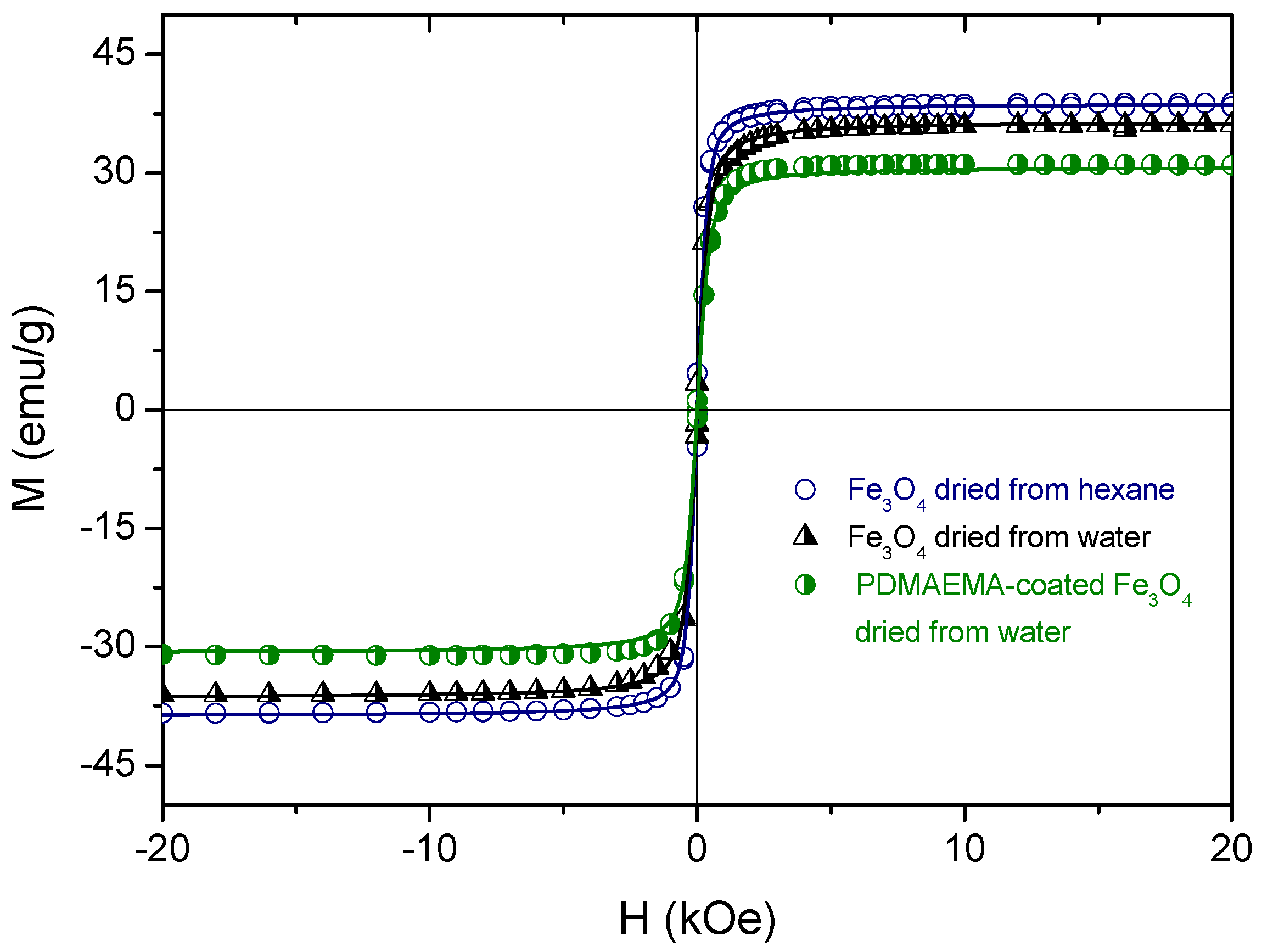
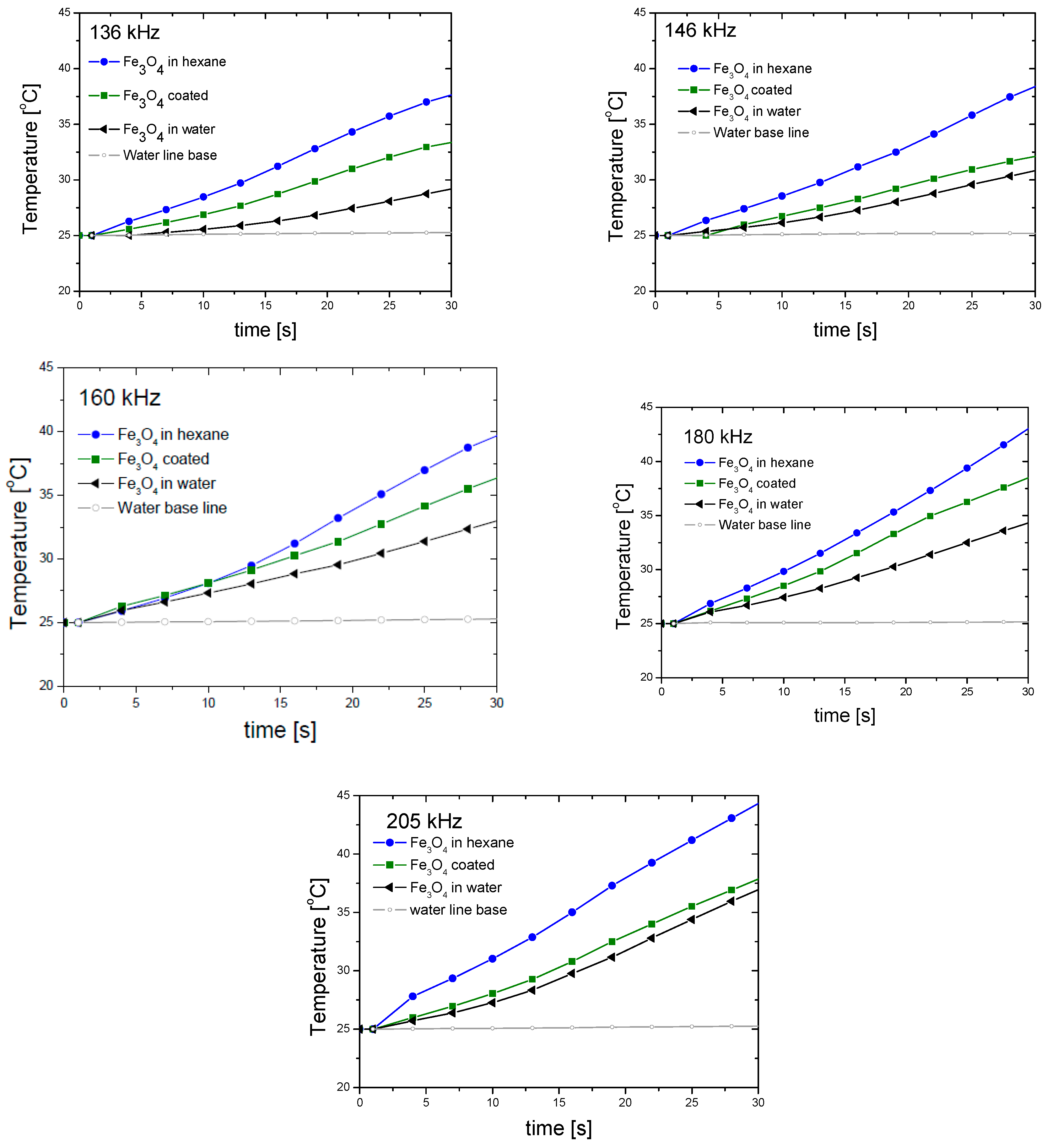
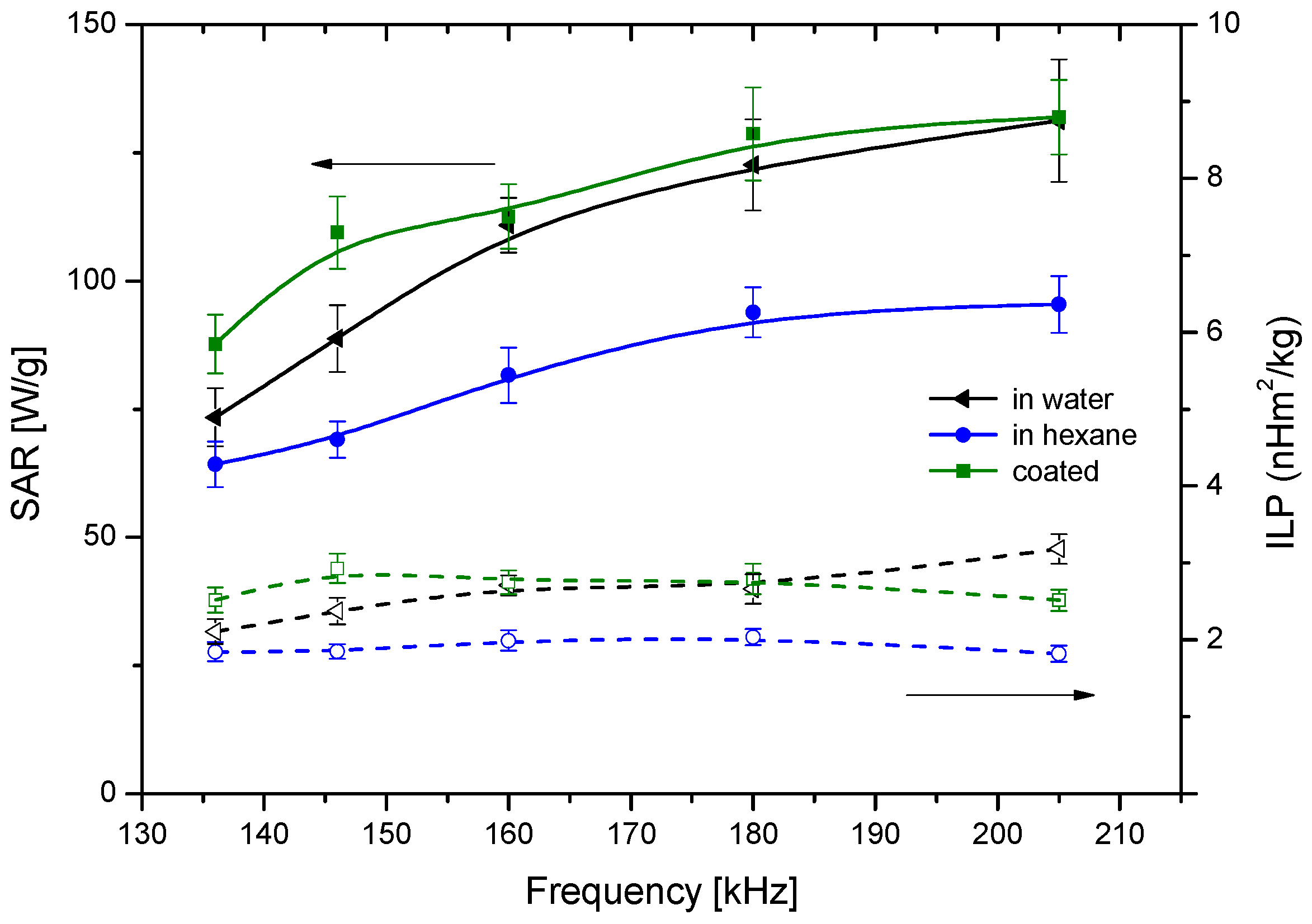
4. Magnetization and Hyperthermia Response
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ortega, D.; Pankhurst, Q.A. Magnetic hyperthermia. In Nanoscience: Nanostructures through Chemistry; Royal Society of Chemistry: London, UK, 2013; Volume 1. [Google Scholar]
- Ramprasad, R.; Zurcher, P.; Petras, M.; Miller, M.; Renaud, P. Magnetic properties of metallic ferromagnetic nanoparticle composites. J. Appl. Phys. 2004, 96, 519. [Google Scholar] [CrossRef]
- Ghobarkar, H.; Schäf, O.; Massiani, Y.; Knauth, P. Hydrothermal synthesis under pressure. The Reconstruction of Natural Zeolites; Springer: Boston, MA, USA, 2003; pp. 19–23. [Google Scholar]
- Laurent, S.D.F.; Port, M.; Roch, A.; Robic, C.; Vander, E.L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D 2009, 42. [Google Scholar] [CrossRef]
- Gómez-Sotomayor, R.; Alhualli, S.; Viota, J.L.; Rudzka, K.; Delgado, A.V. Iron/Magnetite nanoparticles as magnetic delivery systems for antitumor drugs. J. Nanosci. Nanotechnol. 2015, 15, 3507–3524. [Google Scholar] [CrossRef] [PubMed]
- Hervault, A.D.; Alexander, E.; Lim, M.; Boyer, C.; Mott, D.; Maenosono, S.; Thanh, N.T.K. Doxorubicin loaded dual pH- and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale 2016, 8, 12152–12161. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.Y.; Feng, B.; Chen, L.L.; Liu, G.H.; Li, H.Z.; Zheng, Y.; Wei, D.G. Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Eng. J. 2008, 42, 290–300. [Google Scholar] [CrossRef]
