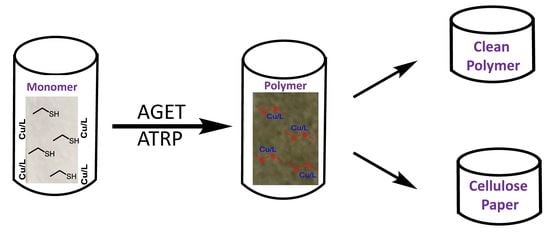
A Facial Strategy for Catalyst and Reducing Agent Synchronous Separation for AGET ATRP Using Thiol-Grafted Cellulose Paper as Reducing Agent
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
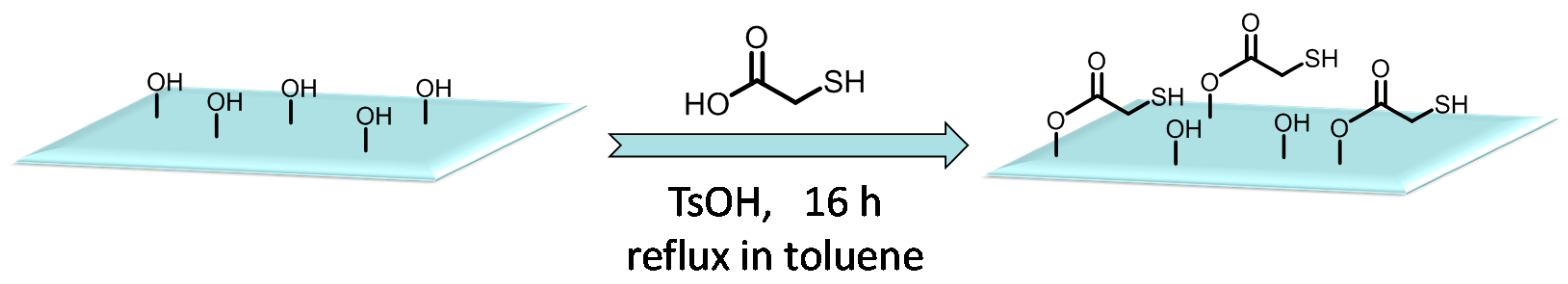
2.2. Synthesis of Biomimetic Reducing Agent, Thiol Functionalized Cellulose Paper (Cell-SH)
2.3. General Procedure of AGET ATRP of MMA with Cell-SH Paper as Reducing Agent
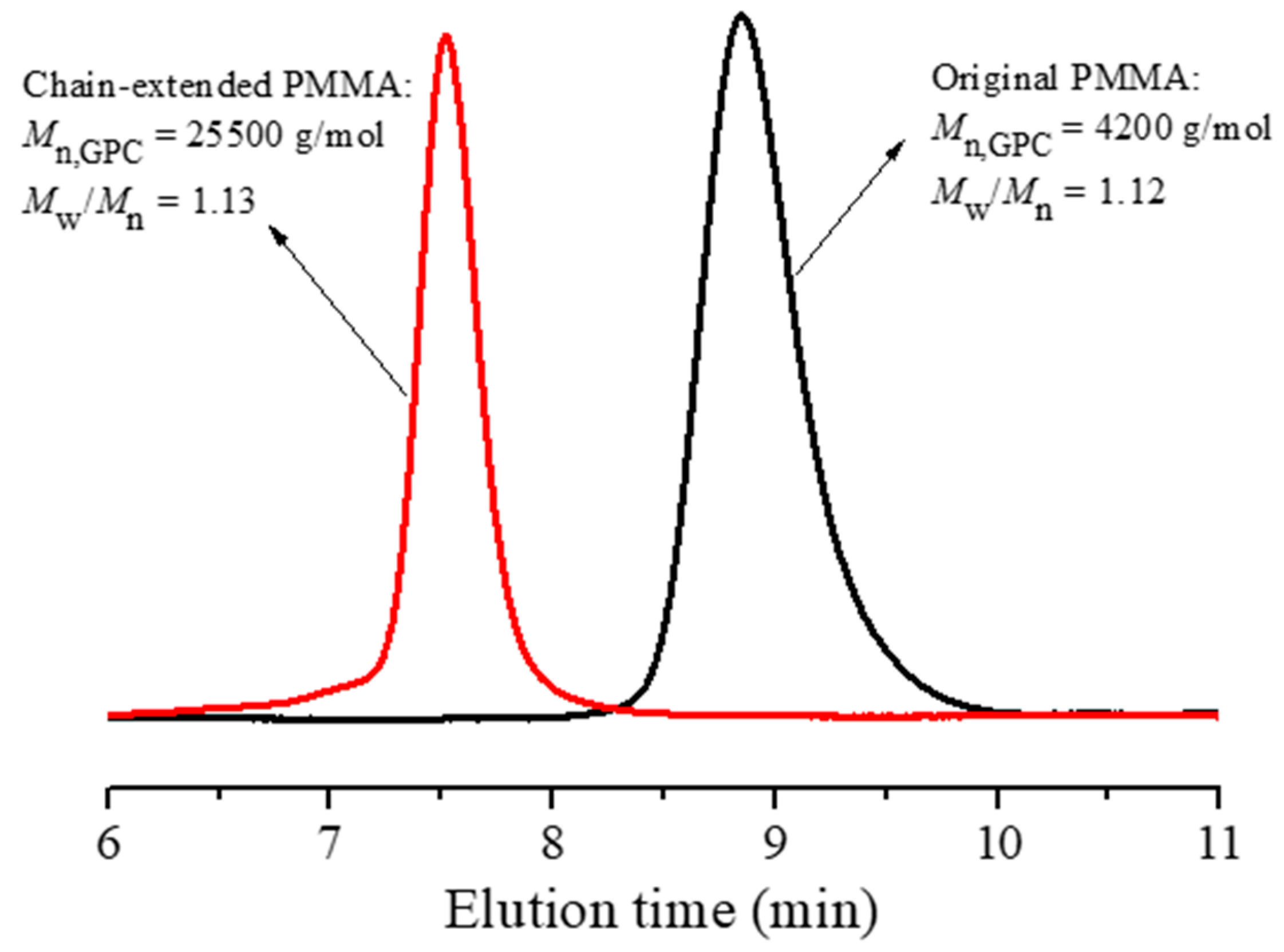
2.4. Chain Extension with PMMA as the Macroinitiator
2.5. Characterization
3. Results and Discussion
3.1. The Properties of Difunctional Reducing Agent, Cell-SH
3.2. The Effect of the Amount of Reducing Agent, Cell-SH and Ligand, PMDETA on the AGET ATRP with Thiol-Functional Cell Paper as Dual Functional Reducing Agent
3.3. Polymerization Kinetics of AGET ATRP of MMA with Cell-SH as Reducing Agent
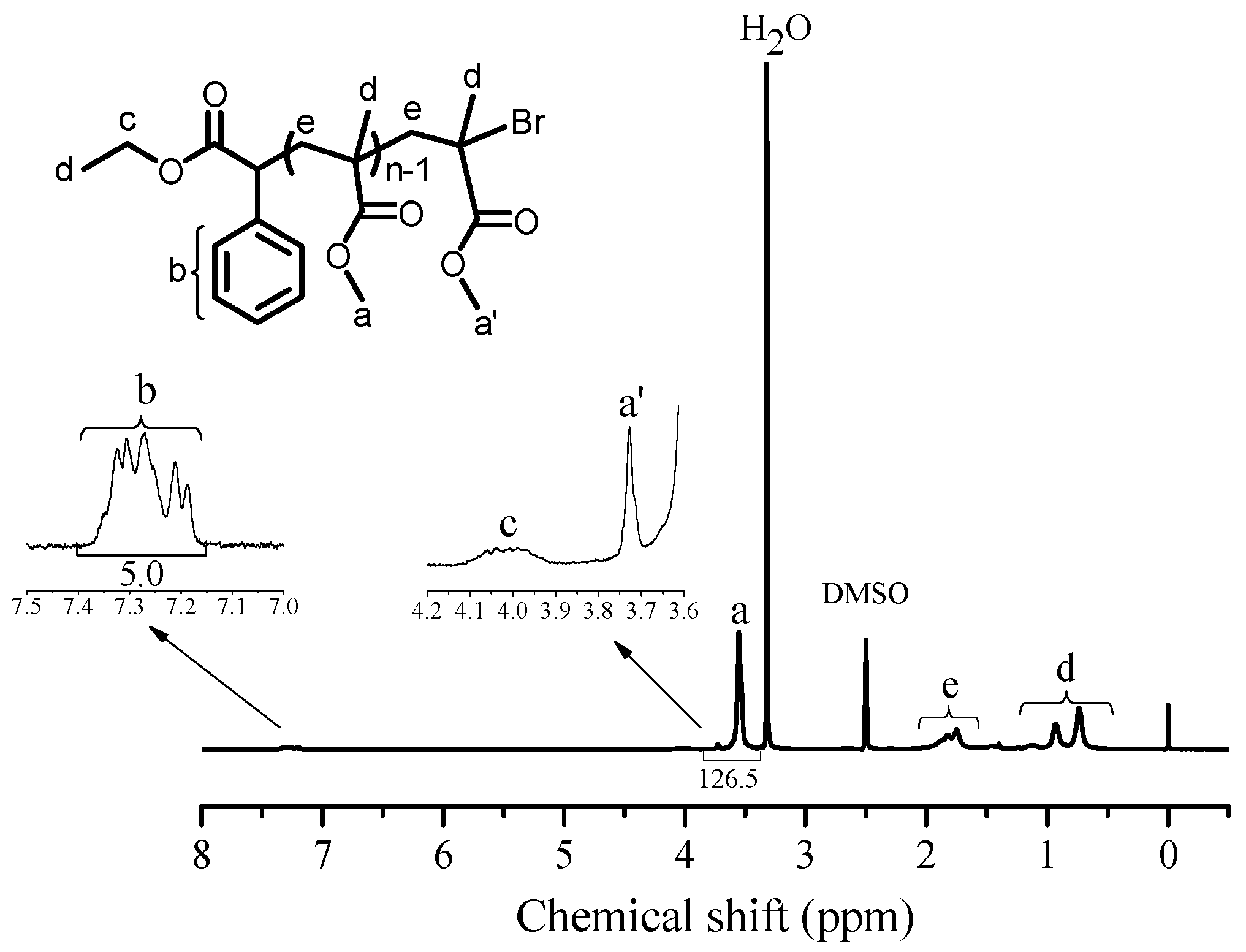
3.4. Analysis of Chain End and Chain Extension
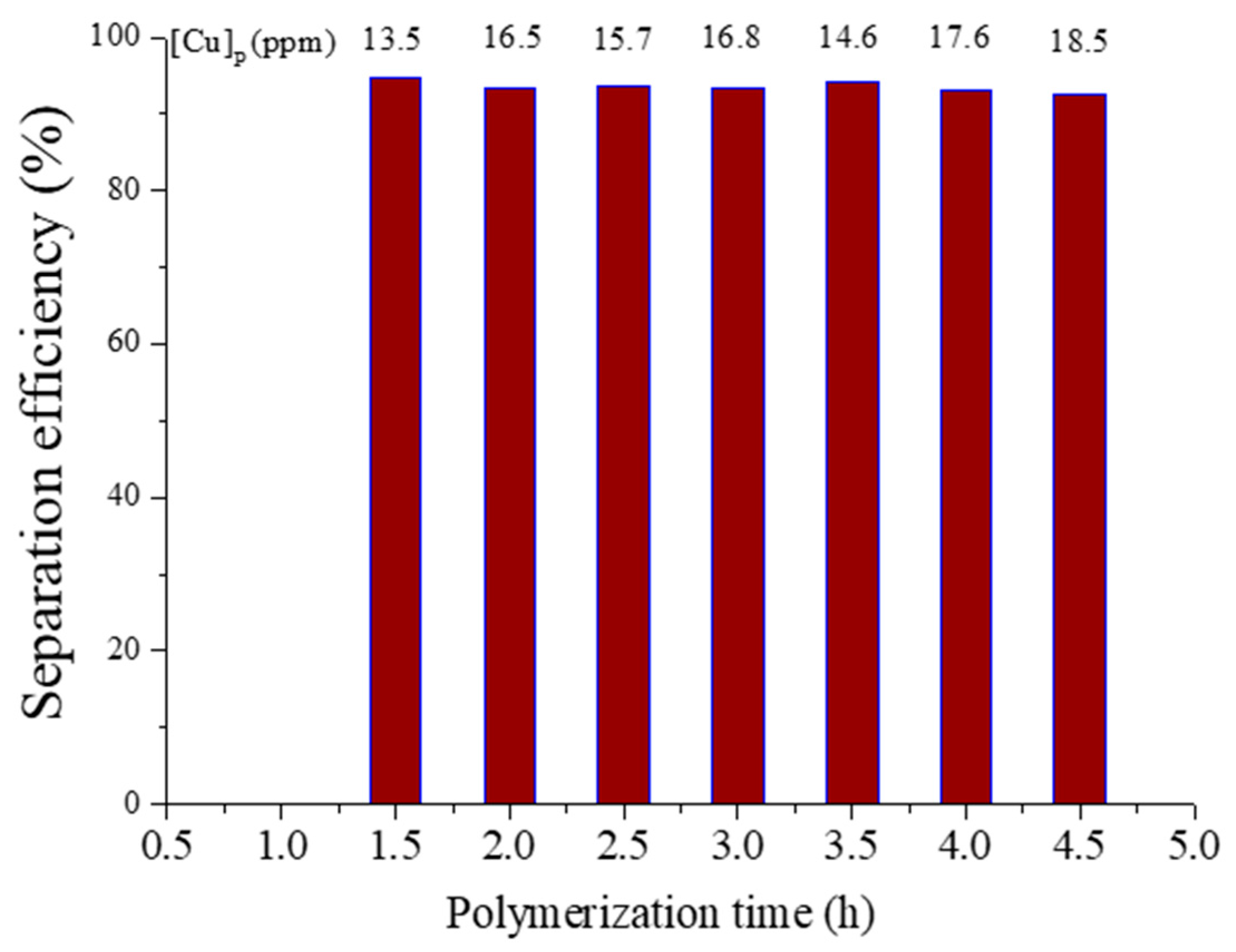
3.5. Synchronous Separation of Metal Catalyst and Byproduct of Reducing Agent
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matyjaszewski, K.; Tsarevsky, N.V. Macromolecular Engineering by Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2014, 136, 6513–6533. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, M.; Sawamoto, M. 50th Anniversary Perspective: Metal-Catalyzed Living Radical Polymerization: Discovery and Perspective. Macromolecules 2017, 50, 2603–2614. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.-F.; Marin, G.B. The strength of multi-scale modeling to unveil the complexity of radical polymerization. Prog. Polym. Sci. 2016, 58, 59–89. [Google Scholar] [CrossRef]
