Preparation of Antibacterial Cellulose Paper Using Layer-by-Layer Assembly for Cooked Beef Preservation at Ambient Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
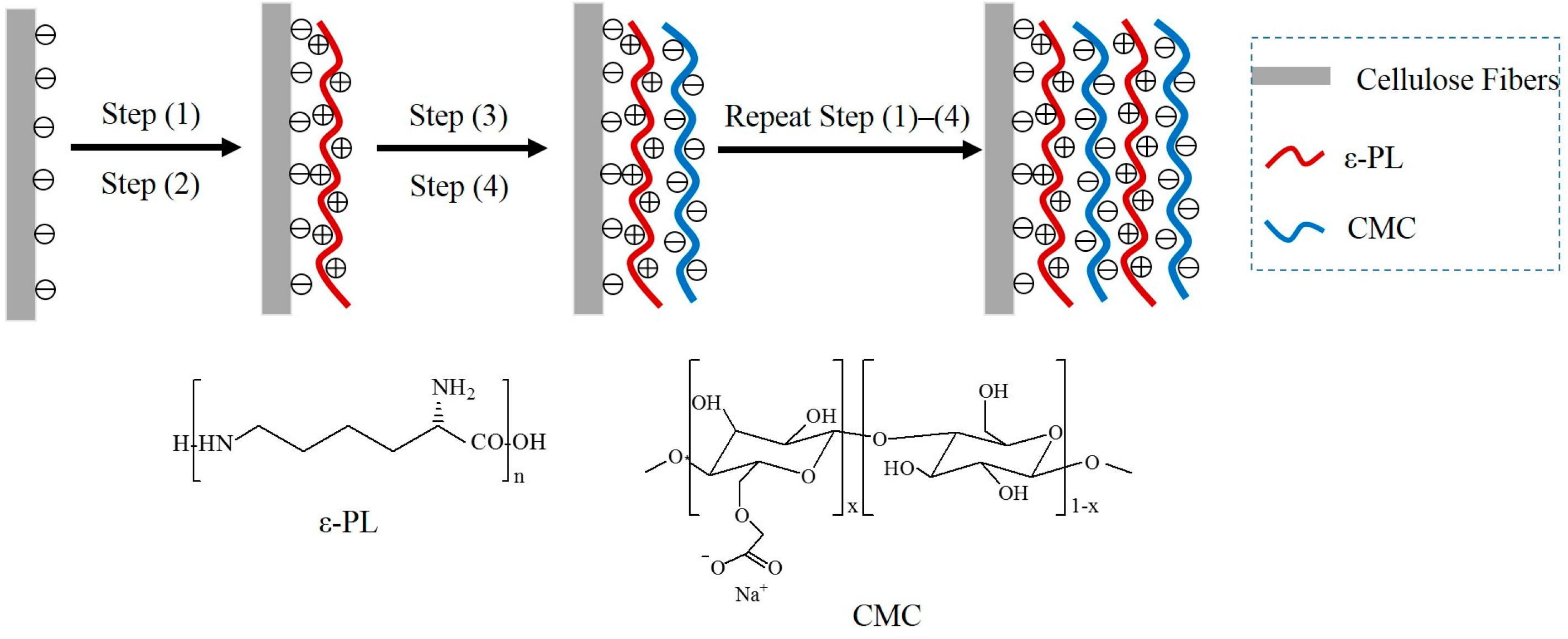
2.2. Preparation of ε-PL/CMC Multilayers on the Cellulose Fiber Surface
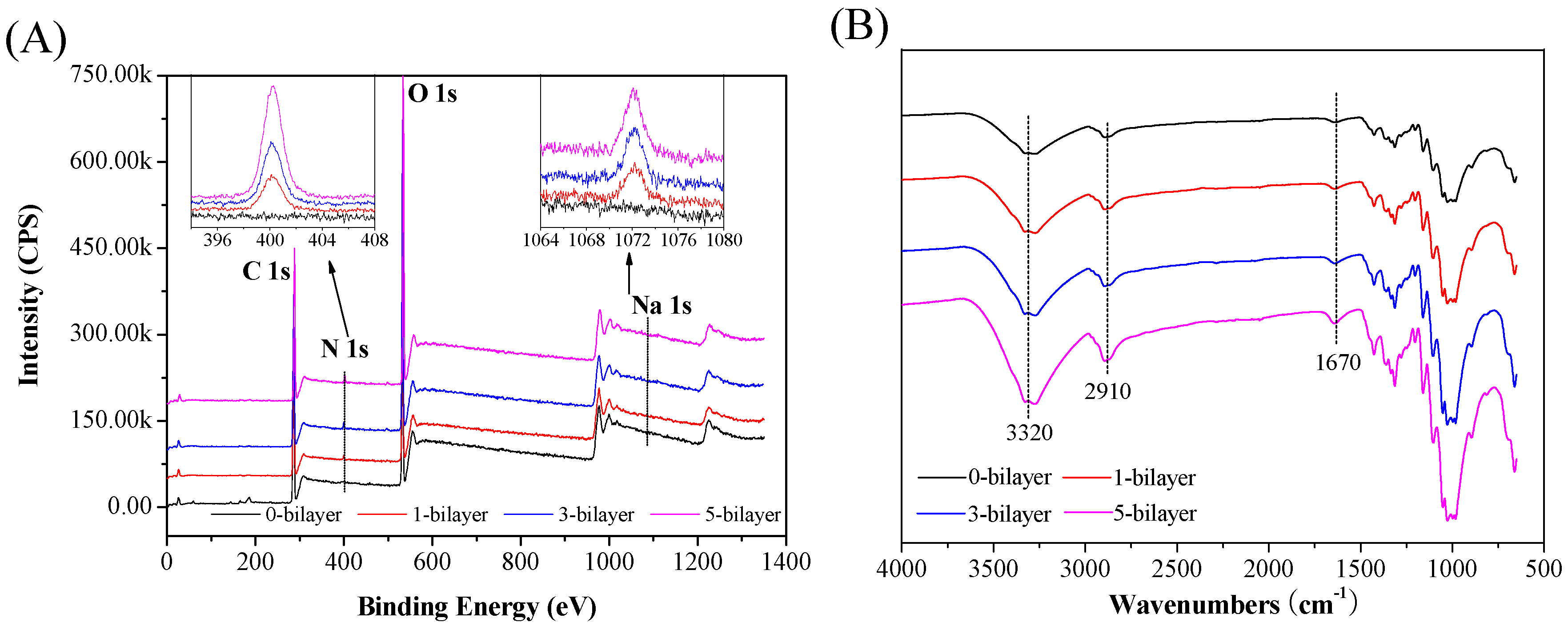
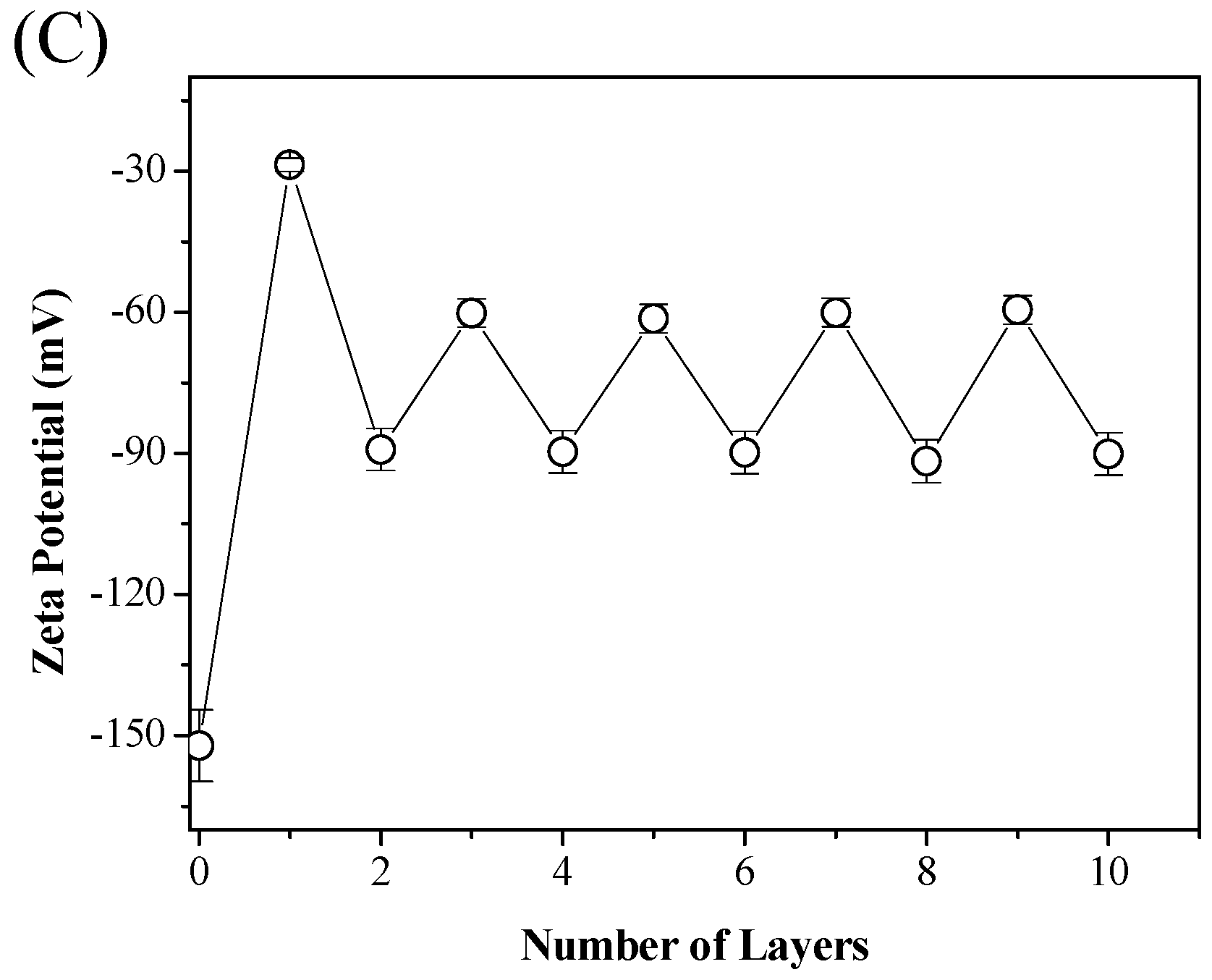
2.3. Characterizations
2.4. Antibacterial Test
2.5. Paper Physical Strength Test
2.6. Antibacterial Paper Applied for Cook Beef Preservation
2.7. Cytotoxicity Assay
3. Results and Discussion
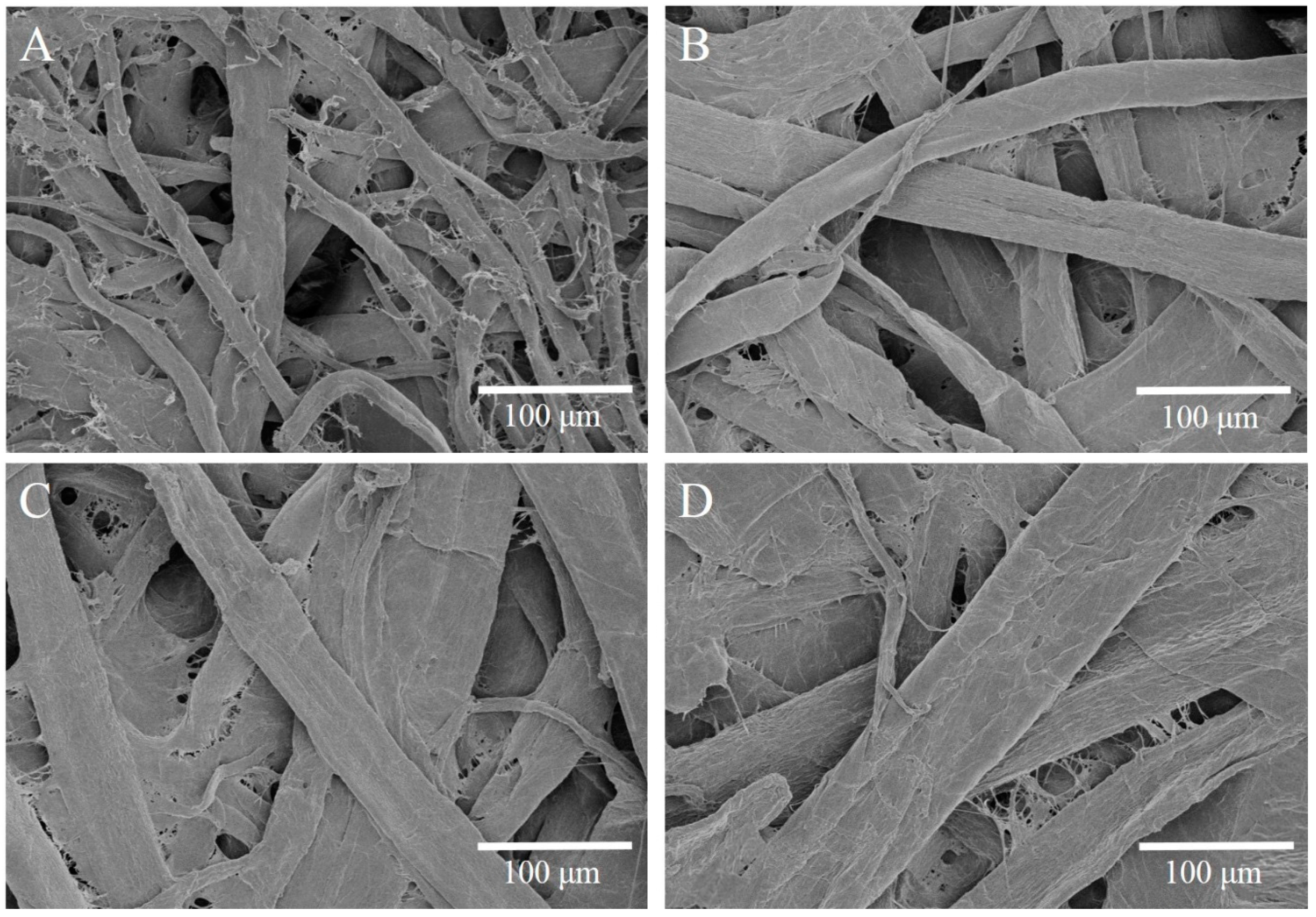
3.1. The Formation of ε-PL/CMC Multilayers on Cellulose Fiber Surfaces
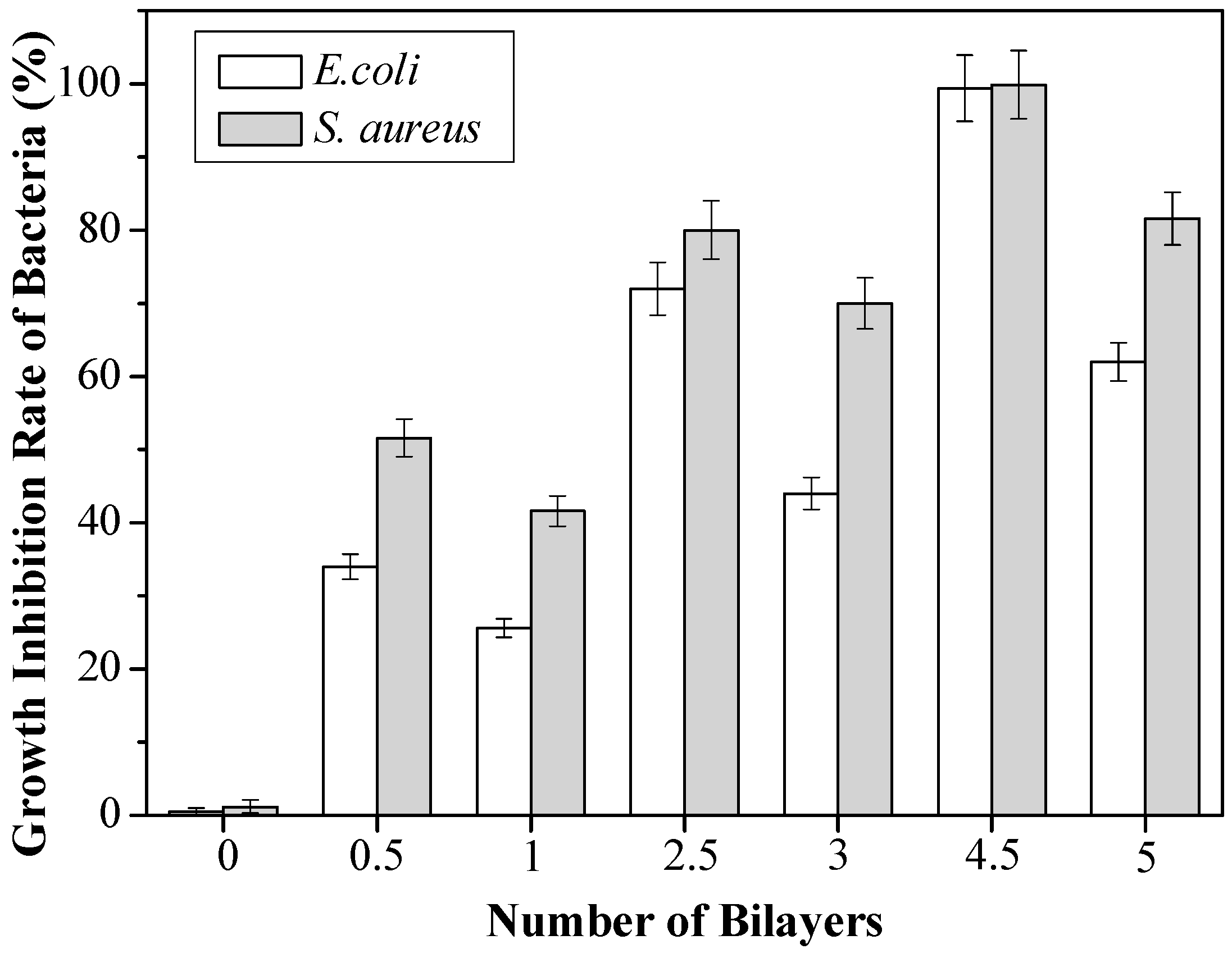
3.2. Antibacterial Activities of Modified Cellulose Paper
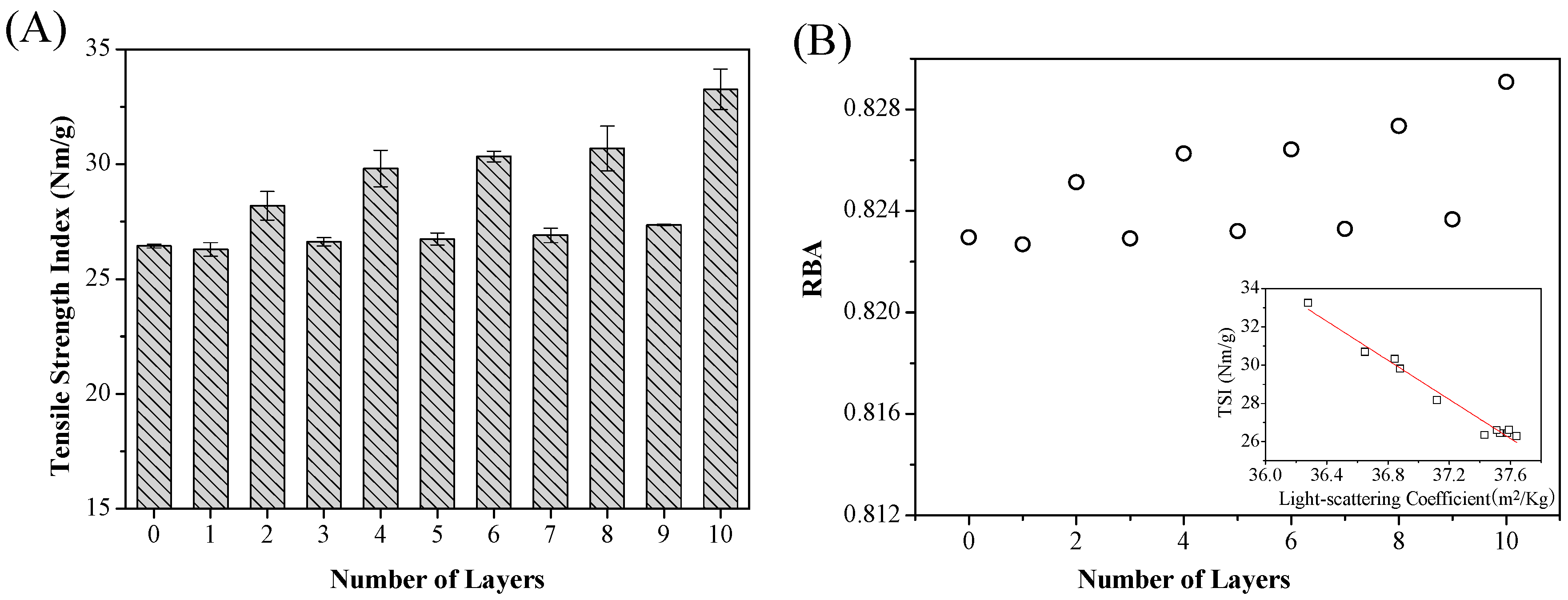
3.3. Tensile Strength of Modified Cellulose Paper
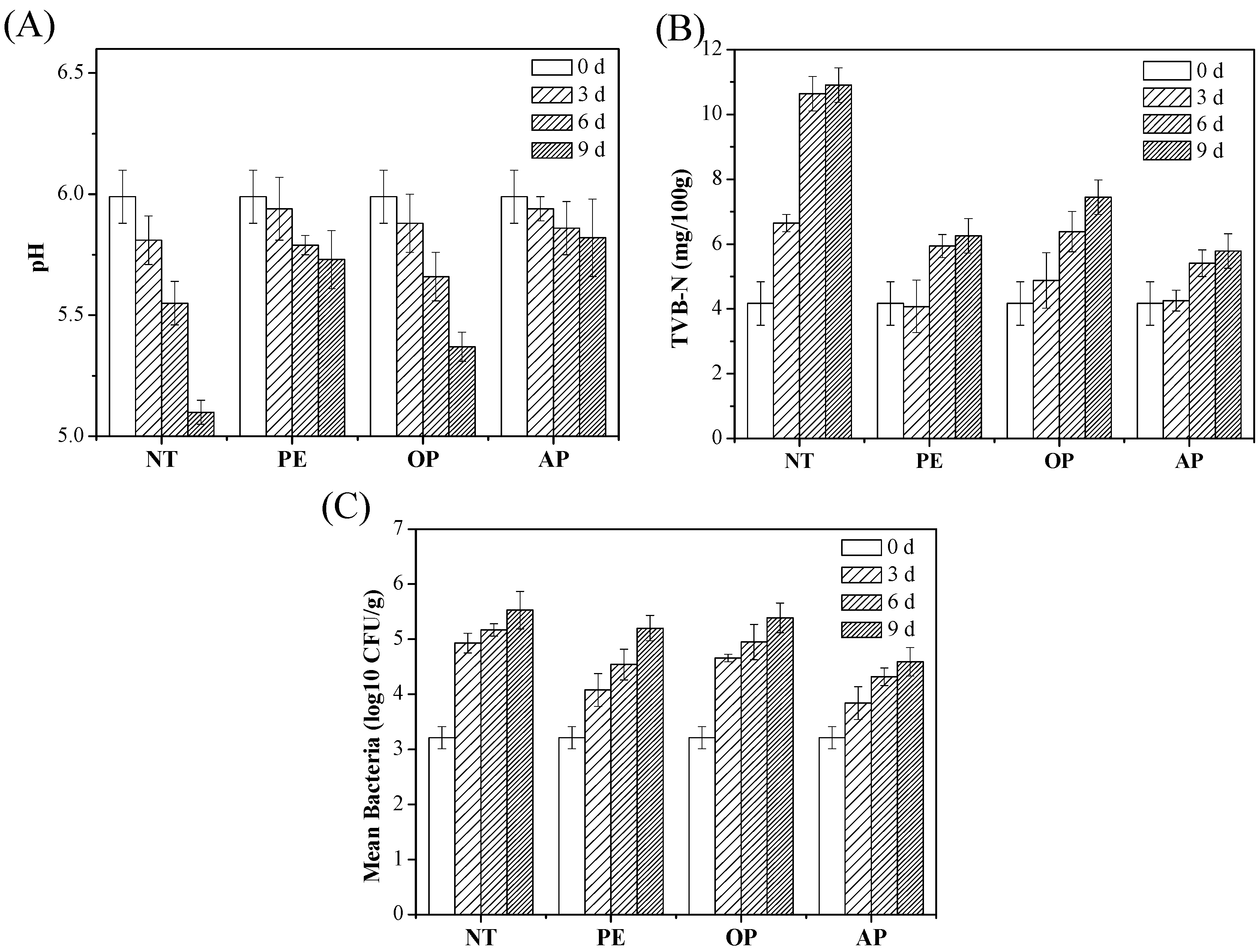
3.4. Application of Antibacterial Cellulose Paper Packaging on Cooked Beef
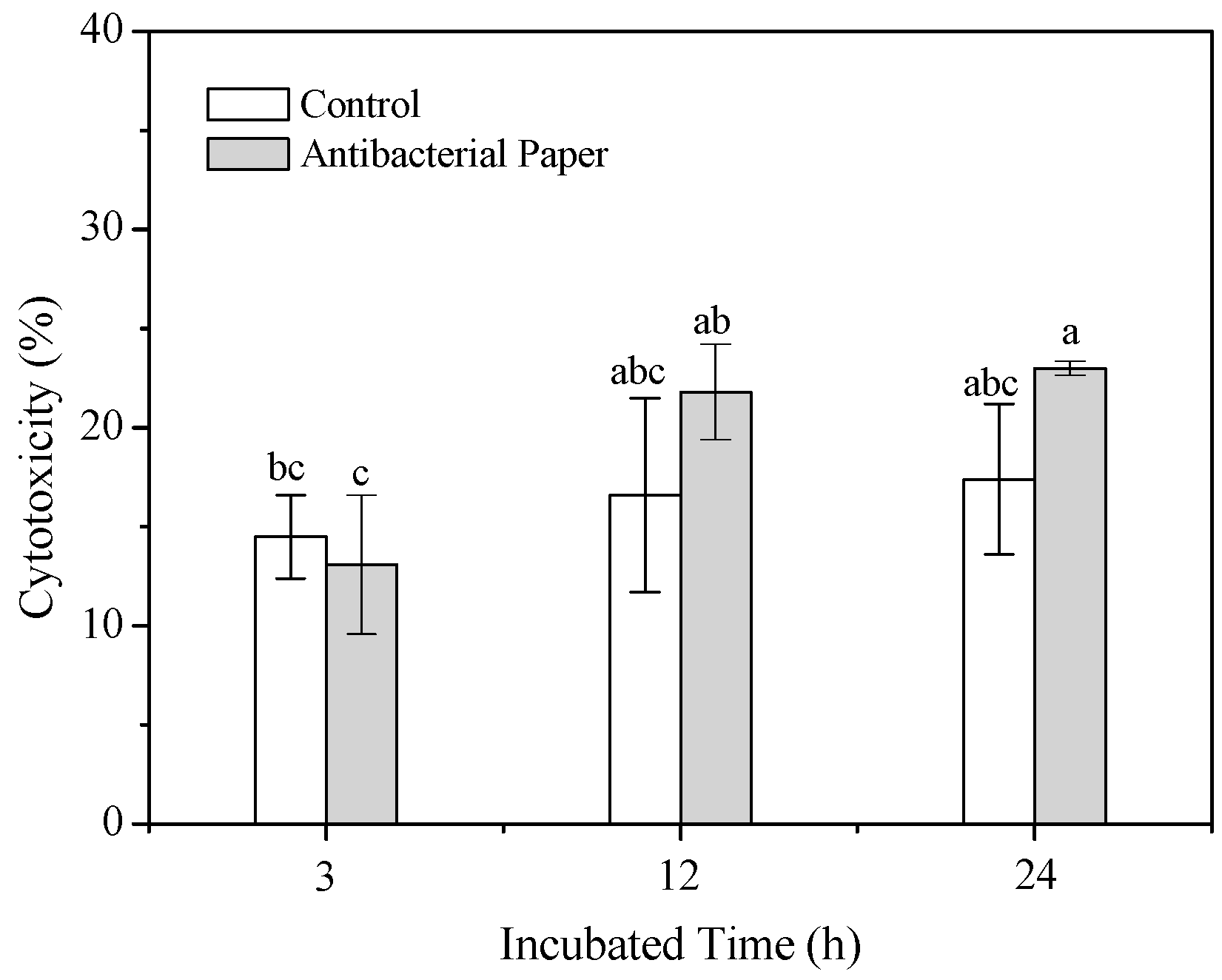
3.5. Cytotoxicity Assay of Preperaed Antibacterial Cellulose Paper
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Romani, V.P.; Prentice-Hernández, C.; Martins, V.G. Active and sustainable materials from rice starch, fish protein and oregano essential oil for food packaging. Ind. Crop. Prod. 2017, 9, 268–274. [Google Scholar] [CrossRef]
- Guart, A.; Wagner, M.; Mezquida, A.; Lacorte, S.; Oehlman, J.; Borrell, A. Migration of plasticisers from TritanTM and polycarbonate bottles and toxicological evaluation. Food Chem. 2013, 141, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crop. Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.S.; Dungani, R.; Paridah, M.T.; Sarker, M.Z.I.; Fazita, M.R.N.; Syakir, M.I.; et al. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Johansson, C.; Bras, J.; Mondragon, I.; Nechita, P.; Plackett, D.; Simon, P.; Svetec, D.G.; Virtanen, S.; Baschetti, M.G.; Breen, C.; et al. Renewable fibers and bio-based materials for packaging applications—A review of recent developments. BioResources 2012, 7, 2506–2552. [Google Scholar] [CrossRef]
- Booshehri, A.Y.; Wang, R.; Xu, R. Simple method of deposition of CuO nanoparticles on a cellulose paper and its antibacterial activity. Chem. Eng. J. 2015, 262, 999–1008. [Google Scholar] [CrossRef]
- Herrera, M.A.; Mathew, A.P.; Oksman, K. Barrier and mechanical properties of plasticized and cross-linked nanocellulose coatings for paper packaging applications. Cellulose 2017, 24, 3969–3980. [Google Scholar] [CrossRef]
- Tankhiwale, R.; Bajpaí, S.K. Graft copolymerization onto cellulose-based filter paper and its further development as silver nanoparticles loaded antibacterial food-packaging material. Colloids Surf. B 2009, 69, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Ei-Samahy, M.A.; Mohamed, S.A.A.; Rehim, M.H.A.; Mohram, M.E. Synthesis of hybrid paper sheets with enhanced air barrier and antimicrobial properties for food packaging. Carbohydr. Polym. 2017, 168, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.H.; Zhou, M.G.; Wang, X.J. Preparation and characterization of durable antibacterial cellulose biomaterials modified with triazine derivatives. Carbohydr. Polym. 2009, 75, 328–332. [Google Scholar] [CrossRef]
- Dong, C.; Ye, Y.; Qian, L.Y.; Zhao, G.L.; He, B.H.; Xiao, H.N. Antibacterial modification of cellulose fibers by grafting β-cyclodextrin and inclusion with ciprofloxacin. Cellulose 2014, 21, 1921–1932. [Google Scholar] [CrossRef]
