High-Tg, Low-Dielectric Epoxy Thermosets Derived from Methacrylate-Containing Polyimides
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
2.3. Synthesis of Hydroxyl Diamine
2.4. Preparation of Poly(hydroxyl imide) P1–OH
2.5. Preparation of Poly(hydroxyl imide) P2–OH
2.6. Preparation of Poly(hydroxyl imides) P3–OH
2.7. Preparation of Poly(methacrylate imides) P1–MMA
2.8. Preparation of Poly(methacrylate imide) P2–MMA
2.9. Preparation of Poly(hydroxyl imides) P3–MMA
2.10. Preparation of Px–MMA/HP7200 Thermosets
2.11. Preparation of P1–OH/HP7200 Thermosets
3. Results and Discussion
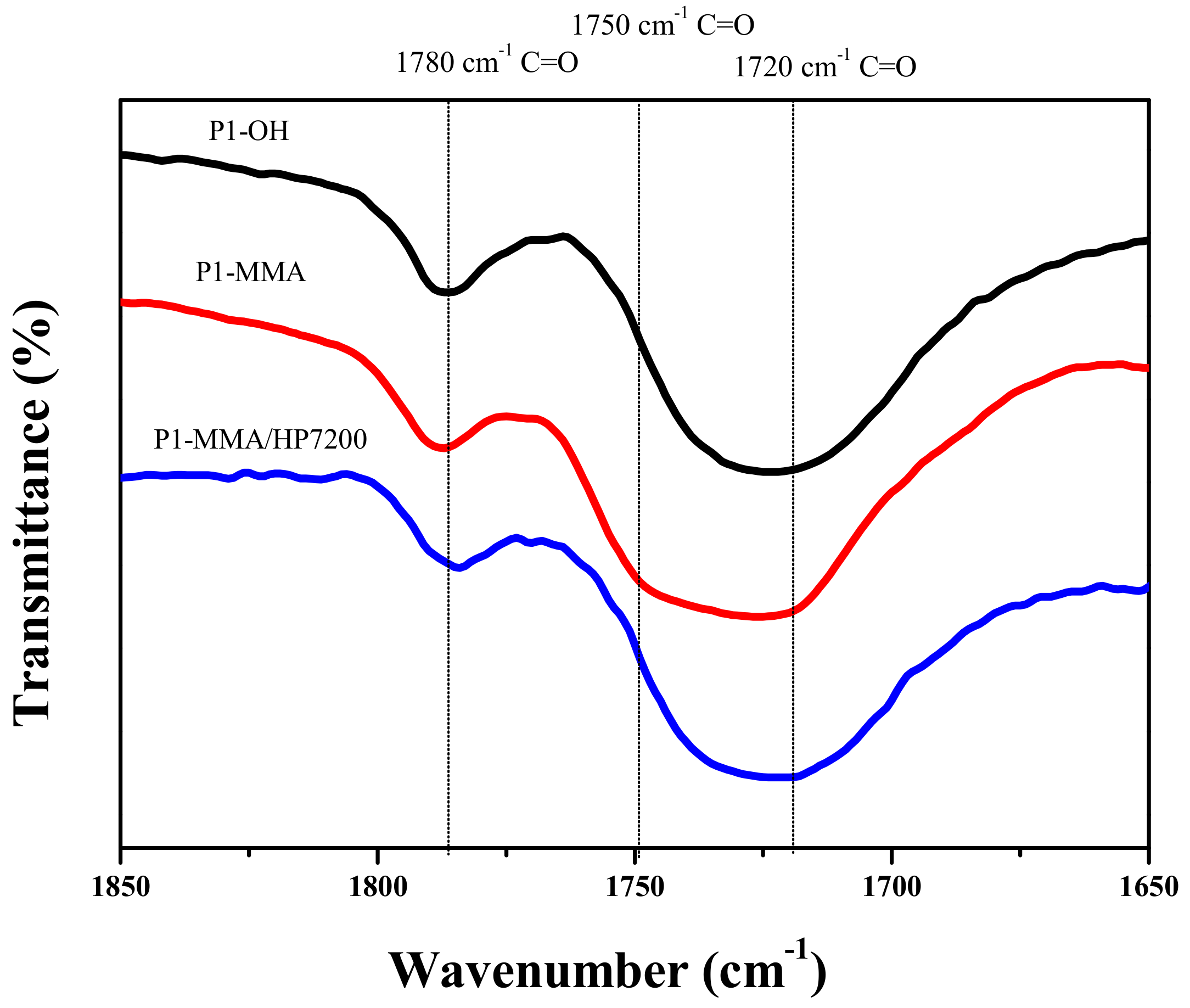
3.1. Synthesis of Px–OH and Px–MMA
3.2. DSC Thermograms of Px–MMA
3.3. Proposed Mechanism
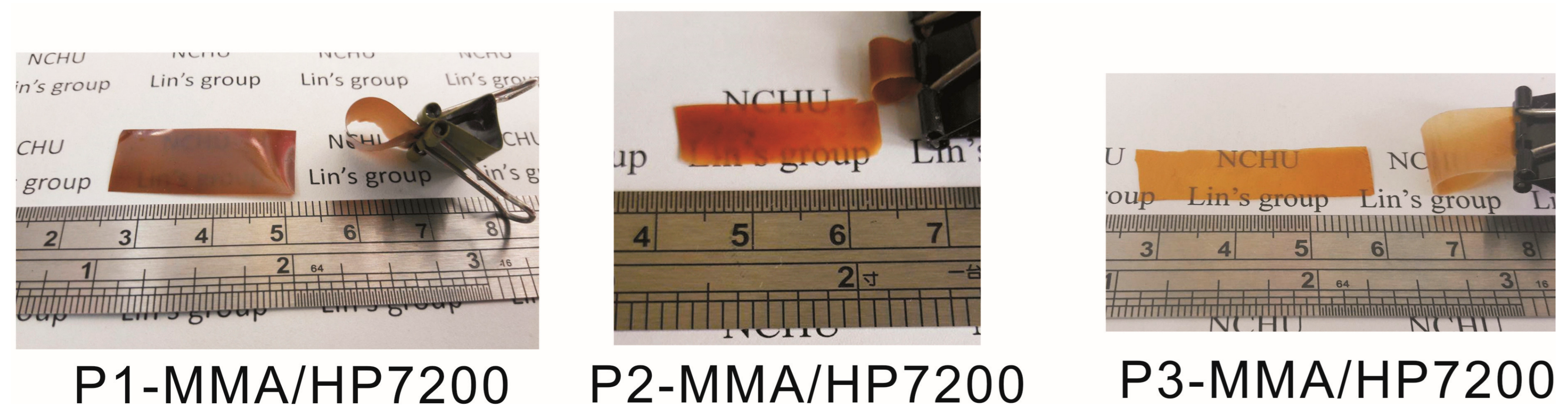
3.4. Photo of Prepared Polymers
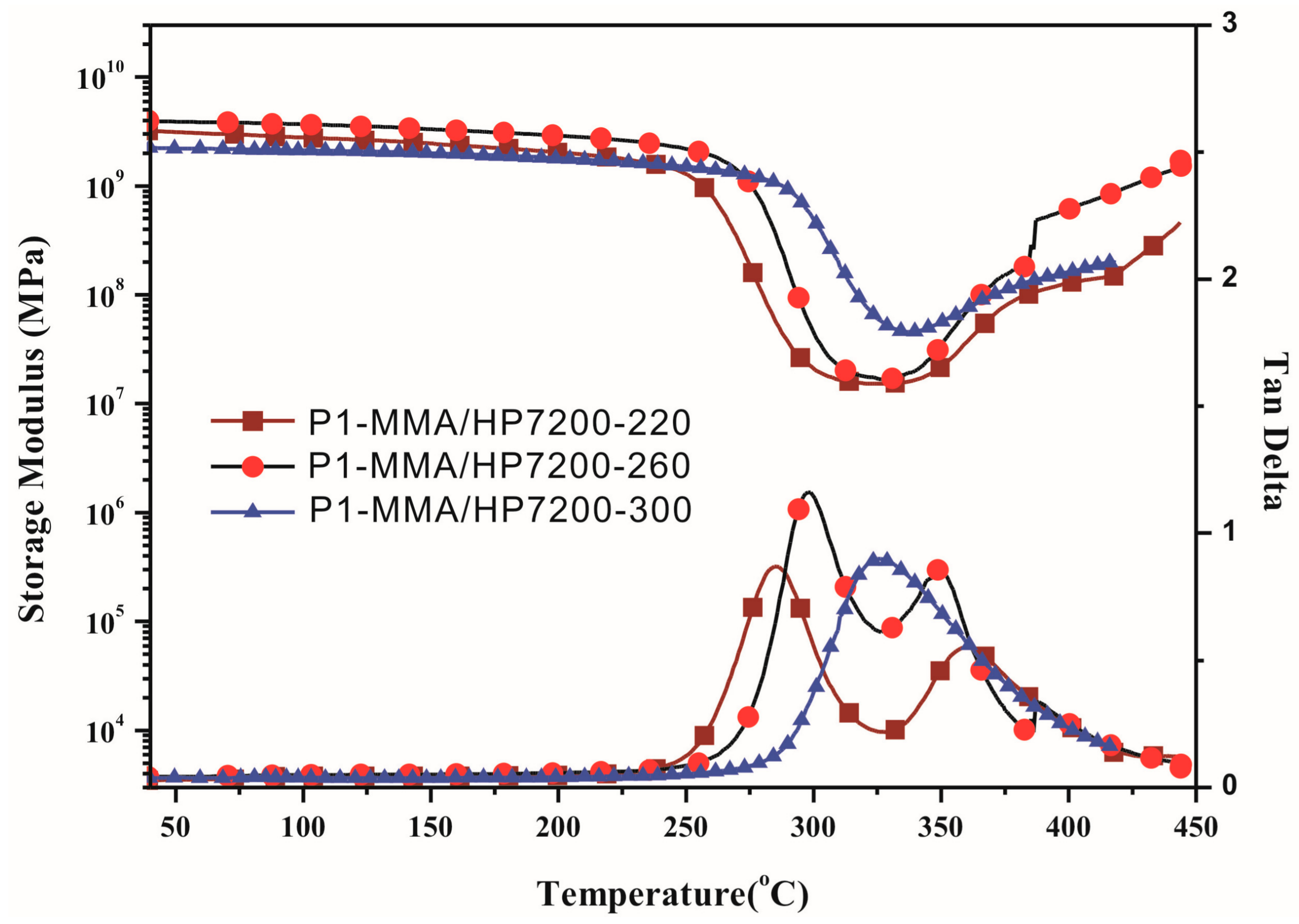
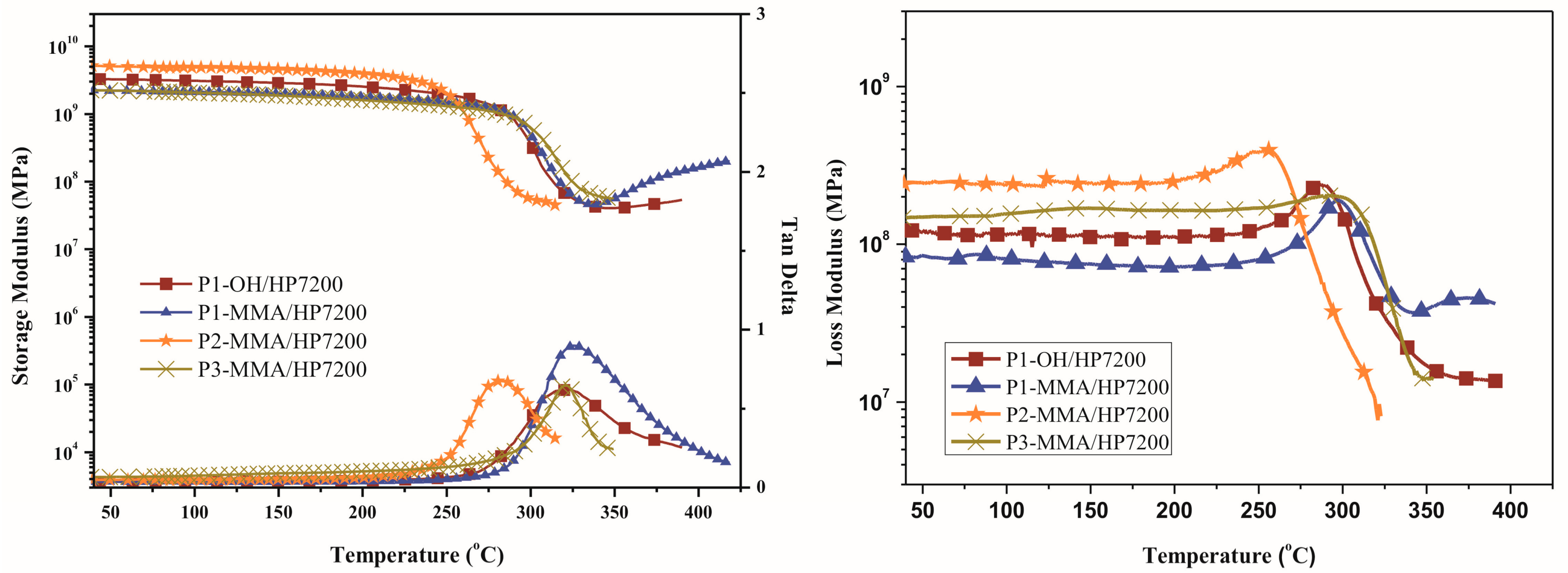
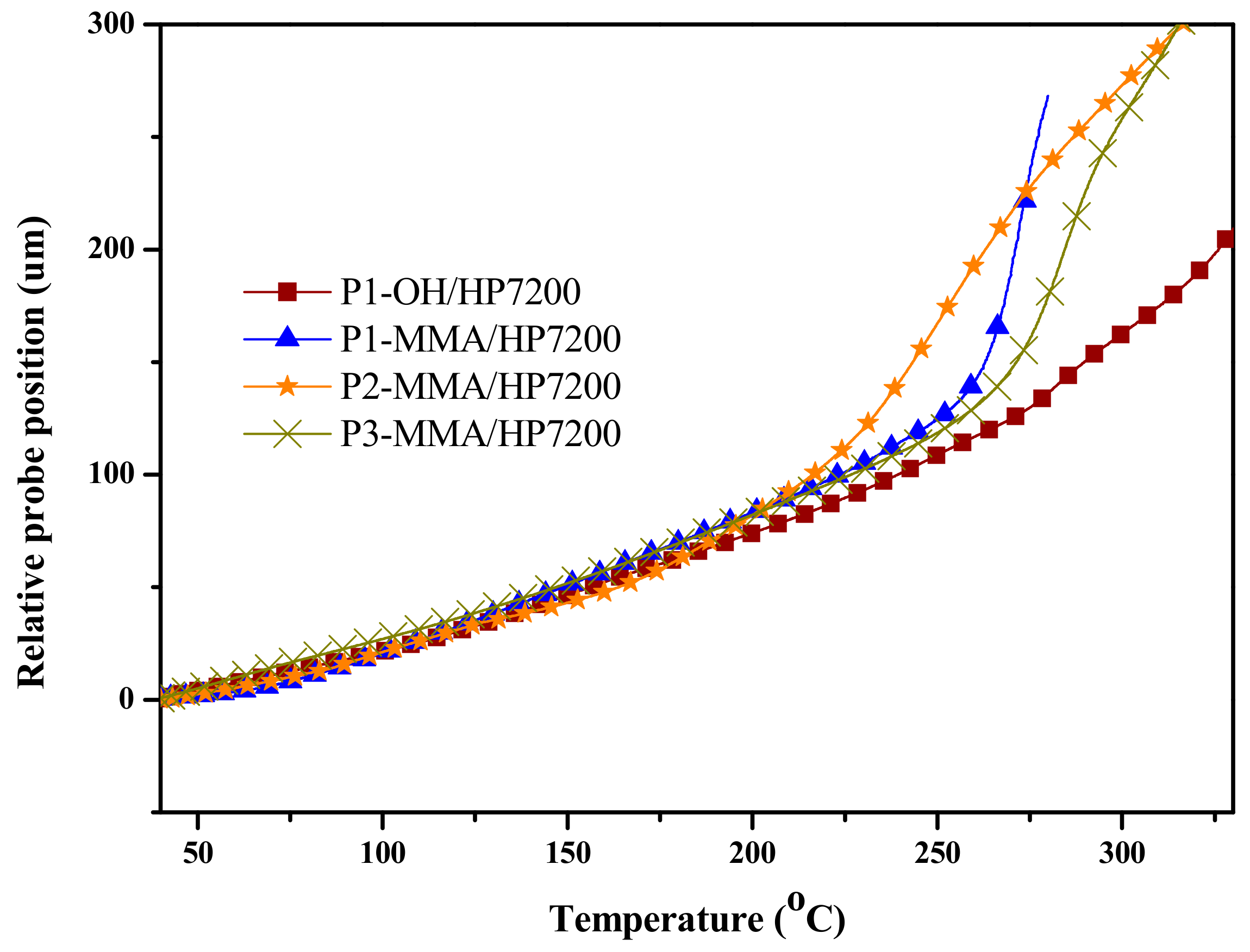
3.5. DMA and TMA Thermograms
3.6. TGA Thermograms
3.7. Contact Angle
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef] [PubMed]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef]
- Carfagna, C.; Amendola, E.; Giamberini, M. Liquid crystalline epoxy based thermosetting polymers. Prog. Polym. Sci. 1997, 22, 1607–1647. [Google Scholar] [CrossRef]
- Chu, W.-C.; Lin, W.-S.; Kuo, S.-W. Flexible epoxy resin formed upon blending with a triblock copolymer through reaction-induced microphase separation. Materials 2016, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Kaji, M.; Nakahara, K.; Ogami, K.; Endo, T. Synthesis of a novel epoxy resin containing diphenylether moiety and thermal properties of its cured polymer with phenol novolac. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3687–3693. [Google Scholar] [CrossRef]
- Endo, T.; Sudo, A. Development and application of novel ring-opening polymerizations to functional networked polymers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4847–4858. [Google Scholar] [CrossRef]
- Liu, J.-G.; Ueda, M. High refractive index polymers: Fundamental research and practical applications. J. Mater. Chem. 2009, 19, 8907–8919. [Google Scholar] [CrossRef]
- Kuo, S.W.; Liu, W.C. Synthesis and characterization of a cured epoxy resin with a benzoxazine monomer containing allyl groups. J. Appl. Polym. Sci. 2010, 117, 3121–3127. [Google Scholar] [CrossRef]
- Konuray, A.O.; Fernandez-Francos, X.; Ramis, X. Analysis of the reaction mechanism of the thiol-epoxy addition initiated by nucleophilic tertiary amines. Polym. Chem. 2017, 8, 5934–5947. [Google Scholar] [CrossRef]
- Fernandez-Francos, X.; Konuray, A.-O.; Belmonte, A.; De la Flor, S.; Serra, A.; Ramis, X. Sequential curing of off-stoichiometric thiol-epoxy thermosets with a custom-tailored structure. Polym. Chem. 2016, 7, 2280–2290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, Y.; Chen, X.; Wen, H.; Xiao, S. Theoretical study on decomposition mechanism of insulating epoxy resin cured by anhydride. Polymers 2017, 9, 341. [Google Scholar] [CrossRef]