- Van Steenberge, P.H.M.; D’hooge, D.R.; Wang, Y.; Zhong, M.; Reyniers, M.-F.; Konkolewicz, D.; Matyjaszewski, K.; Marin, G.B. Linear Gradient Quality of ATRP Copolymers. Macromolecules 2012, 45, 8519–8531. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Matyjaszewski, K. Controlled/“living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Konkolewicz, D.; Magenau, A.J.D.; Averick, S.E.; Simakova, A.; He, H.; Matyjaszewski, K. ICAR ATRP with ppm Cu Catalyst in Water. Macromolecules 2012, 45, 4461–4468. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, L.; Cheng, Z.; Zhu, X. Activators generated by electron transfer for atom transfer radical polymerization: Recent advances in catalyst and polymer chemistry. Polym. Chem. 2012, 3, 2685–2697. [Google Scholar] [CrossRef]
- Jakubowski, W.; Matyjaszewski, K. Activator Generated by Electron Transfer for Atom Transfer Radical Polymerization. Macromolecules 2005, 38, 4139–4146. [Google Scholar] [CrossRef]
- Payne, K.A.; Van Steenberge, P.H.M.; D’Hooge, D.R.; Reyniers, M.-F.; Marin, G.B.; Hutchinson, R.A.; Cunningham, M.F. Controlled synthesis of poly[(butyl methacrylate)-co-(butyl acrylate)] via activator regenerated by electron transfer atom transfer radical polymerization: Insights and improvement. Polym. Int. 2014, 63, 848–857. [Google Scholar] [CrossRef] [Green Version]
- Payne, K.A.; D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.-F.; Cunningham, M.F.; Hutchinson, R.A.; Marin, G.B. ARGET ATRP of Butyl Methacrylate: Utilizing Kinetic Modeling To Understand Experimental Trends. Macromolecules 2013, 46, 3828–3840. [Google Scholar] [CrossRef]
