Passivating ZnO Surface States by C60 Pyrrolidine Tris-Acid for Hybrid Solar Cells Based on Poly(3-hexylthiophene)/ZnO Nanorod Arrays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO NRAs
2.2. Self-Assembly of C60 Pyrrolidine Tris-Acid
2.3. Preparation of P3HT/ZnO Heterojuntions and PV Devices
2.4. Characterizations
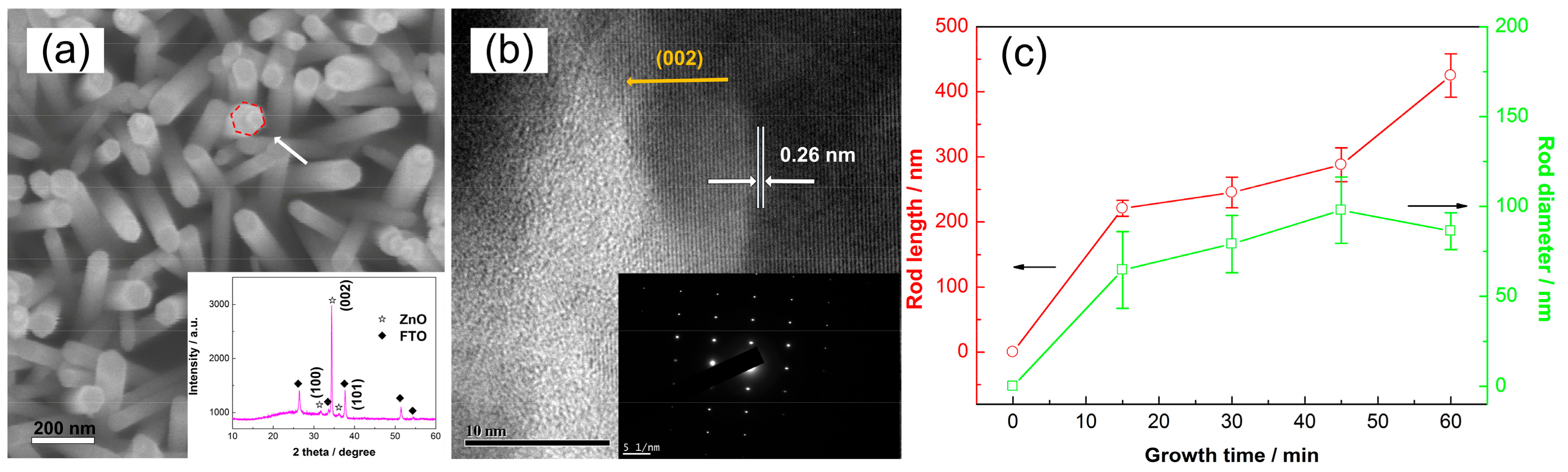
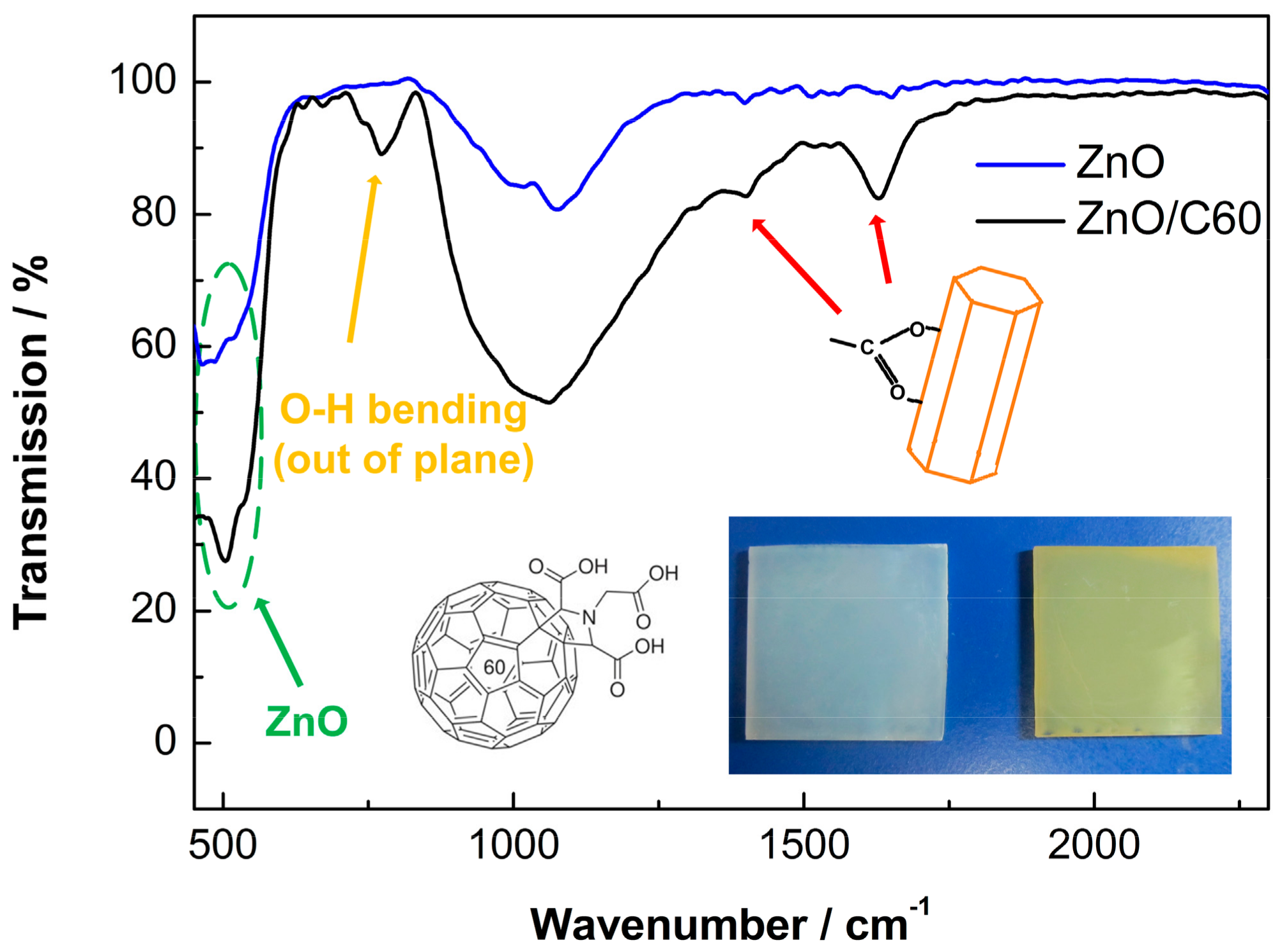
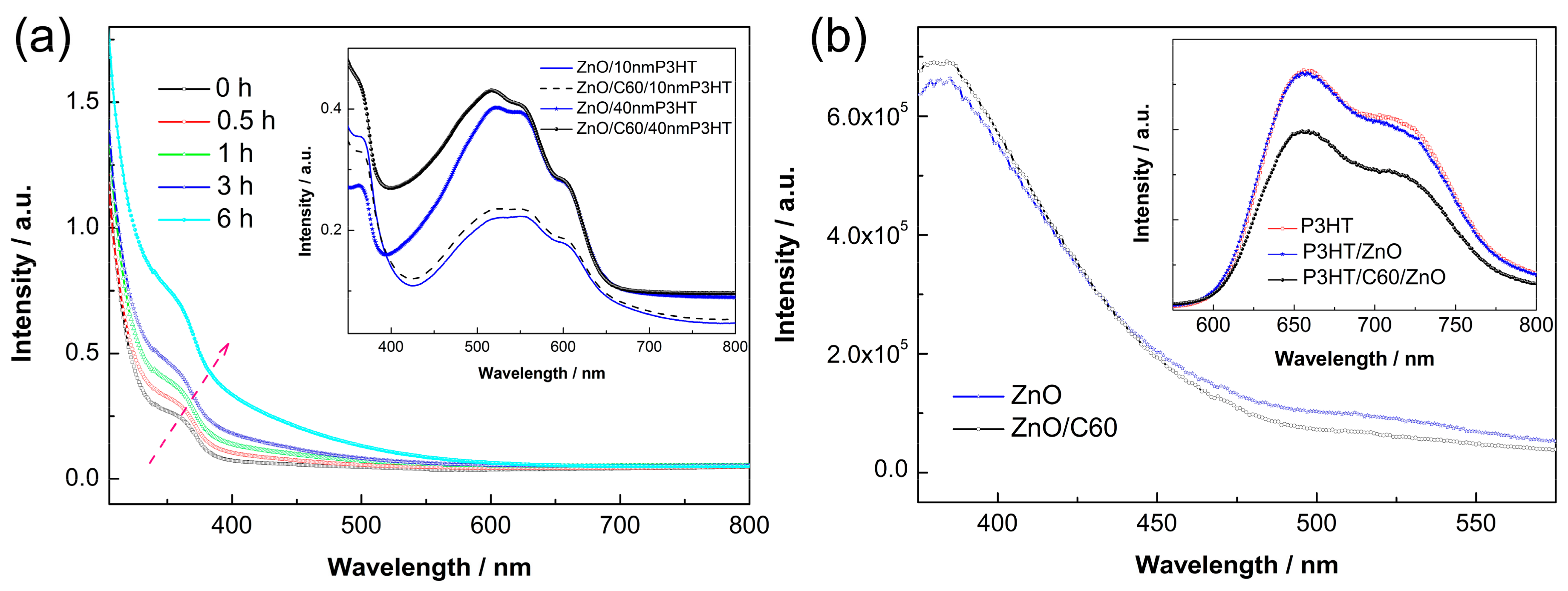
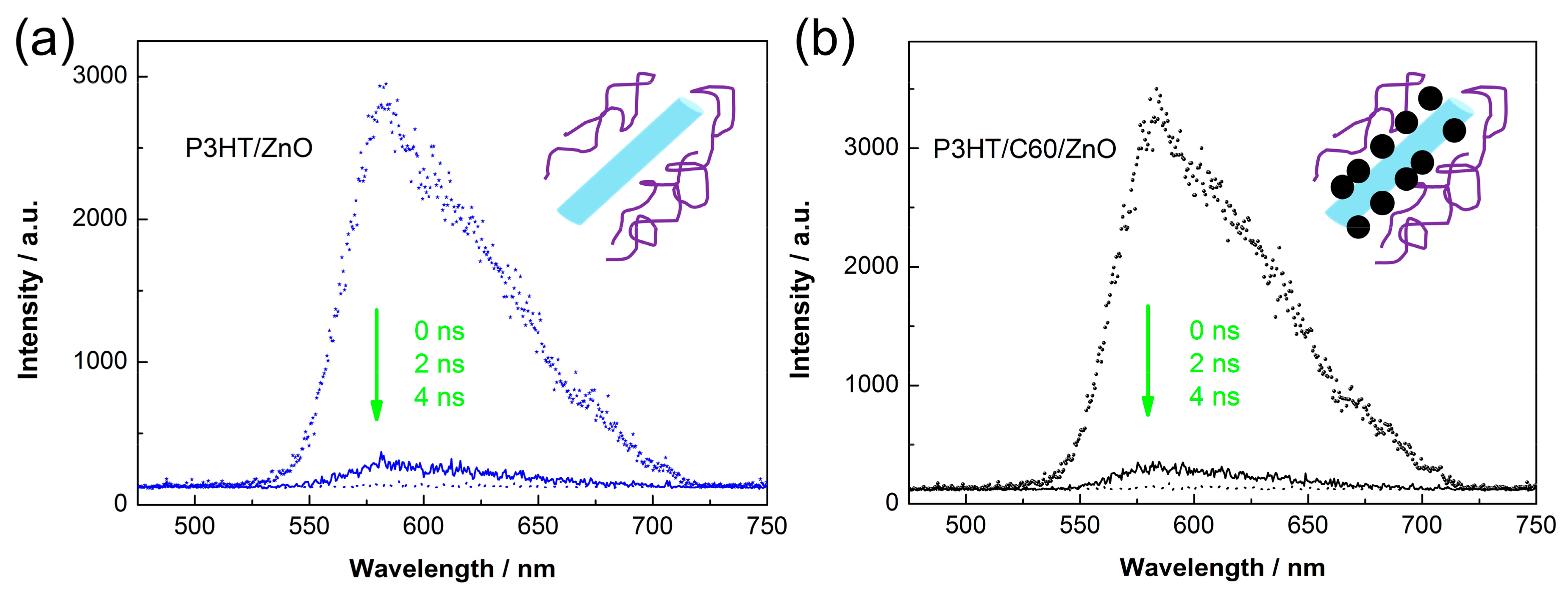
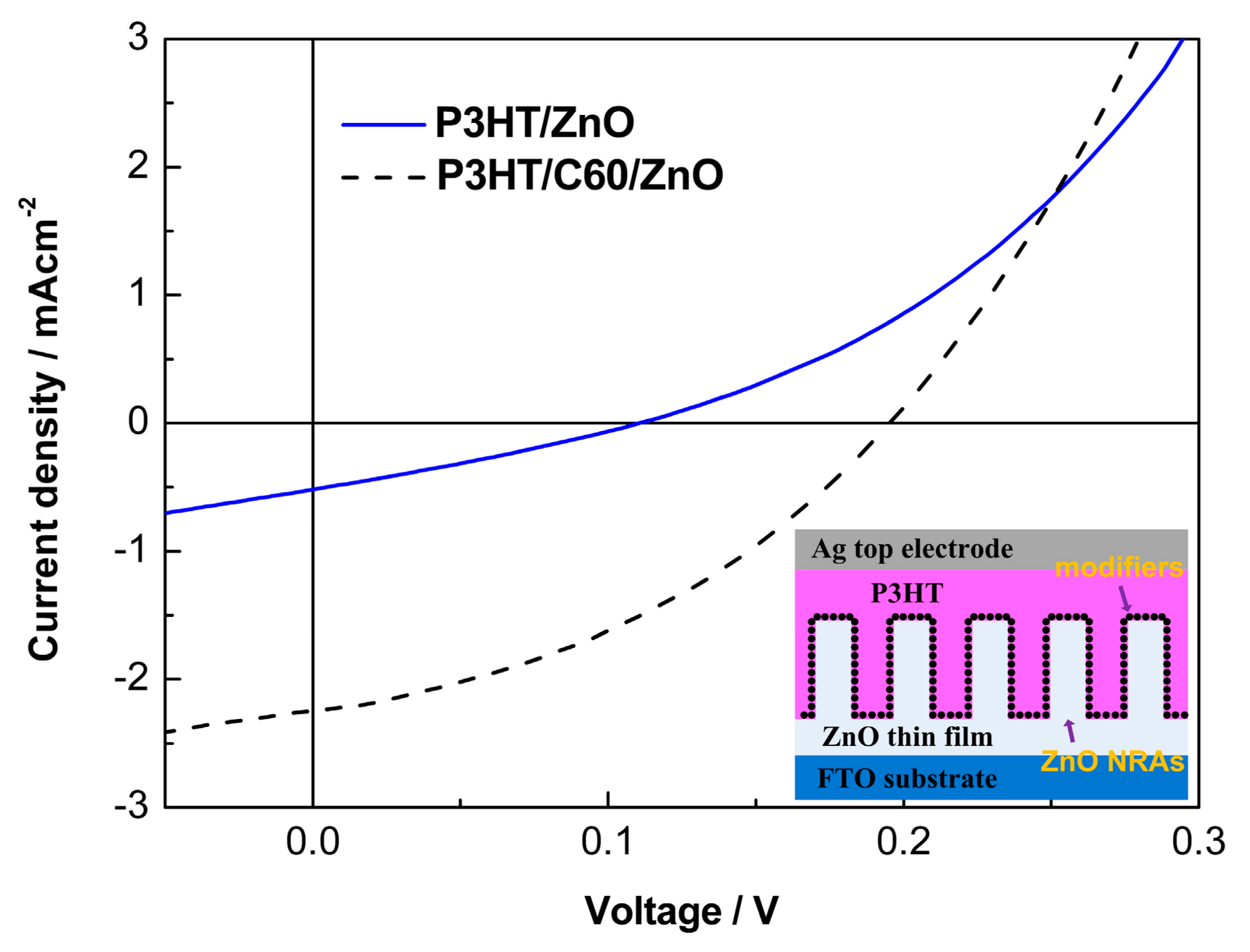
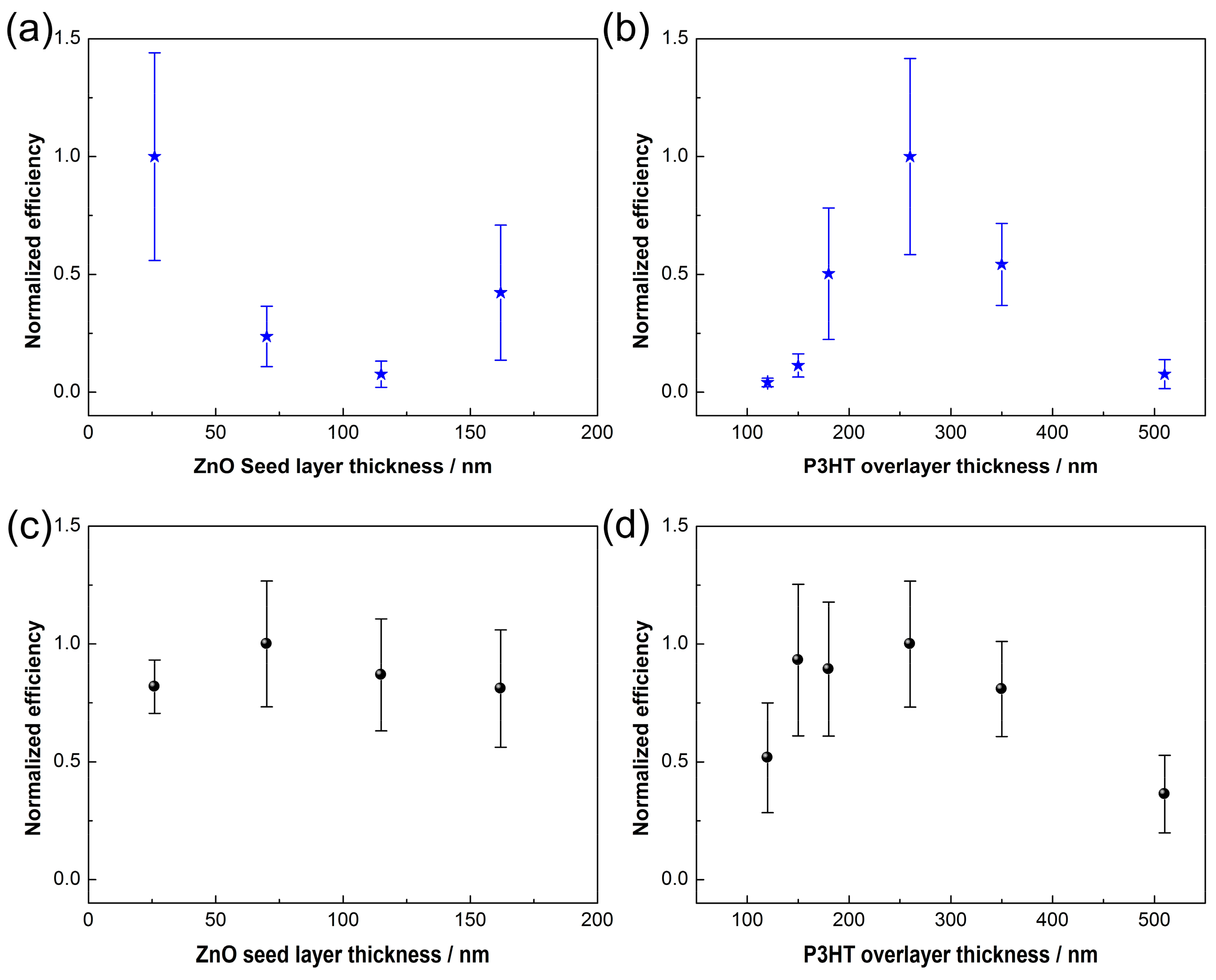
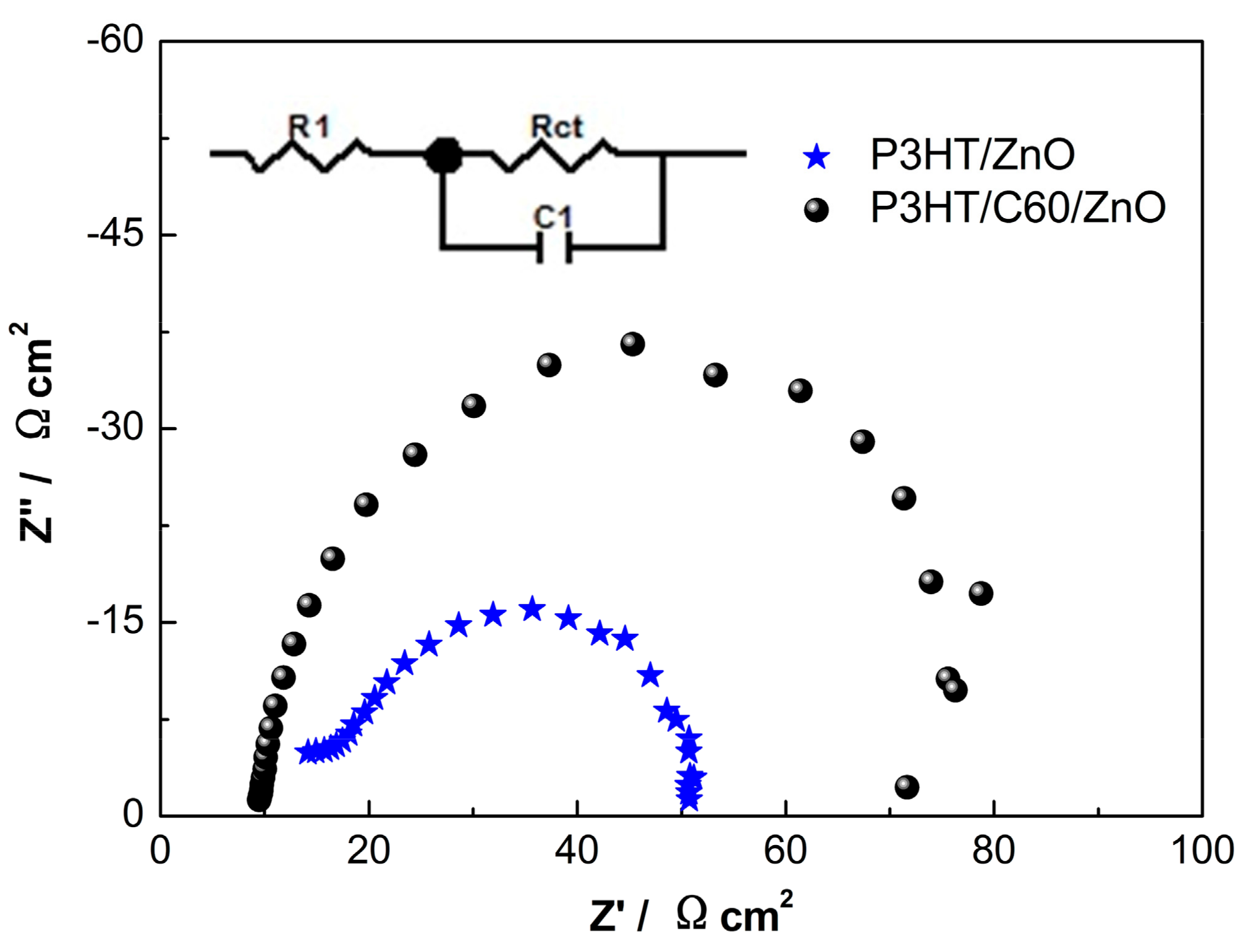
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Q.; Wang, F.; Bai, Y.; Xu, L.; Yang, Y.; Yan, L.; Hu, S.; Zhang, B.; Dai, S.; Tan, Z.A. Decahedral-shaped Au nanoparticles as plasmonic centers for high performance polymer solar cells. Org. Electron. 2017, 43, 33–40. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Shi, Z.; Wang, F.; Zhang, B.; Dai, S.; Tan, Z.A. Engineering the vertical concentration distribution within the polymer: Fullerene blends for high performance inverted polymer solar cells. J. Mater. Chem. A 2017, 5, 2319–2327. [Google Scholar] [CrossRef]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Baek, S.-W.; Park, G.; Noh, J.; Cho, C.; Lee, C.-H.; Seo, M.-K.; Song, H.; Lee, J.-Y. Au@Ag core-shell nanocubes for efficient plasmonic light scattering effect in low bandgap organic solar cells. ACS Nano 2014, 8, 3302–3312. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, W.; Dou, L.; Chang, W.-H.; Duan, H.-S.; Bob, B.; Li, G. High-performance multiple-donor bulk heterojunction solar cells. Nat. Photonics 2015, 9, 190–198. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, C.; Wang, Z.; Zhang, J.; Tang, S.; Wei, W.; Sun, L.; Hao, Y. Efficient indium-tin-oxide free inverted organic solar cells based on aluminum-doped zinc oxide cathode and low-temperature aqueous solution processed zinc oxide electron extraction layer. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Chou, J.-C.; Huang, Y.-C.; Wu, T.-Y.; Liao, Y.-H.; Lai, C.-H.; Chu, C.-M.; Lin, Y.-J. Poly(3,3-dibenzyl-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine)/Platinum Composite Films as Potential Counter Electrodes for Dye-Sensitized Solar Cells. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Ju, X.; Zhang, Y.; Zhao, J. Synthesis, Characterization and application of four novel electrochromic materials employing nitrotriphenylamine unit as the acceptor and different thiophene derivatives as the donor. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Wang, R.; Yan, X.; Yang, X.; Wang, Y.; Li, H.; Sheng, C. Long lived photoexcitation dynamics in pi-conjugated polymer/PbS quantum dot blended films for photovoltaic application. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Bailey, T.S.; Hong, M.; Chen, E.Y.X. Stereoregular brush polymers and graft copolymers by chiral zirconocene-mediated coordination polymerization of P3HT macromers. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Zhang, J.; Wang, Z.; Zhu, L.; Fang, J.; Xia, B.; Wang, Z.; Lu, K.; Ma, W.; et al. Fluorination-enabled optimal morphology leads to over 11% efficiency for inverted small-molecule organic solar cells. Nat. Commun. 2016, 7, 13740. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhang, K.; Jiang, X.