- Kang, Y.; Jin, R.; Wu, Q.; Pu, L.; Song, M.; Cheng, J.; Yu, P. Anhydrides-cured bimodal rubber-like epoxy asphalt composites: From thermosetting to quasi-thermosetting. Polymers 2016, 8, 104. [Google Scholar] [CrossRef]
- Fei, X.; Zhao, F.; Wei, W.; Luo, J.; Chen, M.; Liu, X. Tannic acid as a bio-based modifier of epoxy/anhydride thermosets. Polymers 2016, 8, 314. [Google Scholar] [CrossRef]
- Fernàndez-Francos, X.; Ramis, X.; Serra, A. From curing kinetics to network structure: A novel approach to the modeling of the network buildup of epoxy–anhydride thermosets. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 61–75. [Google Scholar] [CrossRef]
- Riccardi, C.; Dupuy, J.; Williams, R. A simple model to explain the complex kinetic behavior of epoxy/anhydride systems. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2799–2805. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Gao, S.; Liu, X.; Wang, Z.; Yan, J. Bio-based epoxy-anhydride thermosets from six-armed linoleic acid-derived epoxy resin. RSC Adv. 2016, 6, 52549–52555. [Google Scholar] [CrossRef]
- Nakamura, S.; Saegusa, Y.; Yanagisawa, H.; Touse, M.; Shirai, T.; Nishikubo, T. Thermal analysis of epoxy curing using polyfunctional active esters as curing agents. Thermochim. Acta 1991, 183, 269–277. [Google Scholar] [CrossRef]
- Nakamura, S.; Arima, M. Characterization of the network structure o epoxy resins cured with active esters. Int. J. Polym. Anal. Charact. 1995, 1, 75–86. [Google Scholar] [CrossRef]