- Fierens, S.; D’Hooge, D.; Van Steenberge, P.; Reyniers, M.-F.; Marin, G. Exploring the Full Potential of Reversible Deactivation Radical Polymerization Using Pareto-Optimal Fronts. Polymers 2015, 7, 655–679. [Google Scholar] [CrossRef] [Green Version]
- Matyjaszewski, K.; Jakubowski, W.; Min, K.; Tang, W.; Huang, J.; Braunecker, W.A.; Tsarevsky, N.V. Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 15309–15314. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.W.; Wu, J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Highly Active ppm Level Organic Copper Catalyzed Photo-Induced ICAR ATRP of Methyl Methacrylate. Macromol. Rapid Commun. 2014, 35, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, L.; Zhang, Z.; Zhu, J.; Tu, Y.; Cheng, Z.; Zhu, X. Iron-Mediated ICAR ATRP of Methyl Methacrylate. Macromolecules 2011, 44, 3233–3239. [Google Scholar] [CrossRef]
- Kwak, Y.; Magenau, A.J.D.; Matyjaszewski, K. ARGET ATRP of Methyl Acrylate with Inexpensive Ligands and ppm Concentrations of Catalyst. Macromolecules 2011, 44, 811–819. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, S.; Pelton, R. Effect of ligand spacer on silica gel supported atom transfer radical polymerization of methyl methacrylate. Macromolecules 2001, 34, 5812–5818. [Google Scholar] [CrossRef]
- Faucher, S.; Zhu, S. Fundamentals and development of high-efficiency supported catalyst systems for atom transfer radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 553–565. [Google Scholar] [CrossRef]
- Ding, S.; Xing, Y.; Radosz, M.; Shen, Y. Magnetic nanoparticle supported catalyst for atom transfer radical polymerization. Macromolecules 2006, 39, 6399–6405. [Google Scholar] [CrossRef]