-F.; Ying, L.; Huang, F.; Cao, Y. High-performance nonfullerene polymer solar cells based on imide-functionalized wide-bandgap polymers. Adv. Mater. 2017, 29, 1606396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Zhang, M.; Lau, T.-K.; Wu, Y.; Jia, B.; Wang, J.; Yan, C.; Qin, M.; Lu, X.; Zhan, X. Realizing small energy loss of 0.55 eV, high open-circuit voltage >1 V and high efficiency >10% in fullerene-free polymer solar cells via energy driver. Adv. Mater. 2017, 29, 1605216. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Cheng, P.; Mai, J.; Lau, T.-K.; Zhang, Q.; Wang, J.; Yan, C.; Liu, K.; Su, C.-J.; You, W.; et al. Enhancing efficiency and stability of organic solar cells by UV absorbent. Sol. RRL 2017, 1. [Google Scholar] [CrossRef]
- Zhao, F.; Dai, S.; Wu, Y.; Zhang, Q.; Wang, J.; Jiang, L.; Ling, Q.; Wei, Z.; Ma, W.; You, W.; et al. Single-junction binary-blend nonfullerene polymer solar cells with 12.1% efficiency. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.E.; Ruseckas, A.; Samuel, I.D.W. Exciton diffusion measurements in poly(3-hexylthiophene). Adv. Mater. 2008, 20, 3516–3520. [Google Scholar] [CrossRef]
- Wang, Y.; Benten, H.; Ohara, S.; Kawamura, D.; Ohkita, H.; Ito, S. Measurement of exciton diffusion in a well-defined donor/acceptor heterojunction based on a conjugated polymer and cross-linked fullerene derivative. ACS Appl. Mater. Interfaces 2014, 6, 14108–14115. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kramer, E.J.; Heeger, A.J.; Bazan, G.C. Bulk heterojunction solar cells: Morphology and performance relationships. Chem. Rev. 2014, 114, 7006–7043. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shrotriya, V.; Yao, Y.; Huang, J.; Yang, Y. Manipulating regioregular poly(3-hexylthiophene):[6,6]-phenyl-C61-butyric acid methyl ester blends-route towards high efficiency polymer solar cells. J. Mater. Chem. 2007, 17, 3126–3140. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.T.; Hirsch, L.; Wantz, G.; Wuest, J.D. Controlling the morphology and performance of bulk heterojunctions in solar cells. lessons learned from the benchmark poly(3-hexylthiophene):[6,6]-Phenyl-C61-butyric acid methyl ester system. Chem. Rev. 2013, 113, 3734–3765. [Google Scholar] [CrossRef] [PubMed]
- Ganesamoorthy, R.; Sathiyan, G.; Sakthivel, P. Review: Fullerene based acceptors for efficient bulk heterojunction organic solar cell applications. Sol. Energy Mater. Sol. Cells 2017, 161 (Suppl. C), 102–148. [Google Scholar] [CrossRef]
- Peiro, A.M.; Ravirajan, P.; Govender, K.; Boyle, D.S.; O’Brien, P.; Bradley, D.D.C.; Nelson, J.; Durrant, J.R. Hybrid polymer/metal oxide solar cells based on ZnO columnar structures. J. Mater. Chem. 2006, 16, 2088–2096. [Google Scholar] [CrossRef]
- Ravirajan, P.; Peiro, A.M.; Nazeeruddin, M.K.; Graetzel, M.; Bradley, D.D.C.; Durrant, J.R.; Nelson, J. Hybrid polymer/zinc oxide photovoltaic devices with vertically oriented ZnO nanorods and an amphiphilic molecular interface layer. J. Phys. Chem. B 2006, 110, 7635–7639. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.C.; Piris, J.; Collins, R.T.; Shaheen, S.E.; Ginley, D.S. Hybrid photovoltaic devices of polymer and ZnO nanofiber composites. Thin Solid Films 2006, 496, 26–29. [Google Scholar] [CrossRef]
- Huang, J.; Yin, Z.; Zheng, Q. Applications of ZnO in organic and hybrid solar cells. Energy Environ. Sci. 2011, 4, 3861–3877. [Google Scholar] [CrossRef]