- Roy, D.; Knapp, J.S.; Guthrie, J.T.; Perrier, S. Antibacterial cellulose fiber via RAFT surface graft polymerization. Biomacromolecules 2007, 9, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Koepsel, R.R.; Morley, S.W.; Matyjaszewski, K.; Sun, Y.J.; Russell, A.J. Permanent, nonleaching antibacterial surfaces. 1. Synthesis by atom transfer radical polymerization. Biomacromolecules 2004, 5, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Perero, S.; Miola, M.; Maina, G.; Ferri, A.; Ferraris, M.; Balagna, C. Antimicrobial functionalization of cotton fabric with silver nanoclusters/silica composite coating via RF co-sputtering technique. Cellulose 2017, 24, 2331–2345. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process. 1. Consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surface. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Vale, A.C.; Sousa, M.P.; Barbosa, A.M.; Torrado, E.; Mano, J.F.; Alves, N.M. Antibacterial bioadhesive layer-by-layer coatings for orthopedic applications. J. Mater. Chem. B 2016, 4, 5385–5393. [Google Scholar] [CrossRef]
- Chen, X.; Fang, F.; Zhang, X.; Ding, X.; Wang, Y.; Chen, L.; Tian, X. Flame-retardant, electrically conductive and antimicrobial multifunctional coating on cotton fabric via layer-by-layer assembly technique. RSC Adv. 2016, 33, 27669–27676. [Google Scholar] [CrossRef]
- Kiyofumi, K.; Yoshinori, S.; Kunihito, K.; Kei, I. Preparation of pH-Responsive hollow capsules via layer-by-layer assembly of exfoliated layered double hydroxide nanosheets and polyelectrolytes. J. Nanosci. Nanotechnol. 2018, 18, 110–115. [Google Scholar]
- Dubas, S.T.; Kumlangdudsana, P.; Potiyaraj, P. Layer-by-layer deposition of antimicrobial silver nanoparticles on textile fibers. Colloids Surf. A 2006, 289, 105–109. [Google Scholar] [CrossRef]