- Karimi, B.; Zamani, A.; Clark, J.H. A Bipyridyl Palladium Complex Covalently Anchored onto Silica as an Effective and Recoverable Interphase Catalyst for the Aerobic Oxidation of Alcohols. Organometallics 2005, 24, 4695–4698. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Tang, H.D.; Ding, S.J. Catalyst separation in atom transfer radical polymerization. Prog. Polym. Sci. 2004, 29, 1053–1078. [Google Scholar] [CrossRef]
- Faucher, S.; Zhu, S. Location of the Catalytic Site in Supported Atom Transfer Radical Polymerization. Macromol. Rapid Commun. 2004, 25, 991–994. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, S.; Pelton, R. Soluble and recoverable support for copper bromide-mediated living radical polymerization. Macromolecules 2001, 34, 3182–3185. [Google Scholar] [CrossRef]
- Hong, S.C.; Lutz, J.-F.; Inoue, Y.; Strissel, C.; Nuyken, O.; Matyjaszewski, K. Use of an Immobilized/Soluble Hybrid ATRP Catalyst System for the Preparation of Block Copolymers, Random Copolymers, and Polymers with High Degree of Chain End Functionality. Macromolecules 2003, 36, 1075–1082. [Google Scholar] [CrossRef]
- Yang, J.; Ding, S.; Radosz, M.; Shen, Y. Reversible catalyst supporting via hydrogen-bonding-mediated self-assembly for atom transfer radical polymerization of MMA. Macromolecules 2004, 37, 1728–1734. [Google Scholar] [CrossRef]
- Du, X.; Pan, J.; Chen, M.; Zhang, L.; Cheng, Z.; Zhu, X. Thermo-regulated phase separable catalysis (TPSC)-based atom transfer radical polymerization in a thermo-regulated ionic liquid. Chem. Commun. 2014, 50, 9266–9269. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Radosz, M.; Shen, Y. Ionic Liquid Catalyst for Biphasic Atom Transfer Radical Polymerization of Methyl Methacrylate. Macromolecules 2005, 38, 5921–5928. [Google Scholar] [CrossRef]
