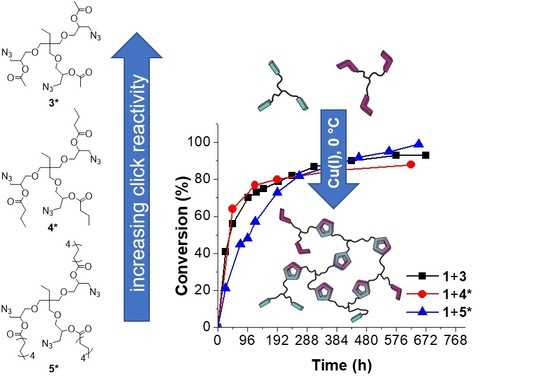
Improving Kinetics of “Click-Crosslinking” for Self-Healing Nanocomposites by Graphene-Supported Cu-Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
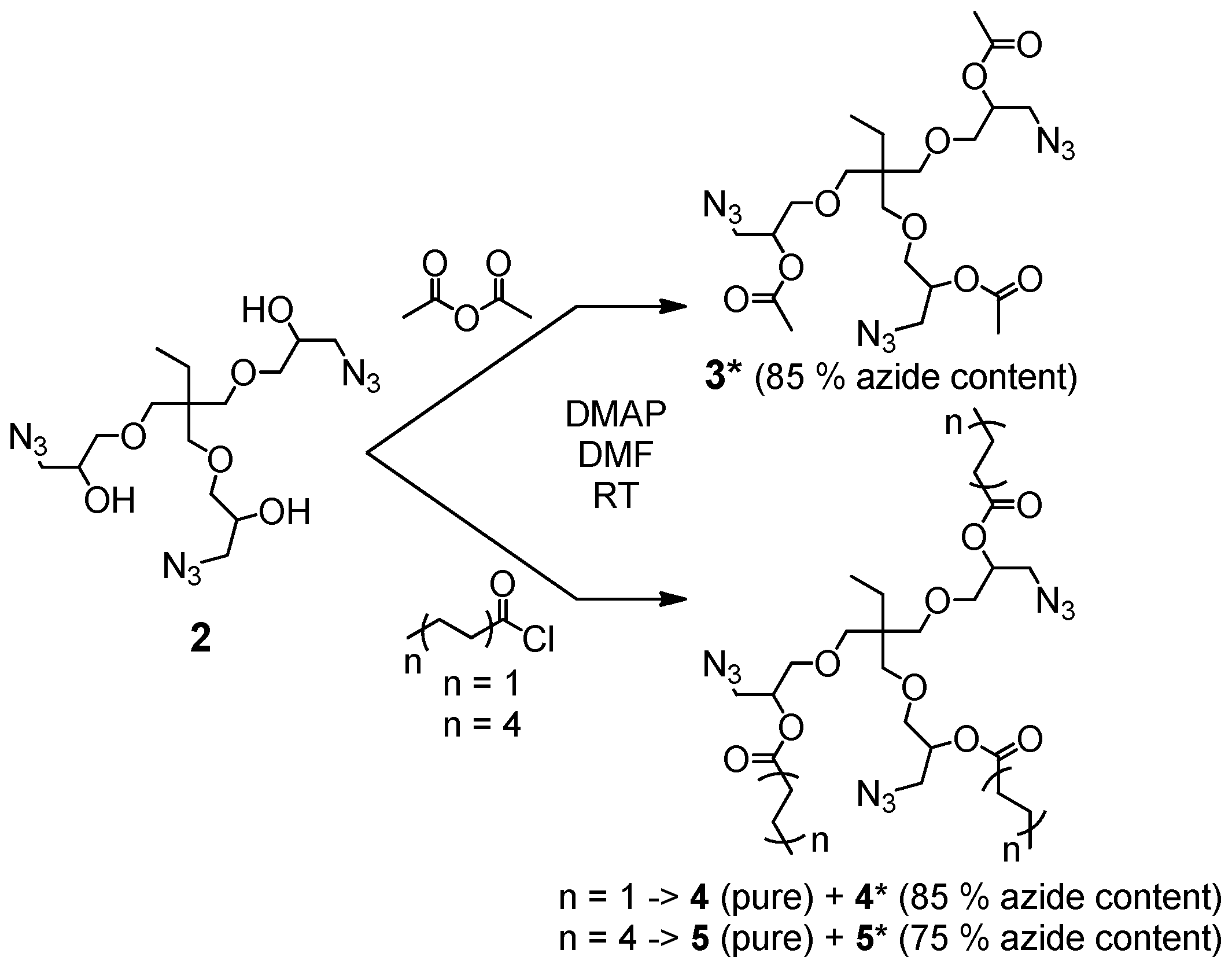
2.3. General Synthesis Procedure for the Preparation of Trivalent Azides
3. Results
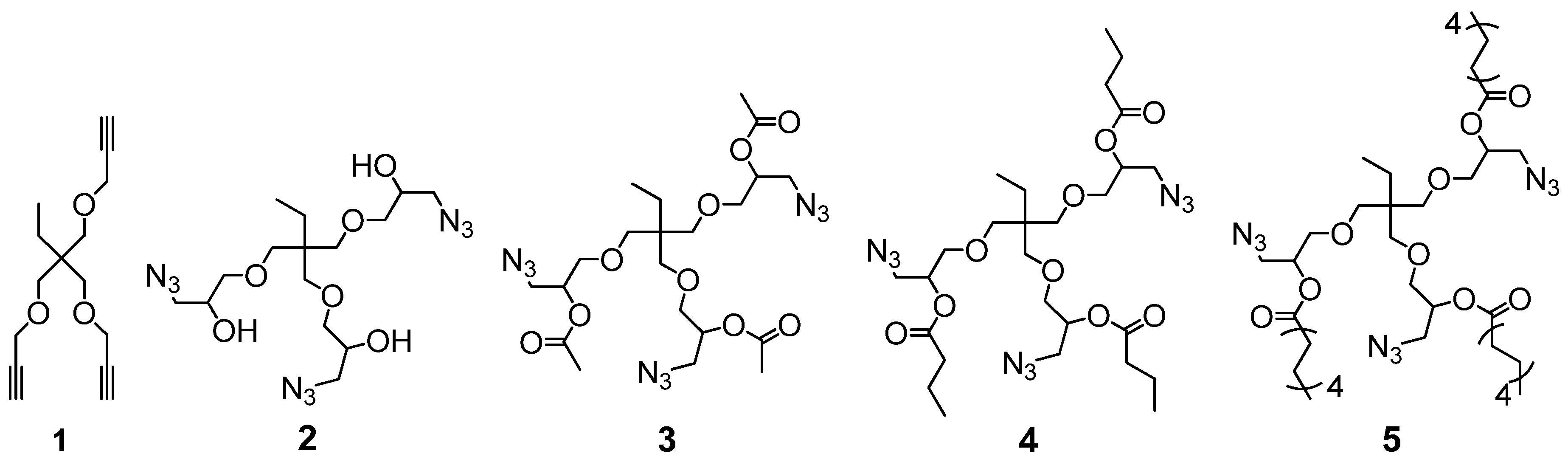
3.1. Synthesis of Trivalent Alkyne and Trivalent Azides
3.2. DSC Investigation of “Click-Crosslinking” Trivalent Alkyne and Trivalent Azides
3.3. DSC Investigation of “Click-Crosslinking” Trivalent Alkyne 1 and Trivalent Azides 3*, 4* and 5* at 0 °C
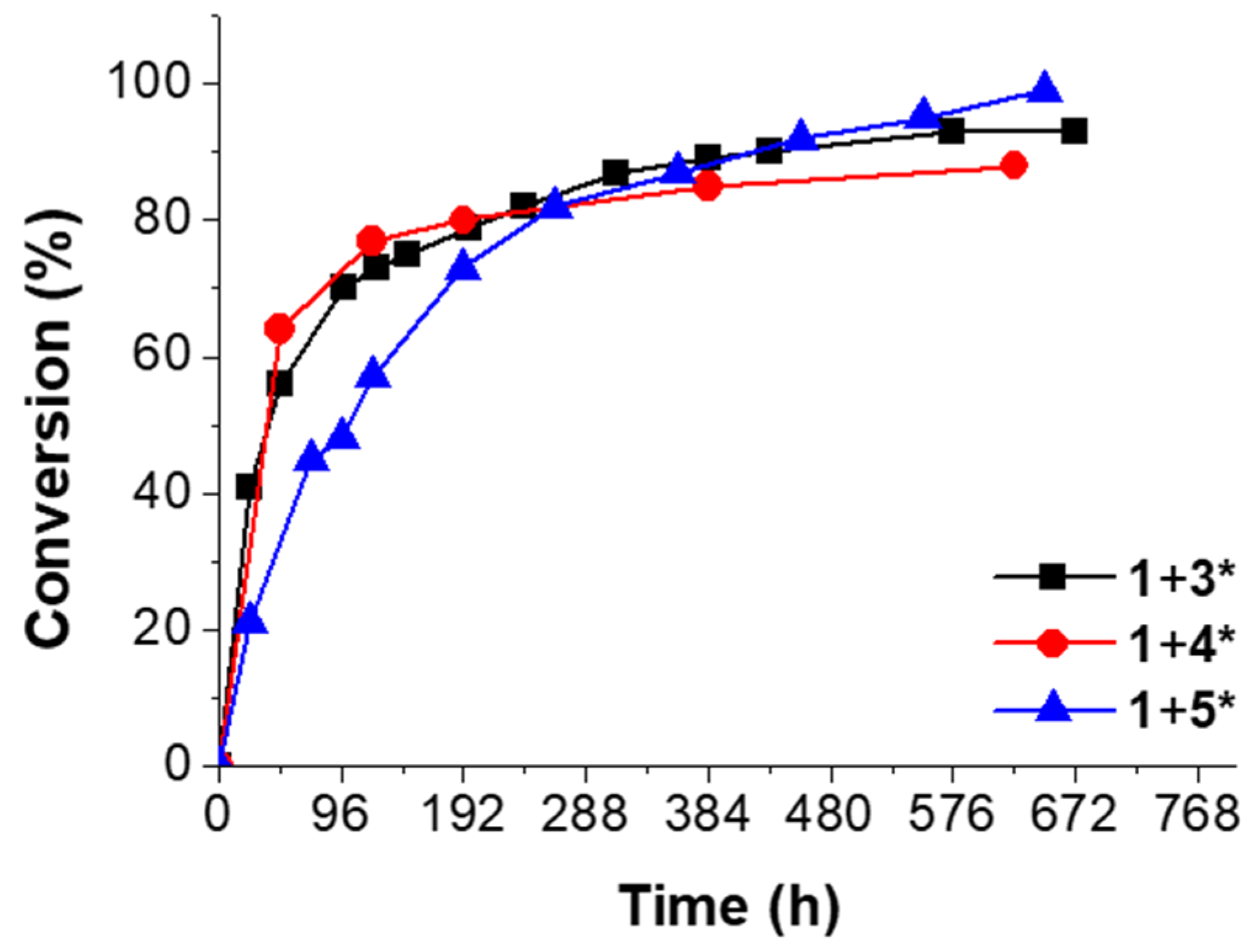
3.4. Rheology Investigation of “Click-Crosslinking” Trivalent Alkyne 1 and Trivalent Azides 4* and 5*
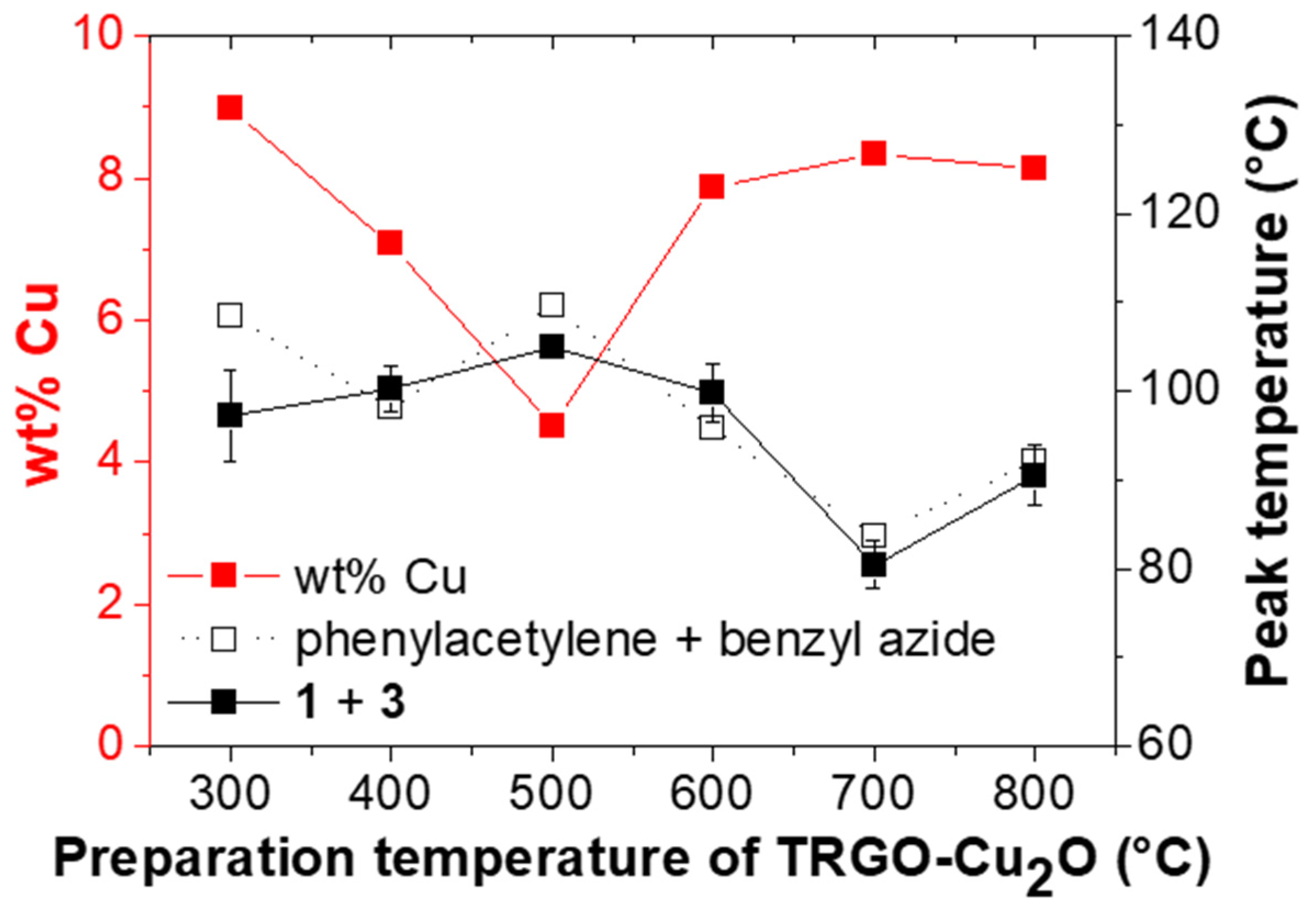
3.5. Synthesis and Characerization of TRGO-Cu2O Prepared at Different Temperatures
3.6. Crosslinking Reactions of Alkynes and Azides in the Presence of TRGO-Cu2O Prepared at Different Temperatures
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–817. [Google Scholar] [CrossRef] [PubMed]
- Michael, P.; Döhler, D.; Binder, W.H. Improving autonomous self-healing via combined chemical/physical principles. Polymer 2015, 69, 216–227. [Google Scholar] [CrossRef]
- Huang, G.C.; Lee, J.K.; Kessler, M.R. Evaluation of norbornene-based adhesives to amine-cured epoxy for self-healing applications. Macromol. Mater. Eng. 2011, 296, 965–972. [Google Scholar] [CrossRef]
- Aïssa, B.; Haddad, E.; Jamroz, W.; Hassani, S.; Farahani, R.D.; Merle, P.G.; Therriault, D. Micromechanical characterization of single-walled carbon nanotube reinforced ethylidene norbornene nanocomposites for self-healing applications. Smart Mater. Struct. 2012, 21, 105028. [Google Scholar] [CrossRef]
- Mauldin, T.C.; Leonard, J.; Earl, K.; Lee, J.K.; Kessler, M.R. Modified rheokinetic technique to enhance the understanding of microcapsule-based self-healing polymers. ACS Appl. Mater. Interfaces 2012, 4, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, M.; Guadagno, L. Healing efficiency of epoxy-based materials for structural applications. Polym. Compos. 2013, 34, 1525–1532. [Google Scholar] [CrossRef]