- Imani, R.; Talaiepour, M.; Dutta, J.; Ghobadinezhad, M.R.; Hemmasi, A.H.; Nazhad, M.M. Production of antibacterial filter paper from wood cellulose. BioResources 2011, 6, 891–900. [Google Scholar]
- Martins, N.C.T.; Freire, C.S.R.; Pinto, R.J.B.; Fernandes, S.C.M.; Neto, C.P.; Silvestre, A.J.D.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Electrostatic assembly of Ag nanoparticles onto nanofibrillated cellulose for antibacterial paper products. Cellulose 2012, 19, 1425–1436. [Google Scholar] [CrossRef]
- Gomes, A.P.; Mano, J.F.; Queiroz, J.A.; Gouveia, I.C. Layer-by-Layer Assembly for Biofunctionalization of Cellulosic Fibers with Emergent Antimicrobial Agents. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials, 1st ed.; Rojas, O., Ed.; Springer: Cham, Switzerland, 2015; Volume 271. [Google Scholar]
- Ahmed, H.B.; Emam, H.E. Layer by layer assembly of nanosilver for high performance cotton fabrics. Fibers Polym. 2016, 17, 418–426. [Google Scholar] [CrossRef]
- Ling, Y.; Luo, Y.; Luo, J.; Wang, X.; Sun, R. Novel antibacterial paper based on quaternized carboxymethyl chitosan/organic montmorillonite/AgNP nanocomposites. Ind. Crop. Prod. 2013, 51, 470–479. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Köller, M.; Epple, M. Toxicity of silver nanoparticles increases during strorage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic potential of silver nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Gaillet, S.; Rouanet, J.M. Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms—A review. Food Chem. Toxicol. 2015, 77, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, P.; Si, C.; Liu, Z. Chitosan nanoparticles: Preparation and application in antibacterial paper. J. Macromol. Sci. B. 2010, 49, 994–1001. [Google Scholar] [CrossRef]