- Das, A.; Theato, P. Activated ester containing polymers: Opportunities and challenges for the design of functional macromolecules. Chem. Rev. 2015, 116, 1434–1495. [Google Scholar] [CrossRef] [PubMed]
- Tikart, F.; Leis, K.H. Epoxy Resin, Styrene-Maleic Anhydride Copolymer and Co-Crosslinking Agent. U.S. Patent 6,509,414 B2, 21 January 2003. [Google Scholar]
- Sukeaki, U.; Satoshi, D.; Katsuji, T. Epoxy Resin Composition for Low-Dielectric Material. Patent JP 2,002,012,650A, January 2002. [Google Scholar]
- Satoshi, D.; Masao, Y.; Koichi, F.; Katsuji, T.; Minoru, T.; Kenichi, K. Epoxy Resin Composition, and Sheet, Preperg-Like Material, Sheet with Metal Foil, Laminated Sheet, Electrical Insulating Material and Resist Material Obtained Therefrom. Patent JP 2,004,277,460A, October 2004. [Google Scholar]
- Takeuchi, K.; Suzuki, E.; Morinaga, K.; Arita, K. Active Ester Resin, Method for Producing the Same, Thermosetting Resin Composition, Cured Product of the Thermosetting Resin Composition, Semiconductor Encapsulating Material, Pre-Preg, Circuit Board, and Build-Up Film. U.S. Patent 8,791,214 B2, 29 July 2014. [Google Scholar]
- Ramos, V.D.; da Costa, H.M.; Soares, V.L.P.; Nascimento, R.S.V. Modification of epoxy resin: A comparison of different types of elastomer. Polym. Test. 2005, 24, 387–394. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Lipic, P.M.; Hajduk, D.A.; Almdal, K.; Bates, F.S. Self-assembly and polymerization of epoxy resin-amphiphilic block copolymer nanocomposites. J. Am. Chem. Soc. 1997, 119, 2749–2750. [Google Scholar] [CrossRef]