- Baeten, L.; Conings, B.; Boyen, H.-G.; D’Haen, J.; Hardy, A.; D’Olieslaeger, M.; Manca, J.V.; Van Bael, M.K. Towards efficient hybrid solar cells based on fully polymer infiltrated ZnO nanorod arrays. Adv. Mater. 2011, 23, 2802–2805. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.C.; Shaheen, S.E.; Collins, R.T.; Ginley, D.S. The effect of atmosphere and ZnO morphology on the performance of hybrid poly(3-hexylthiophene)/ZnO nanofiber photovoltaic devices. J. Phys. Chem. C 2007, 111, 16670–16678. [Google Scholar] [CrossRef]
- Rodnyi, P.A.; Khodyuk, I.V. Optical and luminescence properties of zinc oxide (Review). Opt. Spectrosc. 2011, 111, 776–785. [Google Scholar] [CrossRef]
- Zhang, D.H.; Wang, Q.P.; Xue, Z.Y. Photoluminescence of ZnO films excited with light of different wavelength. Appl. Surf. Sci. 2003, 207, 20–25. [Google Scholar] [CrossRef]
- Ginting, R.T.; Lee, H.B.; Tan, S.T.; Tan, C.H.; Jumali, M.H.H.; Yap, C.C.; Kang, J.-W.; Yahaya, M. A simple approach low-temperature solution process for preparation of bismuth-doped ZnO nanorods and its application in hybrid solar cells. J. Phys. Chem. C 2016, 120, 771–780. [Google Scholar] [CrossRef]
- Zhang, J.; Que, W. Preparation and characterization of sol-gel Al-doped ZnO thin films and ZnO nanowire arrays grown on Al-doped ZnO seed layer by hydrothermal method. Sol. Energy Mater. Sol. Cells 2010, 94, 2181–2186. [Google Scholar] [CrossRef]
- Olson, D.C.; Shaheen, S.E.; White, M.S.; Mitchell, W.J.; van Hest, M.F.A.M.; Collins, R.T.; Ginley, D.S. Band-offset engineering for enhanced open-circuit voltage in polymer-oxide hybrid solar cells. Adv. Funct. Mater. 2007, 17, 264–269. [Google Scholar] [CrossRef]