- Mariconda, A.; Longo, P.; Agovino, A.; Guadagno, L.; Sorrentino, A.; Raimondo, M. Synthesis of ruthenium catalysts functionalized graphene oxide for self-healing applications. Polymer 2015, 69, 330–342. [Google Scholar] [CrossRef]
- Monfared Zanjani, J.S.; Okan, B.S.; Letofsky-Papst, I.; Menceloglu, Y.; Yildiz, M. Repeated self-healing of nano and micro scale cracks in epoxy based composites by tri-axial electrospun fibers including different healing agents. RSC Adv. 2015, 5, 73133–73145. [Google Scholar] [CrossRef]
- Wang, B.; Mireles, K.; Rock, M.; Li, Y.; Thakur, V.K.; Gao, D.; Kessler, M.R. Synthesis and preparation of bio-based ROMP thermosets from functionalized renewable isosorbide derivative. Macromol. Chem. Phys. 2016, 217, 871–879. [Google Scholar] [CrossRef]
- Longo, P.; Mariconda, A.; Calabrese, E.; Raimondo, M.; Naddeo, C.; Vertuccio, L.; Russo, S.; Iannuzzo, G.; Guadagno, L. Development of a new stable ruthenium initiator suitably designed for self-repairing applications in high reactive environments. J. Ind. Eng. Chem. 2017, 54, 234–251. [Google Scholar] [CrossRef]
- Döhler, D.; Michael, P.; Binder, W.H. Autocatalysis in the room temperature copper(I)-catalyzed alkyne-azide “click” cycloaddition of multivalent poly(acrylate)s and poly(isobutylene)s. Macromolecules 2012, 45, 3335–3345. [Google Scholar] [CrossRef]
- Döhler, D.; Michael, P.; Binder, W.H. CuAAC-based click chemistry in self-healing polymers. Acc. Chem. Res. 2017, 50, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Döhler, D.; Rana, S.; Rupp, H.; Bergmann, H.; Behzadi, S.; Crespy, D.; Binder, W.H. Qualitative sensing of mechanical damage by a fluorogenic “click” reaction. Chem. Commun. 2016, 52, 11076–11079. [Google Scholar] [CrossRef] [PubMed]
- Döhler, D.; Zare, P.; Binder, W.H. Hyperbranched polyisobutylenes for self-healing polymers. Polym. Chem. 2014, 5, 992–1000. [Google Scholar] [CrossRef]