- Lipic, P.M.; Bates, F.S.; Hillmyer, M.A. Nanostructured thermosets from self-assembled amphiphilic block copolymer/epoxy resin mixtures. J. Am. Chem. Soc. 1998, 120, 8963–8970. [Google Scholar] [CrossRef]
- Dean, J.M.; Verghese, N.E.; Pham, H.Q.; Bates, F.S. Nanostructure toughened epoxy resins. Macromolecules 2003, 36, 9267–9270. [Google Scholar] [CrossRef]
- Mimura, K.; Ito, H.; Fujioka, H. Improvement of thermal and mechanical properties by control of morphologies in pes-modified epoxy resins. Polymer 2000, 41, 4451–4459. [Google Scholar] [CrossRef]
- Cho, J.B.; Hwang, J.W.; Cho, K.; An, J.H.; Park, C.E. Effects of morphology on toughening of tetrafunctional epoxy resins with poly(ether imide). Polymer 1993, 34, 4832–4836. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Lim, S.H.; Jo, W.H.; Choe, C.R. Effects of mixing temperatures on the morphology and toughness of epoxy/polyamide blends. J. Appl. Polym. Sci. 1999, 72, 1055–1063. [Google Scholar] [CrossRef]
- Li, M.-S.; Ma, C.-C.M.; Chen, J.-L.; Lin, M.-L.; Chang, F.-C. Epoxy-polycarbonate blends catalyzed by a tertiary amine. 1. Mechanism of transesterification and cyclization. Macromolecules 1996, 29, 499–506. [Google Scholar]
- Su, C.C.; Woo, E.M. Chemical interactions in blends of bisphenol a polycarbonate with tetraglycidyl-4,4′-diaminodiphenylmethane epoxy. Macromolecules 1995, 28, 6779–6786. [Google Scholar] [CrossRef]