- Gomes, A.P.; Mano, J.F.; Queiroz, J.A.; Gouveia, I.C. Layer-by-layer deposition of antimicrobial polymers on cellulosic fibers: A new strategy to develop bioactive textiles. Polym. Adv. Technol. 2013, 24, 1005–1010. [Google Scholar] [CrossRef]
- Heydarifard, S.; Pan, Y.; Xiao, H.; Nazhad, M.M.; Shipin, O. Water-resistant cellulosic filter containing non-leaching antimicrobial starch for water purification and disinfection. Carbohydr. Polym. 2017, 163, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, Z.; Wang, H.; Liu, J.; Xiao, H.; Zheng, A.; Guan, Y. Antimicrobial paper obtained by dip-coating with modified guanidine-based particle aqueous dispersion. Cellulose 2017, 24, 3901–3910. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Ikram, S. Chitosan: A natural antimicrobial agent—A review. J. Appl. Chem. 2014, 3, 493–503. [Google Scholar]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Irkin, R.; Esmer, O.K. Novel food packaging systems with natural antimicrobial agents. J. Food Sci. Technol. 2015, 52, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lu, Y.; Liu, X.; Tu, H.; Zhang, J.; Shi, X.; Deng, H.; Du, Y. Layer-by-layer immobilization of quaternized carboxymethyl chitosan/organic rectorite and alginate onto nanofibrous mats and their antibacterial application. Carbohydr. Polym. 2015, 121, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, Y.; Deng, H.; Hu, Y.; Li, B. Antibacterial multilayer films fabricated by layer-by-layer immobilizing lysozyme and gold nanoparticles on nanofibers. Colloids Surf. B 2014, 116, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Guyomard, A.; Dé, E.; Jouenne, T.; Malandain, J.J.; Muller, G.; Glinel, K. Incorporation of a hydrophobic antibacterial peptide into amphiphilic polyelectrolyte multilayers: A bioinspired approach to prepare biocidal thin coatings. Adv. Funct. Mater. 2008, 18, 758–765. [Google Scholar] [CrossRef]