- Haddleton, D.M.; Jackson, S.G.; Bon, S.A.F. Copper(I)-Mediated Living Radical Polymerization under Fluorous Biphasic Conditions. J. Am. Chem. Soc. 2000, 122, 1542–1543. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, L.; Pan, J.; Zhu, J.; Cheng, Z.; Zhu, X. Developing a Synthetic Approach with Thermoregulated Phase-Transfer Catalysis: Facile Access to Metal-Mediated Living Radical Polymerization of Methyl Methacrylate in Aqueous/Organic Biphasic System. Macromolecules 2013, 46, 2060–2066. [Google Scholar] [CrossRef]
- Sarbu, T.; Pintauer, T.; McKenzie, B.; Matyjaszewski, K. Atom transfer radical polymerization of styrene in toluene/water mixtures. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3153–3160. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, L.; Bai, L.; Zhang, Z.; Chen, H.; Cheng, Z.; Zhu, X. Atom transfer radical polymerization of methyl methacrylate with a thermo-responsive ligand: Construction of thermoregulated phase-transfer catalysis in an aqueous-organic biphasic system. Polym. Chem. 2013, 4, 2876–2883. [Google Scholar] [CrossRef]
- Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Highly Efficient and Facile Photocatalytic Recycling System Suitable for ICAR ATRP of Hydrophilic Monomers. Macromol. Rapid Commun. 2016, 37, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.W.; Wu, J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. A Facile Strategy for Catalyst Separation and Recycling Suitable for ATRP of Hydrophilic Monomers Using a Macroligand. Macromol. Rapid Commun. 2016, 37, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Miyake, G.M.; Theriot, J.C. Perylene as an Organic Photocatalyst for the Radical Polymerization of Functionalized Vinyl Monomers through Oxidative Quenching with Alkyl Bromides and Visible Light. Macromolecules 2014, 47, 8255–8261. [Google Scholar] [CrossRef]