- Gragert, M.; Schunack, M.; Binder, W.H. Azide/Alkyne-“Click”-Reactions of encapsulated reagents: Toward self-healing materials. Macromol. Rapid Commun. 2011, 32, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Michael, P.; Binder, W.H. A mechanochemically triggered “click” catalyst. Angew. Chem. Int. Ed. 2015, 54, 13918–13922. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Döhler, D.; Ströhl, D.; Binder, W.H. Chelation-assisted CuAAC of star-shaped polymers enables fast self-healing at low temperatures. Polym. Chem. 2016, 7, 2342–2351. [Google Scholar] [CrossRef]
- Raimondo, M.; De Nicola, F.; Volponi, R.; Binder, W.H.; Michael, P.; Russo, S.; Guadagno, L. Self-repairing CFRPs targeted towards structural aerospace applications. Int. J. Struct. Integr. 2016, 7, 656–670. [Google Scholar] [CrossRef]
- Rana, S.; Döhler, D.; Nia, A.S.; Nasir, M.; Beiner, M.; Binder, W.H. “Click”-triggered self-healing graphene nanocomposites. Macromol. Rapid Commun. 2016, 37, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Schunack, M.; Gragert, M.; Döhler, D.; Michael, P.; Binder, W.H. Low-temperature Cu(I)-catalyzed “click” reactions for self-healing polymers. Macromol. Chem. Phys. 2012, 213, 205–214. [Google Scholar] [CrossRef]
- Shaygan Nia, A.; Rana, S.; Döhler, D.; Noirfalise, X.; Belfiore, A.; Binder, W.H. Click chemistry promoted by graphene supported copper nanomaterials. Chem. Commun. 2014, 50, 15374–15377. [Google Scholar] [CrossRef] [PubMed]
- Shaygan Nia, A.; Rana, S.; Döhler, D.; Osim, W.; Binder, W.H. Nanocomposites via a direct graphene-promoted “click” reaction. Polymer 2015, 79, 21–28. [Google Scholar] [CrossRef]
- Shaygan Nia, A.; Rana, S.; Döhler, D.; Jirsa, F.; Meister, A.; Guadagno, L.; Koslowski, E.; Bron, M.; Binder, W.H. Carbon-supported copper nanomaterials: Recyclable catalysts for huisgen [3 + 2] cycloaddition reactions. Chem. Eur. J. 2015, 21, 10763–10770. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Mauldin, T.C.; Kessler, M.R. Kinetics of bulk azide/alkyne “click” polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4093–4102. [Google Scholar] [CrossRef]