- Bennett, G.S.; Farris, R.J.; Thompson, S.A. Amine-terminated poly(aryl ether ketone)-epoxy/amine resin systems as tough high performance materials. Polymer 1991, 32, 1633–1641. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Motto, O.; Rocca, A.; Carter, J.T.; McGrail, P.T.; Acierno, D. New high-performance thermoplastic toughened epoxy thermosets. Polymer 1997, 38, 4345–4348. [Google Scholar] [CrossRef]
- Lin, C.H.; Wang, Y.R.; Feng, Y.R.; Wang, M.W.; Juang, T.Y. An approach of modifying poly(aryl ether ketone) to phenol-containing poly(aryl ether) and its application in preparing high-performance epoxy thermosets. Polymer 2013, 54, 1612–1620. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, J.C.; Huang, C.M.; Jehng, J.M.; Chang, H.C.; Juang, T.Y.; Su, W.C. Side-chain phenol-functionalized poly(ether sulfone) and its contribution to high-performance and flexible epoxy thermosets. Polymer 2013, 54, 6936–6941. [Google Scholar] [CrossRef]
- Gaw, K.; Jikei, M.; Kakimoto, M.-A.; Imai, Y.; Mochjizuki, A. Adhesion behaviour of polyamic acid cured epoxy. Polymer 1997, 38, 4413–4415. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Synthesis and characterization of epoxy film cured with reactive polyimide. Polymer 1999, 40, 6557–6563. [Google Scholar] [CrossRef]
- Yuan, W.; Feng, J.; Judeh, Z.; Dai, J.; Chan-Park, M.B. Use of polyimide-graft-bisphenol a diglyceryl acrylate as a reactive noncovalent dispersant of single-walled carbon nanotubes for reinforcement of cyanate ester/epoxy composite. Chem. Mater. 2010, 22, 6542–6554. [Google Scholar] [CrossRef]
- Yu, H.S.; Yamashita, T.; Horie, K. Synthesis and chemically amplified photo-cross-linking reaction of a polyimide containing an epoxy group. Macromolecules 1996, 29, 1144–1150. [Google Scholar] [CrossRef]