- Theriot, J.C.; Lim, C.-H.; Yang, H.; Ryan, M.D.; Musgrave, C.B.; Miyake, G.M. Organocatalyzed atom transfer radical polymerization driven by visible light. Science 2016, 352, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Fang, C.; Fantin, M.; Malhotra, N.; So, W.Y.; Peteanu, L.A.; Isse, A.A.; Gennaro, A.; Liu, P.; Matyjaszewskit, K. Mechanism of Photoinduced Metal-Free Atom Transfer Radical Polymerization: Experimental and Computational Studies. J. Am. Chem. Soc. 2016, 138, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Cheng, Z.; Zhu, X. Metal-free photoinduced electron transfer-atom transfer radical polymerization (PET-ATRP) via a visible light organic photocatalyst. Polym. Chem. 2016, 7, 689–700. [Google Scholar] [CrossRef]
- Treat, N.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G.; Barton, B.E.; de Alaniz, J.R.; Fors, B.P.; Hawker, C.J. Metal-Free Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-Y.; Jiang, N.; Zhang, J.; Wei, X.; Lin, H.-H.; Yu, X.-Q. The Oxidative Damage of Plasmid DNA by Ascorbic Acid Derivatives in vitro: The First Research on the Relationship between the Structure of Ascorbic Acid and the Oxidative Damage of Plasmid DNA. Chem. Biodivers. 2006, 3, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Rull-Barrull, J.; D’Halluin, M.; Le Grognec, E.; Felpin, F.X. A paper-based biomimetic device for the reduction of Cu(II) to Cu(I)–application to the sensing of Cu(II). Chem. Commun. 2016, 52, 6569–6572. [Google Scholar] [CrossRef] [PubMed]
- Rull-Barrull, J.; D’Halluin, M.; Le Grognec, E.; Felpin, F.X. Harnessing the Dual Properties of Thiol-Grafted Cellulose Paper for Click Reactions: A Powerful Reducing Agent and Adsorbent for Cu. Angew. Chem. Int. Ed. 2016, 55, 13549–13552. [Google Scholar] [CrossRef] [PubMed]