- Zhu, X.; Jun Loh, X. Layer-by-layer assemblies for antibacterial applications. Biomater. Sci. 2015, 3, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Y.; Huang, Q. Synthesis and characterization of novel antimicrobial emulsifiers from ε-polylysine. J. Agric. Food. Chem. 2010, 58, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.; Shen, M.; Van, Y. Microbial synthesis of poly(ε-lysine) and its various applications. Bioresour. Technol. 2006, 97, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Najjar, M.B.; Kashtanov, D.; Chikindas, M.L. ε-Poly-l-lysine and nisin A act synergistically against Gram-positive food-borne pathogens Bacillus cereus and Listeria monocytogenes. Lett. Appl. Microbiol. 2007, 45, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Geornaras, I.; Yoon, Y.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Antimicrobial activity of ε-polylysine against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in various food extracts. J. Food Sci. 2007, 72, M330–M334. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, K.; Hamano, Y.; Katano, H. Antimicrobial activity of ε-Poly-l-lysine after forming a water-insoluble complex with an anionic surfactant. Biomacromolecules 2017, 18, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, J.; Han, Q.; Dai, Z.; Liu, W.; Mo, H. Effects of ε-polylysine on physicochemical characteristics of chilled pork. Food Bioprocess Technol. 2014, 7, 2507–2515. [Google Scholar] [CrossRef]