- Lee, H.B.; Ginting, R.T.; Tan, S.T.; Tan, C.H.; Alshanableh, A.; Oleiwi, H.F.; Yap, C.C.; Jumali, M.H.H.; Yahaya, M. Controlled defects of fluorine-incorporated ZnO nanorods for photovoltaic enhancement. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Swain, B.S.; Amassian, A. 16.1% efficient hysteresis-free mesostructured perovskite solar cells based on synergistically improved ZnO nanorod arrays. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Obuchovsky, S.; Deckman, I.; Moshonov, M.; Peretz, T.S.; Ankonina, G.; Savenije, T.J.; Frey, G.L. Atomic layer deposition of zinc oxide onto and into P3HT for hybrid photovoltaics. J. Mater. Chem. C 2014, 2, 8903–8910. [Google Scholar] [CrossRef]
- Beek, W.J.E.; Slooff, L.H.; Wienk, M.M.; Kroon, J.M.; Janssen, R.A.J. Hybrid solar cells using a zinc oxide precursor and a conjugated polymer. Adv. Funct. Mater. 2005, 15, 1703–1707. [Google Scholar] [CrossRef]
- Whittaker-Brooks, L.; McClain, W.E.; Schwartz, J.; Loo, Y.-L. Donor-acceptor interfacial interactions dominate device performance in hybrid P3HT-ZnO nanowire-array solar cells. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Allen, C.G.; Baker, D.J.; Albin, J.M.; Oertli, H.E.; Gillaspie, D.T.; Olson, D.C.; Furtak, T.E.; Collins, R.T. Surface modification of ZnO using triethoxysilane-based molecules. Langmuir 2008, 24, 13393–13398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Baldelli, S. Alkanethiol monolayers at reduced and oxidized zinc surfaces with corrosion proctection: A sum frequency generation and electrochemistry investigation. J. Phys. Chem. B 2006, 110, 24062–24069. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Que, W.; Fei, D.; Xie, H.; He, Z. Effect of TiO2 shell layer prepared by wet-chemical method on the photovoltaic performance of ZnO nanowires arrays-based quantum dot sensitized solar cells. Electrochim. Acta 2013, 99, 204–210. [Google Scholar] [CrossRef]