- Sheng, X.; Rock, D.M.; Mauldin, T.C.; Kessler, M.R. Evaluation of different catalyst systems for bulk polymerization through “click” chemistry. Polymer 2011, 52, 4435–4441. [Google Scholar] [CrossRef]
- Vasiliu, S.; Kampe, B.; Theil, F.; Dietzek, B.; Döhler, D.; Michael, P.; Binder, W.H.; Popp, J. Insights into the mechanism of polymer coating self-healing using raman spectroscopy. Appl. Spectrosc. 2014, 68, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Döhler, D.; Peterlik, H.; Binder, W.H. A dual crosslinked self-healing system: Supramolecular and covalent network formation of four-arm star polymers. Polymer 2015, 69, 264–273. [Google Scholar] [CrossRef]
- Cao, S.; Li, S.; Li, M.; Xu, L.; Ding, H.; Xia, J.; Zhang, M.; Huang, K. A thermal self-healing polyurethane thermoset based on phenolic urethane. Polym. J. 2017, 49, 775–781. [Google Scholar] [CrossRef]
- Haghayegh, M.; Mirabedini, S.M.; Yeganeh, H. Preparation of microcapsules containing multi-functional reactive isocyanate-terminated-polyurethane-prepolymer as healing agent, part II: Corrosion performance and mechanical properties of a self-healing coating. RSC Adv. 2016, 6, 50874–50886. [Google Scholar] [CrossRef]
- Haghayegh, M.; Mirabedini, S.M.; Yeganeh, H. Microcapsules containing multi-functional reactive isocyanate-terminated polyurethane prepolymer as a healing agent. Part 1: Synthesis and optimization of reaction conditions. J. Mater. Sci. 2016, 51, 3056–3068. [Google Scholar] [CrossRef]
- Hillewaere, X.K.D.; Teixeira, R.F.A.; Nguyen, L.-T.T.; Ramos, J.A.; Rahier, H.; Du Prez, F.E. Autonomous self-healing of epoxy thermosets with thiol-isocyanate chemistry. Adv. Funct. Mater. 2014, 24, 5575–5583. [Google Scholar] [CrossRef]
- Keller, M.W.; Hampton, K.; McLaury, B. Self-healing of erosion damage in a polymer coating. Wear 2013, 307, 218–225. [Google Scholar] [CrossRef]
- McIlroy, D.A.; Blaiszik, B.J.; Caruso, M.M.; White, S.R.; Moore, J.S.; Sottos, N.R. Microencapsulation of a reactive liquid-phase amine for self-healing epoxy composites. Macromolecules 2010, 43, 1855–1859. [Google Scholar] [CrossRef]