- Chheda, A.H.; Vernekar, M.R. Improved production of natural food preservative ε-poly-l-lysine using a novel producer Bacillus cereus. Food Biosci. 2014, 7, 56–63. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Dong, F.; Tian, A.; Li, Z.; Dai, Y. Physical, mechanical and antimicrobial properties of starch films incorporated with ε-poly-l-lysine. Food Chem. 2015, 166, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhong, Z.; Xu, H. Multifunctional wool fiber treated with ε-polylysine. Korean J. Chem. Eng. 2012, 29, 507–512. [Google Scholar] [CrossRef]
- Xing, T.; Li, X.; Guo, S.; Tang, R.; Cai, J.; Zhou, S. Preparation and properties of silk fabric grafted with ε-polylysine by tyrosinase. Text. Res. J. 2015, 85, 1743–1748. [Google Scholar] [CrossRef]
- Marais, A.; Utsel, S.; Gustafsson, E.; Wågberg, L. Towards a super-strainable paper using the layer-by-layer technique. Carbohydr. Polym. 2014, 100, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, L. Antimicrobial and antioxidant surface modification of cellulose fibers using layer-by-layer deposition of chitosan and lignosulfonates. Carbohydr. Polym. 2015, 124, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Guan, Y.; Ziaee, Z.; He, B.; Zheng, A.; Xiao, H. Rendering cellulose fibers antimicrobial using cationic β-cyclodextrin-based polymers included with antibiotics. Cellulose 2009, 16, 309–317. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Q.; Wan, X.; Zhao, J. Determination of total volatile basic nitrogen (TVB-N) content and Warner–Bratzler shear force (WBSF) in pork using Fourier transform near infrared (FT-NIR) spectroscopy. Food Chem. 2011, 126, 1354–1360. [Google Scholar] [CrossRef]