- Roca, A.G.; Costo, R.; Rebolledo, A.F.; Veintemillas-Verdaguer, S.; Tartaj, P.; Gonzalez-Carreno, T.; Morales, M.P.; Serna, C.J. Progress in the preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D 2009, 42. [Google Scholar] [CrossRef]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.M.; Rainer, H. Nanotoxicological classification system (NCS)—A guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur. J. Pharm. Biopharm. 2013, 84, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Tonga, G.Y.; Solfiell, D.; Rotello, V.M. Inorganic nanosystems for therapeutic delivery: Status and prospects. Adv. Drug Deliv. Rev. 2013, 65, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol. J. 2011, 6, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Yiu, H.H.P.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar]
- Mehdaoui, B.; Meffre, A.; Carrey, J.; Lachaize, S.; Lacroix, L.M.; Gougeon, M.; Chaudret, B.; Respaud, M. Optimal size of nanoparticles for magnetic hyperthermia: A combined theoretical and experimental study. Adv. Funct. Mater. 2011, 21, 4573–4581. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Zarghami, N.; Mikaeili, H.; Asgari, D.; Goganian, A.M.; Khiabani, H.K.; Samiei, M.; Davaran, S. Synthesis, characterization, and in vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled delivery of doxorubicin. Nanotechnol. Sci. Appl. 2012, 5, 13–25. [Google Scholar] [PubMed]
- Lien, Y.H.; Wu, T.M. Preparation and characterization of thermosensitive polymers grafted onto silica-coated iron oxide nanoparticles. J. Colloid Interface Sci. 2008, 326, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ortega, F.; Parra-Ruiz, F.J.; Averick, S.E.; Rodriguez, G.; Aguilar, M.R.; Matyjaszewski, K.; Roman, J.S. Smart heparin-based bioconjugates synthesized by a combination of ATRP and click chemistry. Polym. Chem. 2013, 4, 28002814. [Google Scholar] [CrossRef]