- Park, J.I.; Choe, A.; Kim, M.P.; Ko, H.; Lee, T.H.; Noh, S.M.; Kim, J.C.; Cheong, I.W. Water-adaptive and repeatable self-healing polymers bearing bulky urea bonds. Polym. Chem. 2018, 9, 11–19. [Google Scholar] [CrossRef]
- Schüssele, A.C.; Nübling, F.; Thomann, Y.; Carstensen, O.; Bauer, G.; Speck, T.; Mülhaupt, R. Self-healing rubbers based on NBR blends with hyperbranched polyethylenimines. Macromol. Mater. Eng. 2012, 297, 411–419. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zechel, S.; Geitner, R.; Abend, M.; Siegmann, M.; Enke, M.; Kuhl, N.; Klein, M.; Vitz, J.; Gräfe, S.; Dietzek, B.; et al. Intrinsic self-healing polymers with a high E-modulus based on dynamic reversible urea bonds. NPG Asia Mater. 2017, 9, 420. [Google Scholar] [CrossRef]
- Akiyama, T.; Ushio, A.; Itoh, Y.; Kawaguchi, Y.; Matsumoto, K.; Jikei, M. Synthesis and healing properties of poly(arylether sulfone)-poly(alkylthioether) multiblock copolymers containing disulfide bonds. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3545–3553. [Google Scholar] [CrossRef]
- Billiet, S.; Van Camp, W.; Hillewaere, X.K.D.; Rahier, H.; Du Prez, F.E. Development of optimized autonomous self-healing systems for epoxy materials based on maleimide chemistry. Polymer 2012, 53, 2320–2326. [Google Scholar] [CrossRef]
- Chakma, P.; Rodrigues Possarle, L.H.; Digby, Z.A.; Zhang, B.; Sparks, J.L.; Konkolewicz, D. Dual stimuli responsive self-healing and malleable materials based on dynamic thiol-Michael chemistry. Polym. Chem. 2017, 8, 6534–6543. [Google Scholar] [CrossRef]
- Kuhl, N.; Geitner, R.; Vitz, J.; Bode, S.; Schmitt, M.; Popp, J.; Schubert, U.S.; Hager, M.D. Increased stability in self-healing polymer networks based on reversible Michael addition reactions. J. Appl. Polym. Sci. 2017, 134, 44805. [Google Scholar] [CrossRef]
- Pepels, M.; Filot, I.; Klumperman, B.; Goossens, H. Self-healing systems based on disulfide-thiol exchange reactions. Polym. Chem. 2013, 4, 4955–4965. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kamada, J.; Koynov, K.; Mohin, J.; Nicolaÿ, R.; Zhang, Y.; Balazs, A.C.; Kowalewski, T.; Matyjaszewski, K. Self-healing polymer films based on thiol–disulfide exchange reactions and self-healing kinetics measured using atomic force microscopy. Macromolecules 2011, 45, 142–149. [Google Scholar] [CrossRef]