- Lee, M.W.; Hung, C.L.; Cheng, J.C.; Wang, Y.J. A new anti-adhesion film synthesized from polyaglacturonic acid with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide crosslinker. Biomaterials 2005, 26, 3793–3799. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Farnood, R. Cellulose fibre networks reinforced with carboxymethyl cellulose/chitosan complex layer-by-layer. Carbohydr. Polym. 2014, 114, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fu, S.; Peng, L.; Zhan, H. Surface modification of cellulose fibers with layer-by-layer self-assembly of lignosulfonate and polyelectrolyte: Effects on fibers wetting properties and paper strength. Cellulose 2012, 19, 533–546. [Google Scholar] [CrossRef]
- Yingguad, S.; Ruamsin, S.; Reekprkhon, D.; Douglas, S.; Pongamphai, S.; Siripatrawan, U. Effect of chitosan coating and vacuum packaging on the quality of refrigerated grilled pork. Packag. Technol. Sci. 2006, 19, 149–157. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, X.; Li, W.; Song, R.; Li, J.; Li, Y.; Li, B.; Liu, S. Green and biodegradable composite films with novel antimicrobial performance based on cellulose. Food Chem. 2016, 197, 250–256. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Cui, R.; Peng, L.; Cai, S.; Li, P.; Lan, T. Preparation of Antibacterial Cellulose Paper Using Layer-by-Layer Assembly for Cooked Beef Preservation at Ambient Temperature. Polymers 2018, 10, 15. https://doi.org/10.3390/polym10010015
Li H, Cui R, Peng L, Cai S, Li P, Lan T. Preparation of Antibacterial Cellulose Paper Using Layer-by-Layer Assembly for Cooked Beef Preservation at Ambient Temperature. Polymers. 2018; 10(1):15. https://doi.org/10.3390/polym10010015
Chicago/Turabian StyleLi, Hui, Rongqi Cui, Lincai Peng, Shengbao Cai, Pan Li, and Tianqing Lan. 2018. "Preparation of Antibacterial Cellulose Paper Using Layer-by-Layer Assembly for Cooked Beef Preservation at Ambient Temperature" Polymers 10, no. 1: 15. https://doi.org/10.3390/polym10010015