- Zhong, P.; Que, W.; Liang, Y.N.; Yin, X.; Liao, Y.; Kong, L.B.; Hu, X. Origin of the boosted exciton separation at fullerene molecule modified poly(3-hexylthiophene)/ZnO interfaces. RSC Adv. 2013, 3, 17904–17913. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Wu, Y.; Min, C.; Fang, J. High performance polymer solar cells with a polar fullerene derivative as the cathode buffer layer. J. Mater. Chem. A 2013, 1, 12413–12416. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Wang, G.; Li, P.; Zhang, W.; Jiu, T.; Jiang, N.; Fang, J. Highly efficient inverted polymer solar cells using fullerene derivative modified TiO2 nanorods as the buffer layer. RSC Adv. 2014, 4, 19529–19532. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Sun, C.; Wang, G.; Li, J.; Jiu, T.; Fang, J. Performance enhancement of inverted polymer solar cells with fullerene ester derivant-modified ZnO film as cathode buffer layer. Sol. Energy Mater. Sol. Cells 2014, 126 (Suppl. C), 36–41. [Google Scholar] [CrossRef]
- Said, A.J.; Poize, G.; Martini, C.; Ferry, D.; Marine, W.; Giorgio, S.; Fages, F.; Hocq, J.; Bouclé, J.; Nelson, J.; et al. Hybrid bulk heterojunction solar cells based on P3HT and porphyrin-modified ZnO nanorods. J. Phys. Chem. C 2010, 114, 11273–11278. [Google Scholar] [CrossRef]