- Yue, H.-B.; Fernández-Blázquez, J.P.; Beneito, D.F.; Vilatela, J.J. Real time monitoring of click chemistry self-healing in polymer composites. J. Mater. Chem. A 2014, 2, 3881–3887. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.H.; Vuluga, D.; Lecamp, L.; Burel, F. Photoinitiated thiol-epoxy addition for the preparation of photoinduced self-healing fatty coatings. RSC Adv. 2016, 6, 32098–32105. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Cao, G.S.; Qiu, W.L.; Rong, M.Z.; Zhang, M.Q. Self-healing polyvinyl chloride (PVC) based on microencapsulated nucleophilic thiol-click chemistry. Polymer 2015, 69, 1–9. [Google Scholar] [CrossRef]
- Chung, U.S.; Min, J.H.; Lee, P.-C.; Koh, W.-G. Polyurethane matrix incorporating PDMS-based self-healing microcapsules with enhanced mechanical and thermal stability. Colloids Surf. A 2017, 518, 173–180. [Google Scholar] [CrossRef]
- Wei, K.; Gao, Z.; Liu, H.; Wu, X.; Wang, F.; Xu, H. Mechanical activation of platinum—acetylide complex for olefin hydrosilylation. ACS Macro Lett. 2017, 6, 1146–1150. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Peptides, the Wave of the Future; American Peptide Society: San Diego, CA, USA, 2001. [Google Scholar]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ Chemistry in polymer and materials science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. “Click”-chemistry in polymer and material science: An update. Macromol. Rapid Commun. 2008, 29, 952–981. [Google Scholar] [CrossRef]
- Zhao, Y.; Döhler, D.; Lv, L.-P.; Binder, W.H.; Landfester, K.; Crespy, D. Facile phase-separation approach to encapsulate functionalized polymers in core–shell nanoparticles. Macromol. Chem. Phys. 2014, 215, 198–204. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Huisgen, R. Kinetics and reaction mechanisms: Selected examples from the experience of forty years. Pure Appl. Chem 1989, 61, 613–628. [Google Scholar] [CrossRef]