- Hoogenboom, R. Temperature-responsive polymers: Properties, synthesis and applications. In Smart Polymers and Their Applications; Román, J.S., Aguilar, M.R., Eds.; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Reyes-Ortega, F. pH-responsive polymers: Properties, synthesis and applications. In Smart Polymers and Their Applications; Román, J.S., Aguilar, M.R., Eds.; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules 2011, 16, 9755–9765. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Zhao, J. Properties of poly (N,N-dimethylaminoethyl methacrylate)/polysulfone positively charged composite nanofiltration membrane. J. Membr. Sci. 2004, 239, 183–188. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed]
- Formica, D.; Silvestri, S. Biological effects of exposure to magnetic resonance imaging: An overview. Biomed. Eng. Online 2004, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, G.; Delgado, A.V.; Kujda, M.; Ramos-Tejada, M.M. Magnetic hyperthermia with magnetite nanoparticles: Electrostatic and polymeric stabilization. Colloid Polym. Sci. 2016, 294, 1541–1550. [Google Scholar] [CrossRef]
- Dong, Z.X.; Wei, H.; Mao, J.; Wang, D.P.; Yang, M.Q.; Bo, S.Q.; Ji, X.L. Synthesis and responsive behavior of poly(N,N-dimethylaminoethyl methacrylate) brushes grafted on silica nanoparticles and their quaternized derivatives. Polymer 2012, 53, 2074–2084. [Google Scholar] [CrossRef]