- Wang, P.J.; Lin, C.H.; Chang, S.L.; Shih, S.-J. Facile, efficient synthesis of a phosphinated hydroxyl diamine and properties of its high-performance poly(hydroxyl imides) and polyimide-SIO2 hybrids. Polym. Chem. 2012, 3, 2867–2874. [Google Scholar] [CrossRef]
- Chen, C.H.; Gu, Z.C.; Lin, C.H. Reaction mechanism of methacrylate and epoxy and its application in preparing low-dielectric epoxy thermoset with flexibility. 2017. submitted. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy, 4th ed.; Brooks/Cole: Pacific Grove, CA, USA, 2010; p. 68. [Google Scholar]
- Maier, G. Low dielectric constant polymers for microelectronics. Prog. Polym. Sci. 2001, 26, 3–65. [Google Scholar] [CrossRef]
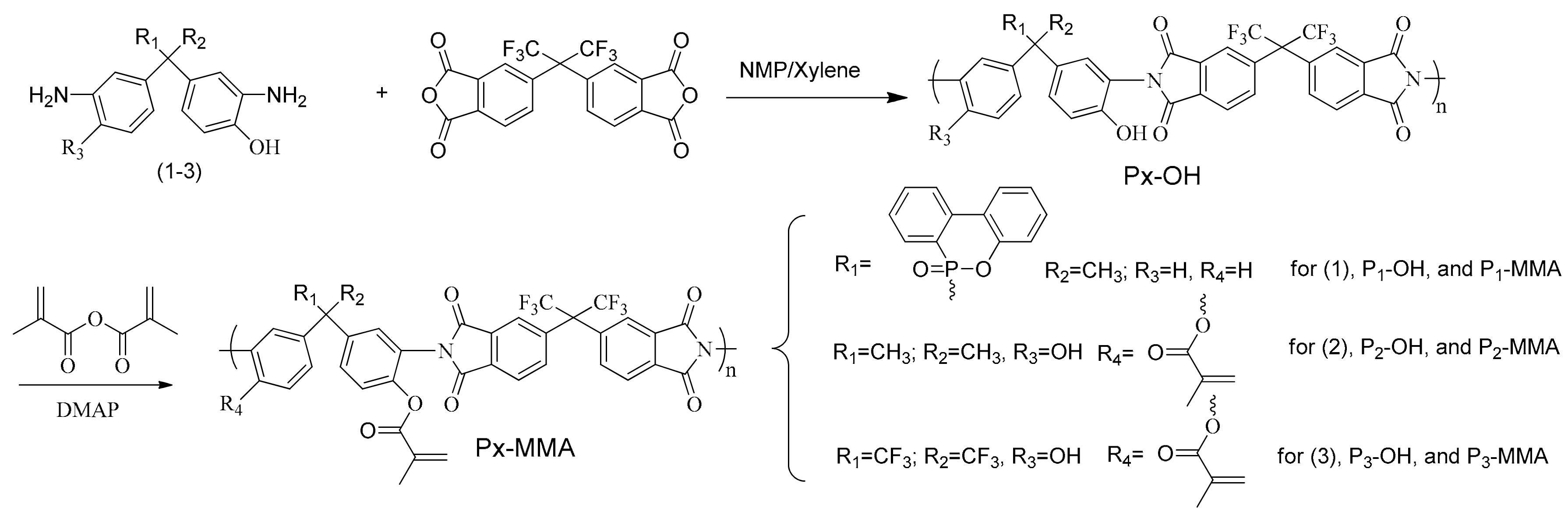
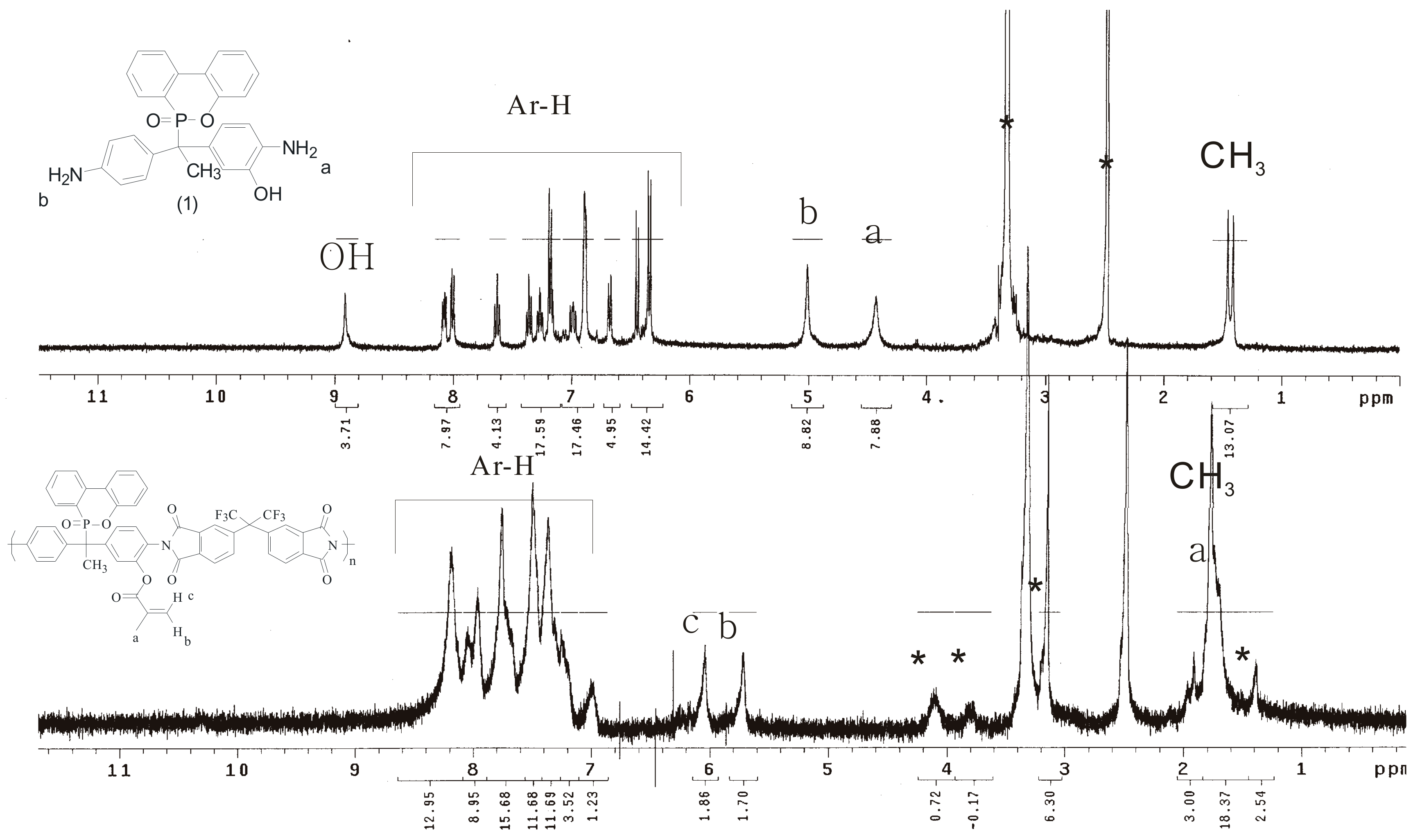
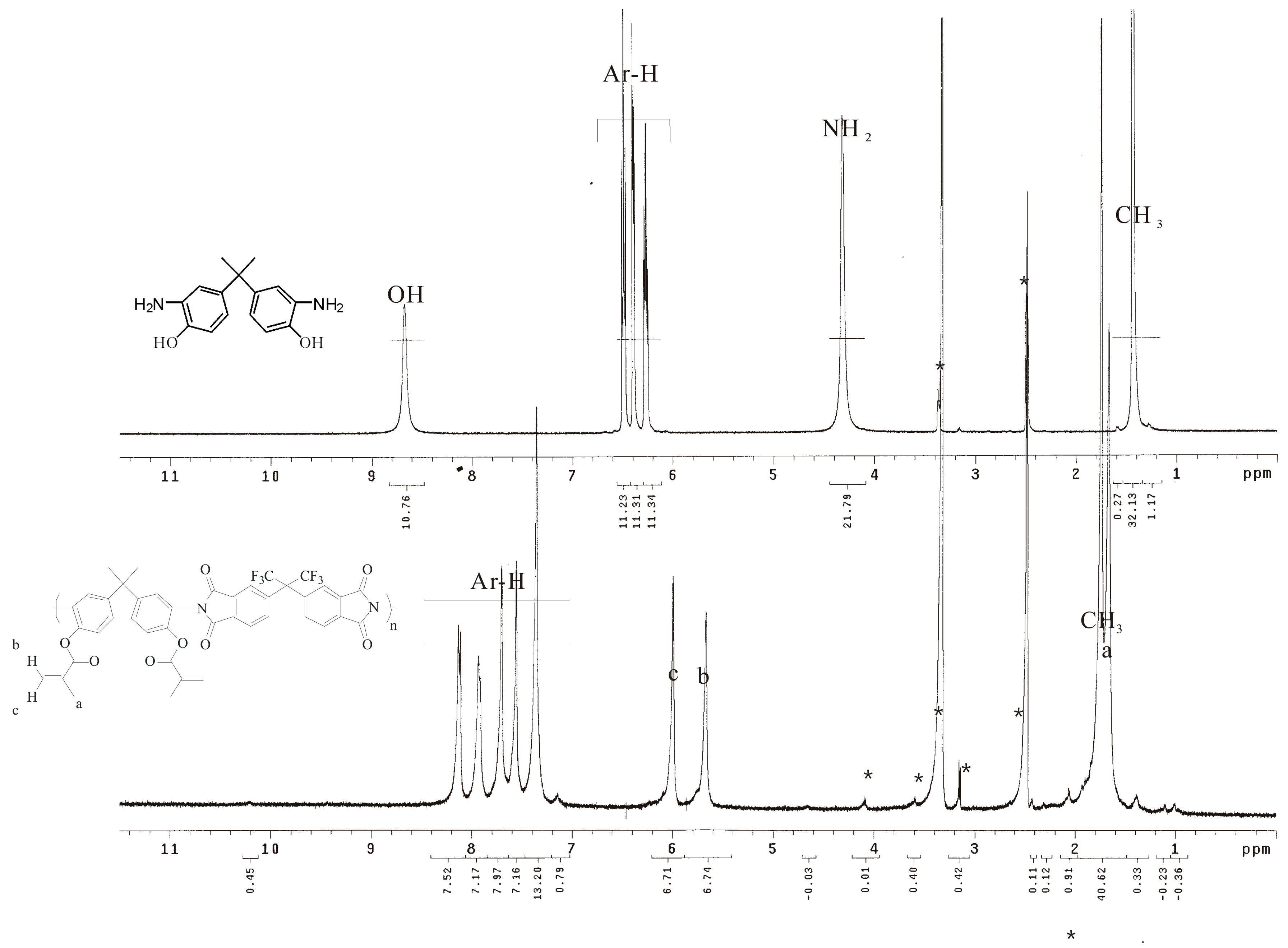
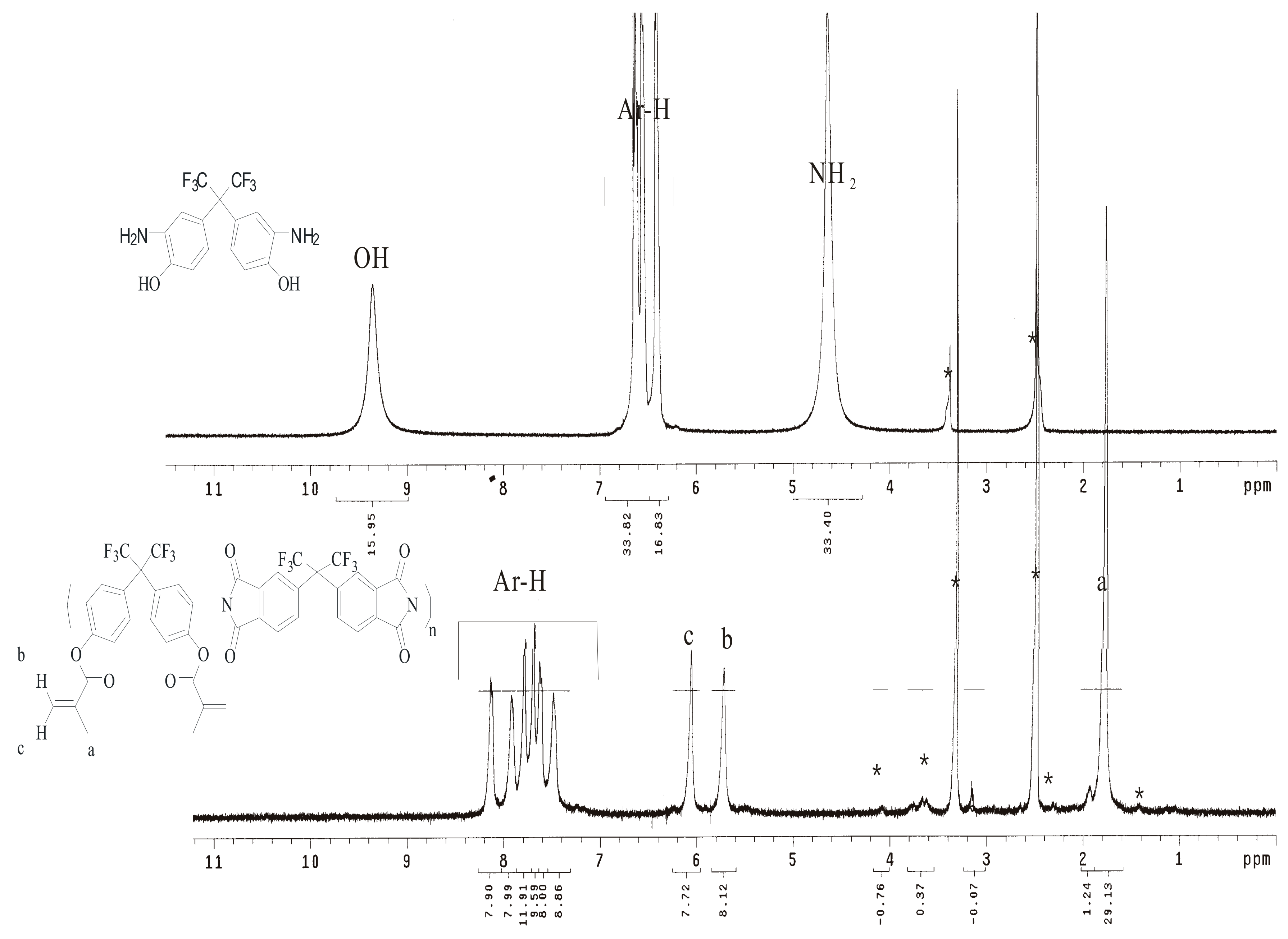


| Run | Catalyst | Temperature (°C) | Time (h) | Status a |
|---|---|---|---|---|
| 1 | DMAP (2 mole %) | 100 | 12 | Residual phenolic |
| 2 | DMAP (5 mole %) | 100 | 12 | Residual phenolic |
| 3 | Sodium acetate (1 wt. %) | 100 | 12 | Residual phenolic |
| 4 | Sodium acetate (1 wt. %) | 80 | 12 | Side reactions b |
| 5 | DMAP (2 mole %) | 80 | 12 | Residual phenolic |
| 6 | DMAP (2 mole %) | 50 | 12 | Side reactions b |
| 7 | DMAP (2 mole %) | 50 | 24 | Side reactions b |
| 8 | DMAP (2 mole %) | 25 | 24 | Completed |