- Chan, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004, 6, 2853–2855. [Google Scholar] [CrossRef] [PubMed]
- Gorman, I.E.; Willer, R.L.; Kemp, L.K.; Storey, R.F. Development of a triazole-cure resin system for composites: Evaluation of alkyne curatives. Polymer 2012, 53, 2548–2558. [Google Scholar] [CrossRef]
- Kantheti, S.; Sarath, P.S.; Narayan, R.; Raju, K.V.S.N. Synthesis and characterization of triazole rich polyether polyols using click chemistry for highly branched polyurethanes. React. Funct. Polym. 2013, 73, 1597–1605. [Google Scholar] [CrossRef]
- Bond, G.C.; Keane, M.A.; Kral, H.; Lercher, J.A. Compensation phenomena in heterogeneous catalysis: General principles and a possible explanation. Cat. Rev. 2000, 42, 323–383. [Google Scholar] [CrossRef]
- Mulokozi, A.M. Kinetic parameters in heterogeneous kinetics. Thermochim. Acta 1992, 197, 363–372. [Google Scholar] [CrossRef]
- Nonahal, M.; Rastin, H.; Saeb, M.R.; Sari, M.G.; Moghadam, M.H.; Zarrintaj, P.; Ramezanzadeh, B. Epoxy/PAMAM dendrimer-modified graphene oxide nanocomposite coatings: Nonisothermal cure kinetics study. Prog. Org. Coat. 2018, 114, 233–243. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Kyotani, T.; Suzuki, K.-Y.; Yamashita, H.; Tomita, A. Formation of carbon-metal composites from metal ion exchanged graphite oxide. Tanso 1993, 1993, 255–265. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene-based nanoarchitectures. anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J. Phys. Chem. Lett. 2010, 1, 520–527. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Grácio, J. Surface modification of graphene nanosheets with gold nanoparticles: The role of oxygen moieties at graphene surface on gold nucleation and growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Gan, Y.; Sun, L.; Banhart, F. One- and two-dimensional diffusion of metal atoms in graphene. Small 2008, 4, 587–591. [Google Scholar] [CrossRef] [PubMed]

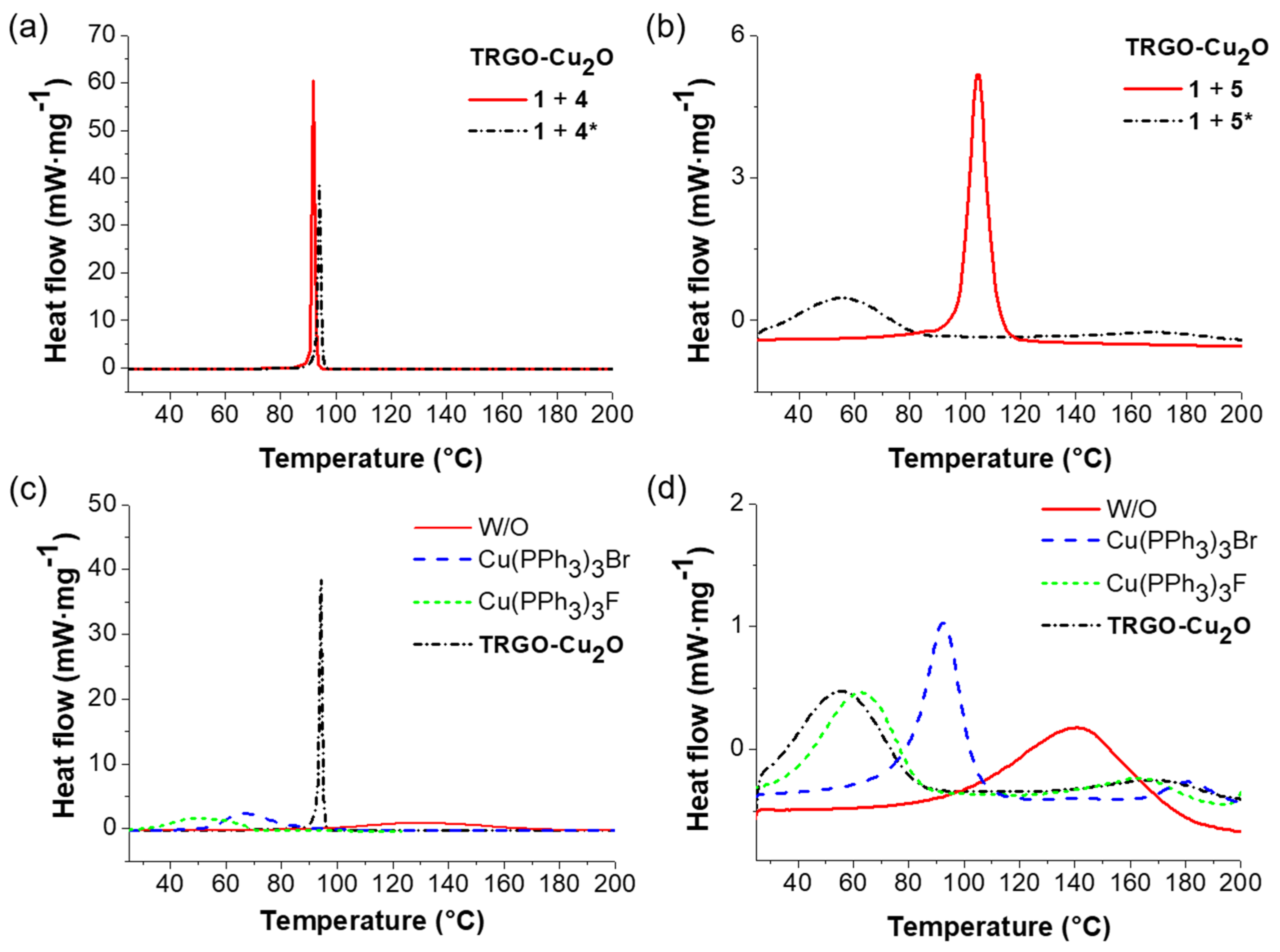