- Park, J.W.; Bae, K.H.; Kim, C.; Park, T.G. Clustered magnetite nanocrystals cross-linked with PEI for efficient siRNA delivery. Biomacromolecules 2011, 12, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gao, C.; Xu, W.J. Amphibious polymer-functionalized CdTe quantum dots: Synthesis, thermo-responsive self-assembly, and photoluminescent properties. J. Mater. Chem. 2009, 19, 5655–5664. [Google Scholar] [CrossRef]
- Hemery, G.; Garanger, E.L.S.; Wong, A.D.; Gillies, E.R.; Pedrono, B.; Bayle, T.; Jacob, D.; Sandre, O. Thermosensitive polymer grafted iron oxide nanoparticles studied by in situ dynamic light backscattering under magnetic hyperthermia. J. Phys. D 2015, 48. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Porshokouh, Z.N.; Kalappattil, V.; Torres, D.; Phan, M.H.; Garaio, E.; Garcia, J.A.; Llamazares, J.L.S.; Srikanth, H. Tunable high aspect ratio iron oxide nanorods for enhanced hyperthermia. J. Phys. Chem. C. 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- O’Brien, R.W.; White, L.R. Electrophoretic mobility of a spherical colloidal particle. J. Chem. Soc. Faraday Trans. Mol. Chem. Phys. 1978, 74, 1607–1626. [Google Scholar] [CrossRef]
- Márquez, T.C.F.; Cotto, M.; Polanco, R.; Roque, R.; Fierro, P.; Sanz, J.; Elizalde, E.; Morant, C. Synthesis and characterization of monodisperse magnetite hollow microspheres. Soft Nanosci. Lett. 2011, 1, 25–32. [Google Scholar] [CrossRef]
- Viota, J.L.; Arroyo, F.J.; Delgado, A.V.; Horno, J. Electrokinetic characterization of magnetite nanoparticles functionalized with amino acids. J. Colloid Interface Sci. 2010, 344, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Yañez-Macias, R.; Alvarez-Moises, I.; Perevyazko, I.; Lezov, A.; Guerrero-Santos, R.; Schubert, U.S.; Guerrero-Sanchez, C. Effect of the degree of quaternization and molar mass on the cloud point of Poly[2-(dimethylamino)ethyl methacrylate] aqueous solutions: A systematic investigation. Macromol. Chem. Phys. 2017, 218. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, W.; Yuan, J.; Hong, X. Preparation of double-responsive SiO2-g-PDMAEMA nanoparticles via ATRP. Mater. Lett. 2008, 62, 1372–1375. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Ferrohydrodynamics; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Cullity, B.D. Introduction to Magnetic Materials; Addison–Wesley: Reading, MA, USA, 1974. [Google Scholar]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Appl. Phys. D 2014, 47. [Google Scholar] [CrossRef]
- Purushotham, S.; Ramanujan, R.V. Thermoresponsive magnetic composite nanomaterials for multimodal cancer therapy. Acta Biomater. 2010, 6, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, B.B.; Muthukumaran, T.; Philip, J. Magnetic hyperthermia in phosphate coated iron oxide nanofluids. J. Magn. Magn. Mater. 2016, 407, 101–113. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Ortega, F.; Delgado, Á.V.; Schneider, E.K.; Checa Fernández, B.L.; Iglesias, G.R. Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties. Polymers 2018, 10, 10. https://doi.org/10.3390/polym10010010
Reyes-Ortega F, Delgado ÁV, Schneider EK, Checa Fernández BL, Iglesias GR. Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties. Polymers. 2018; 10(1):10. https://doi.org/10.3390/polym10010010
Chicago/Turabian StyleReyes-Ortega, Felisa, Ángel V. Delgado, Elena K. Schneider, B. L. Checa Fernández, and G. R. Iglesias. 2018. "Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties" Polymers 10, no. 1: 10. https://doi.org/10.3390/polym10010010