| Sample | Tg (°C) a | Tg (°C) b | Tg (°C) c | CTE (10−6/°C) d | Td5% (°C) e | Char Yield (%) f | ||
|---|---|---|---|---|---|---|---|---|
| N2 | Air | N2 | Air | |||||
| P1–OH/HP7200 | 323 | 288 | 266 | 55 | 464 | 466 | 43 | 19 |
| P1–MMA/HP7200 | 325 | 297 | 263 | 48 | 444 | 460 | 47 | 16 |
| P2–MMA/HP7200 | 282 | 257 | 227 | 50 | 431 | 421 | 26 | 0 |
| P3–MMA/HP7200 | 320 | 300 | 274 | 54 | 433 | 433 | 41 | 0 |
| Sample | Contact Angle (Deg.) | Dielectric Constant | Dissipation Factor (×10−3) |
|---|---|---|---|
| P1–OH/HP7200 | 68.7 | 3.4 ± 0.005 | 16.7 ± 0.03 |
| P1–MMA/HP7200 | 87.1 | 3.2 ± 0.003 | 12.0 ± 0.02 |
| P2–MMA/HP7200 | 91.1 | 3.0 ± 0.004 | 11.0 ± 0.02 |
| P3–MMA/HP7200 | 92.2 | 2.9 ± 0.005 | 10.2 ± 0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Lee, K.-W.; Lin, C.-H.; Ho, M.-J.; Hsu, M.-F.; Hsiang, S.-J.; Huang, N.-K.; Juang, T.-Y. High-Tg, Low-Dielectric Epoxy Thermosets Derived from Methacrylate-Containing Polyimides. Polymers 2018, 10, 27. https://doi.org/10.3390/polym10010027
Chen C-H, Lee K-W, Lin C-H, Ho M-J, Hsu M-F, Hsiang S-J, Huang N-K, Juang T-Y. High-Tg, Low-Dielectric Epoxy Thermosets Derived from Methacrylate-Containing Polyimides. Polymers. 2018; 10(1):27. https://doi.org/10.3390/polym10010027
Chicago/Turabian StyleChen, Chien-Han, Kuan-Wei Lee, Ching-Hsuan Lin, Ming-Jaan Ho, Mao-Feng Hsu, Shou-Jui Hsiang, Nan-Kun Huang, and Tzong-Yuan Juang. 2018. "High-Tg, Low-Dielectric Epoxy Thermosets Derived from Methacrylate-Containing Polyimides" Polymers 10, no. 1: 27. https://doi.org/10.3390/polym10010027