| Entry | Azide | Catalyst | Mass (%) | Tonset 1 (°C) | Tp 1 (°C) | ΔH 2 (kJ∙mol−1) | Ea, app (kJ∙mol−1) | Conversion 3 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 3* | W/O | - | 91 | 133 | 205 | 83 | 78 |
| 2 | Cu(PPh3)3Br | 3.4 | 59 | 74 | 185 | 87 | 71 | |
| 3 | Cu(PPh3)3F | 3.2 | 39 | 66 | 191 | 83 | 73 | |
| 4 | TRGO-Cu2O | 7.4 | 51 | 63 | 177 | 55 | 67 | |
| 5 | 4 | TRGO-Cu2O | 6.7 | 91 | 92 | 233 | 104 | 89 |
| 6 | 4* | W/O | - | 94 | 125 | 227 | 76 | 87 |
| 7 | Cu(PPh3)3Br | 2.9 | 54 | 66 | 223 | 70 | 85 | |
| 8 | Cu(PPh3)3F | 3.2 | 32 | 50 | 192 | 61 | 73 | |
| 9 | TRGO-Cu2O | 6.9 | 93 | 94 | 211 | 97 | 81 | |
| 10 | 5 | TRGO-Cu2O | 5.0 | 98 | 105 | 248 | 133 | 95 |
| 11 | 5* | W/O | - | 102 | 141 | 174 | 100 | 66 |
| 12 | Cu(PPh3)3Br | 2.2 | 80 | 93 | 149 | 55 | 57 | |
| 13 | Cu(PPh3)3F | 2.4 | 38 | 62 | 140 | 103 | 53 | |
| 14 | TRGO-Cu2O | 5.2 | 32 | 56 | 127 | 85 | 48 |
| Entry | Catalyst | Tonset 1 (°C) | Tp 1 (°C) | ∆H 2 (kJ⋅mol−1) | Conversion 3 (%) |
|---|---|---|---|---|---|
| 1 | W/O | 96 | 130 | 75 | 29 |
| 2 | TRGO-Cu2O (300 °C) | 82 | 97 | 108 | 41 |
| 3 | TRGO-Cu2O (400 °C) | 80 | 100 | 101 | 38 |
| 4 | TRGO-Cu2O (500 °C) | 90 | 105 | 107 | 41 |
| 5 | TRGO-Cu2O (600 °C) | 91 | 100 | 115 | 44 |
| 6 | TRGO-Cu2O (700 °C) | 64 | 80 | 107 | 41 |
| 7 | TRGO-Cu2O (800 °C) | 72 | 91 | 113 | 43 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargarfard, N.; Diedrich, N.; Rupp, H.; Döhler, D.; Binder, W.H. Improving Kinetics of “Click-Crosslinking” for Self-Healing Nanocomposites by Graphene-Supported Cu-Nanoparticles. Polymers 2018, 10, 17. https://doi.org/10.3390/polym10010017
Kargarfard N, Diedrich N, Rupp H, Döhler D, Binder WH. Improving Kinetics of “Click-Crosslinking” for Self-Healing Nanocomposites by Graphene-Supported Cu-Nanoparticles. Polymers. 2018; 10(1):17. https://doi.org/10.3390/polym10010017
Chicago/Turabian StyleKargarfard, Neda, Norman Diedrich, Harald Rupp, Diana Döhler, and Wolfgang H. Binder. 2018. "Improving Kinetics of “Click-Crosslinking” for Self-Healing Nanocomposites by Graphene-Supported Cu-Nanoparticles" Polymers 10, no. 1: 17. https://doi.org/10.3390/polym10010017