- Musselman, K.P.; Albert-Seifried, S.; Hoye, R.L.Z.; Sadhanala, A.; Munoz-Rojas, D.; MacManus-Driscoll, J.L.; Friend, R.H. Improved exciton dissociation at semiconducting polymer:ZnO donor:Acceptor interfaces via nitrogen doping of ZnO. Adv. Funct. Mater. 2014, 24, 3562–3570. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Chen, L.; Chen, Y.; Li, F.; Wang, P. Interfacial nanostructuring of ZnO nanoparticles by fullerene surface functionalization for “annealing-gree” hybrid bulk heterojunction solar cells. J. Phys. Chem. C 2012, 116, 3486–3491. [Google Scholar] [CrossRef]
- Zhong, P.; Ma, X.; Chen, X.; Zhong, R.; Liu, X.; Ma, D.; Zhang, M.; Li, Z. Morphology-controllable polycrystalline TiO2 nanorod arrays for efficient charge collection in dye-sensitized solar cells. Nano Energy 2015, 16, 99–111. [Google Scholar] [CrossRef]


| Samples | A0 | A2 | η |
|---|---|---|---|
| P3HT/ZnO | 209,542 | 12,294 | 0.941 |
| P3HT/C60/ZnO | 253,133 | 14,224 | 0.944 |
| Samples | R1/Ω·cm2 | Rct/Ω·cm2 | C1/μF·cm−2 | τ/μs |
|---|---|---|---|---|
| P3HT/ZnO | 12.3 | 44.2 | 2.99 | 91.2 |
| P3HT/C60/ZnO | 10.1 | 76.5 | 2.84 | 135.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, P.; Ma, X.; Xi, H. Passivating ZnO Surface States by C60 Pyrrolidine Tris-Acid for Hybrid Solar Cells Based on Poly(3-hexylthiophene)/ZnO Nanorod Arrays. Polymers 2018, 10, 4. https://doi.org/10.3390/polym10010004
Zhong P, Ma X, Xi H. Passivating ZnO Surface States by C60 Pyrrolidine Tris-Acid for Hybrid Solar Cells Based on Poly(3-hexylthiophene)/ZnO Nanorod Arrays. Polymers. 2018; 10(1):4. https://doi.org/10.3390/polym10010004
Chicago/Turabian StyleZhong, Peng, Xiaohua Ma, and He Xi. 2018. "Passivating ZnO Surface States by C60 Pyrrolidine Tris-Acid for Hybrid Solar Cells Based on Poly(3-hexylthiophene)/ZnO Nanorod Arrays" Polymers 10, no. 1: 4. https://doi.org/10.3390/polym10010004