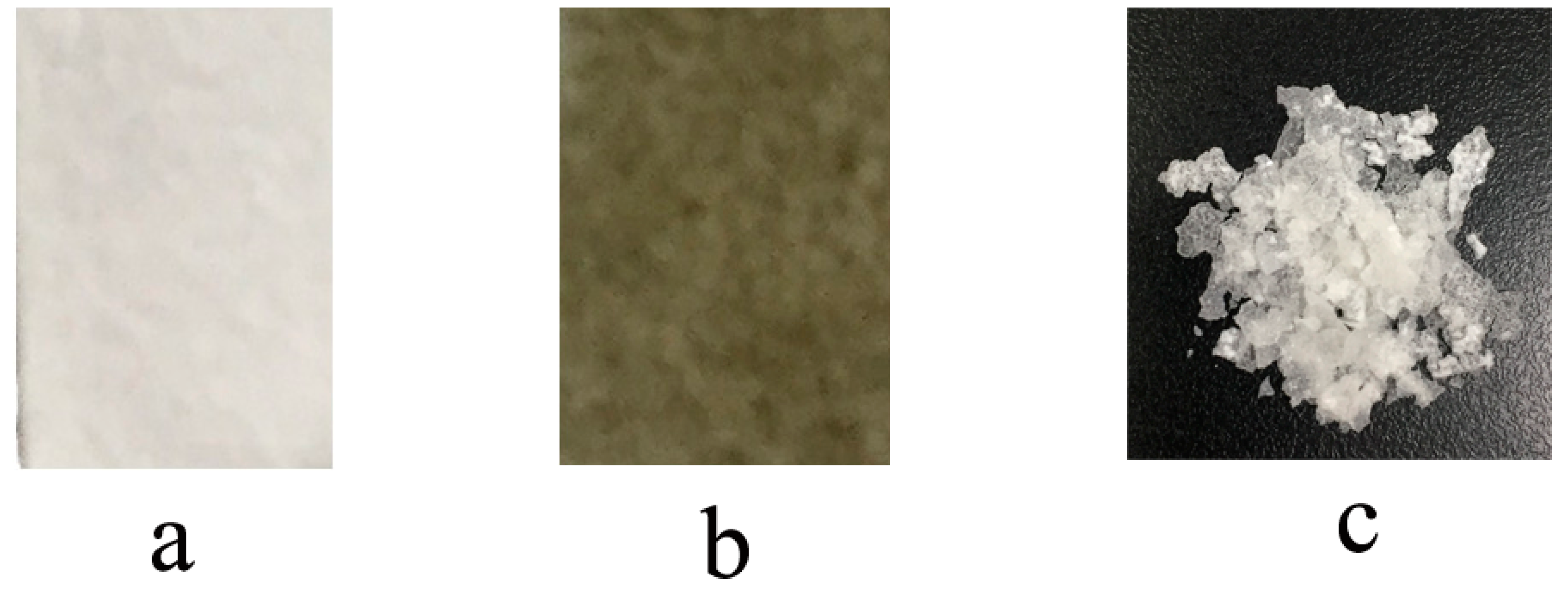
| Entry | m(Cell-SH) (mg) | Time (h) | Conv. (%) | Mn,th (g·mol−1) | Mn,GPC (g·mol−1) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | 0 | 17 | 25.9 | 10,400 | 89,000 | 2.11 |
| 2 | 25 | 2 | 28.8 | 11,500 | 23,200 | 1.08 |
| 3 | 50 | 2 | 32.2 | 12,300 | 27,900 | 1.12 |
| 4 | 50 | 17 | >99 | 40,000 | 56,900 | 1.28 |
| Entry | Volume (mL) | X | Conv. (%) | Mn,th (g·mol−1) | Mn,GPC (g·mol−1) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | 4.9 | 400/1/0.5/0.5 | 0 | NA | NA | NA |
| 2 | 9.8 | 400/1/0.5/1 | 45.9 | 18,400 | 25,500 | 1.09 |
| 3 | 14.7 | 400/1/0.5/1.5 | 70.2 | 28,100 | 39,000 | 1.25 |
| 4 | 19.6 | 400/1/0.5/2 | 78.2 | 31,300 | 45,200 | 1.28 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Han, J.; Cao, L.; Bao, Y.; Shi, J.; Zhang, J.; Ni, L.; Chen, J. A Facial Strategy for Catalyst and Reducing Agent Synchronous Separation for AGET ATRP Using Thiol-Grafted Cellulose Paper as Reducing Agent. Polymers 2018, 10, 26. https://doi.org/10.3390/polym10010026
Jiang X, Han J, Cao L, Bao Y, Shi J, Zhang J, Ni L, Chen J. A Facial Strategy for Catalyst and Reducing Agent Synchronous Separation for AGET ATRP Using Thiol-Grafted Cellulose Paper as Reducing Agent. Polymers. 2018; 10(1):26. https://doi.org/10.3390/polym10010026
Chicago/Turabian StyleJiang, Xiaowu, Jie Han, Lunan Cao, Yan Bao, Jian Shi, Jing Zhang, Lingli Ni, and Jing Chen. 2018. "A Facial Strategy for Catalyst and Reducing Agent Synchronous Separation for AGET ATRP Using Thiol-Grafted Cellulose Paper as Reducing Agent" Polymers 10, no. 1: 26. https://doi.org/10.3390/polym10010026