Thermal, Mechanical and Optical Features of Aluminosilicate-Coated Cotton Textiles via the Crosslinking Method
Abstract
:1. Introduction
2. Experimental
2.1. Materials
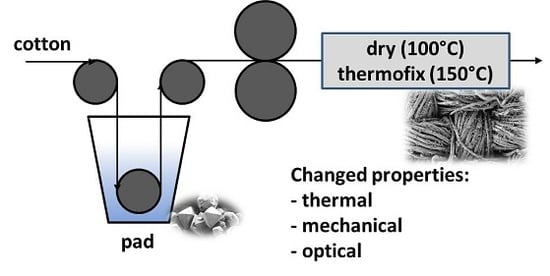
2.2. Preparation of Aluminosilicate Coated Textile
2.3. Analytical Procedure
2.3.1. X-ray Powder Diffraction (XRD)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. Optical Properties
2.3.5. Ultraviolet Protective Properties
2.3.6. Mechanical Properties and Crease Recovery Angle
2.3.7. Thermogravimetric Analysis and Vertical Burning Test
3. Results and Discussion
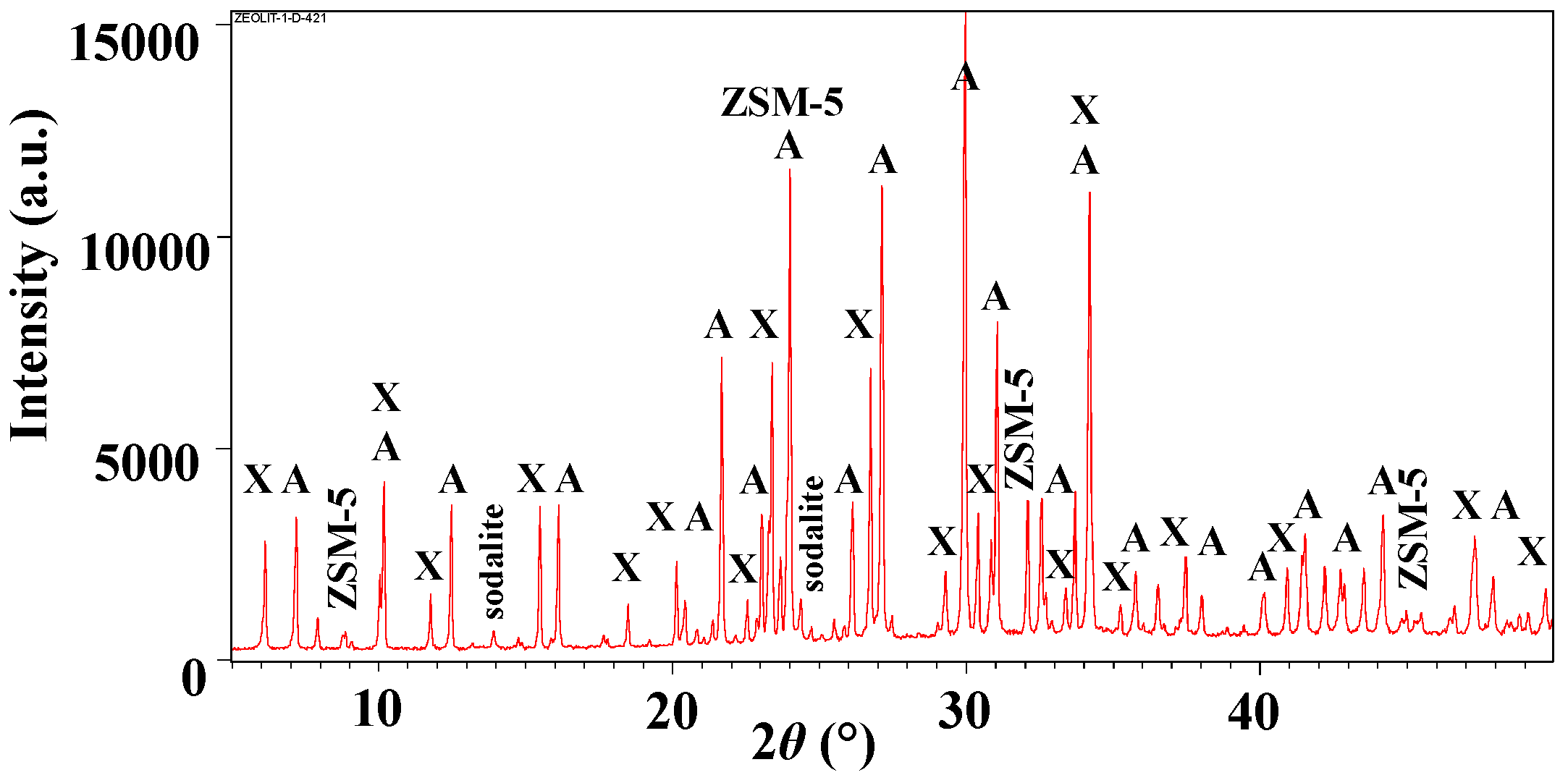
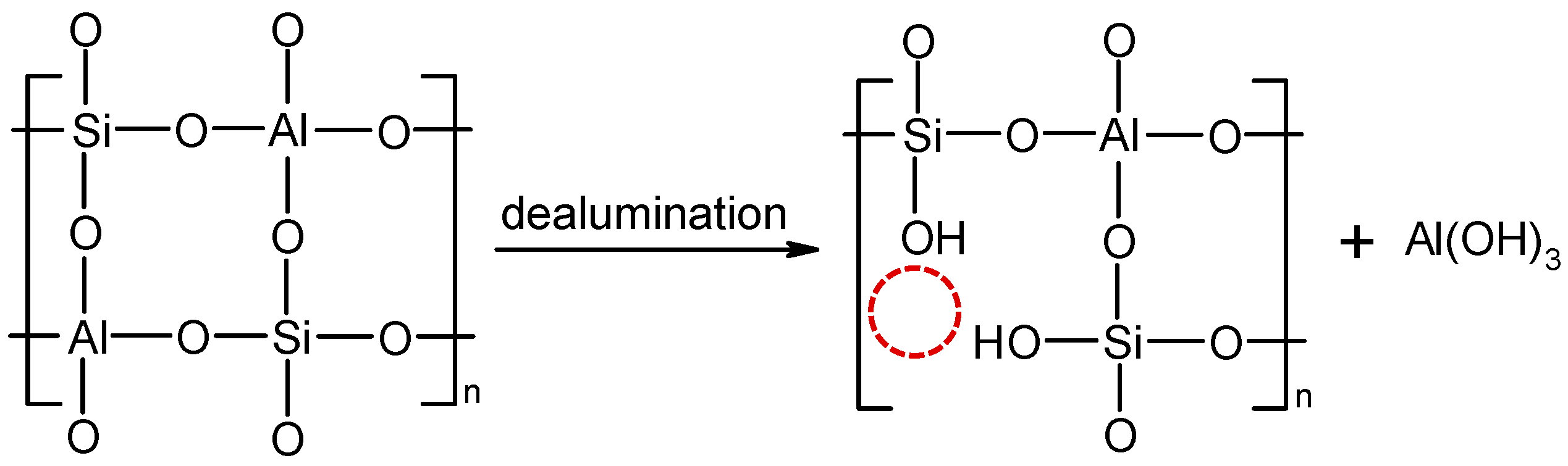
3.1. Analysis of Aluminosilicate Particles
3.2. Morphological and Chemical Characterisation of Zeolite-Modified Cotton
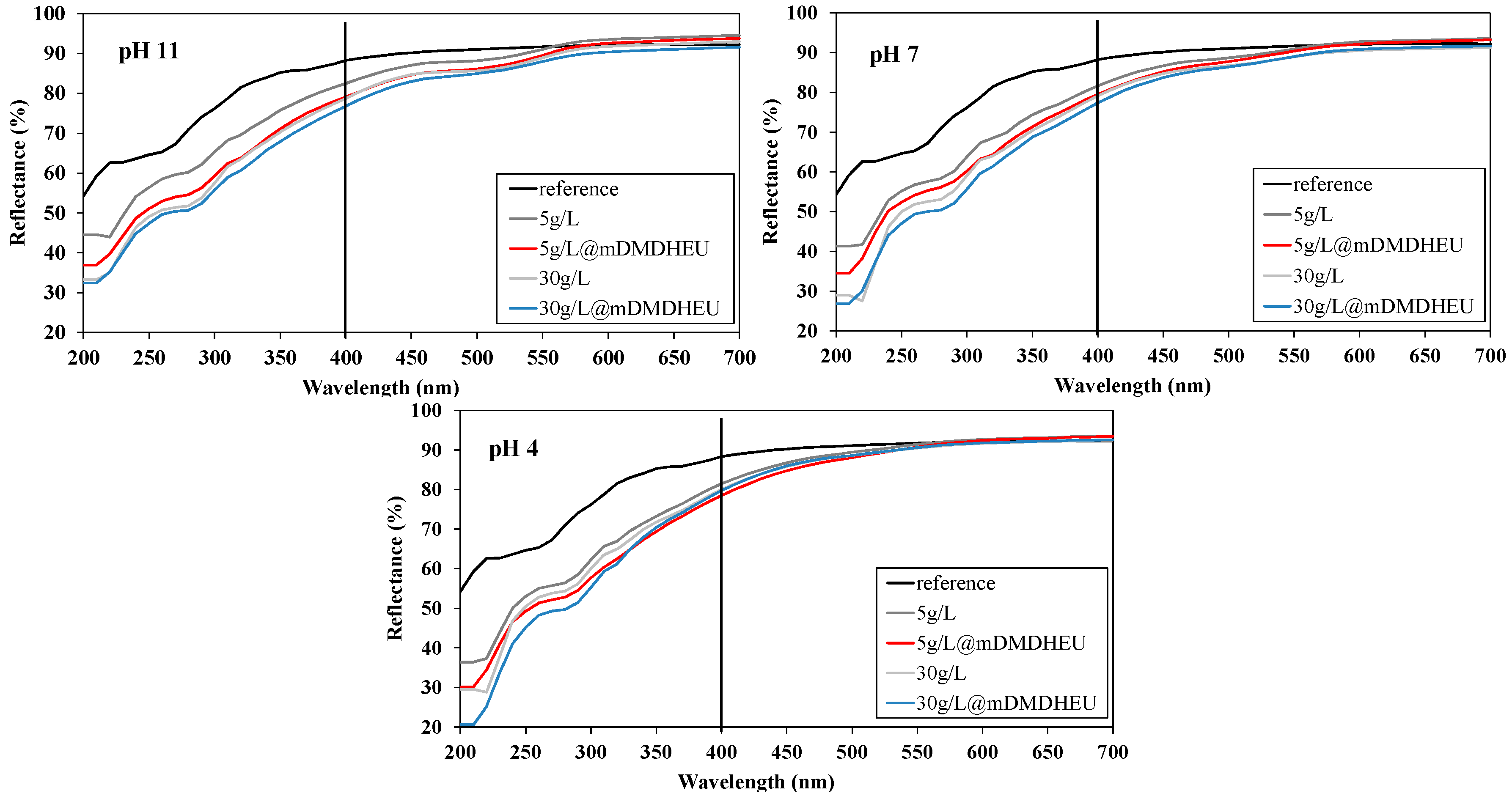
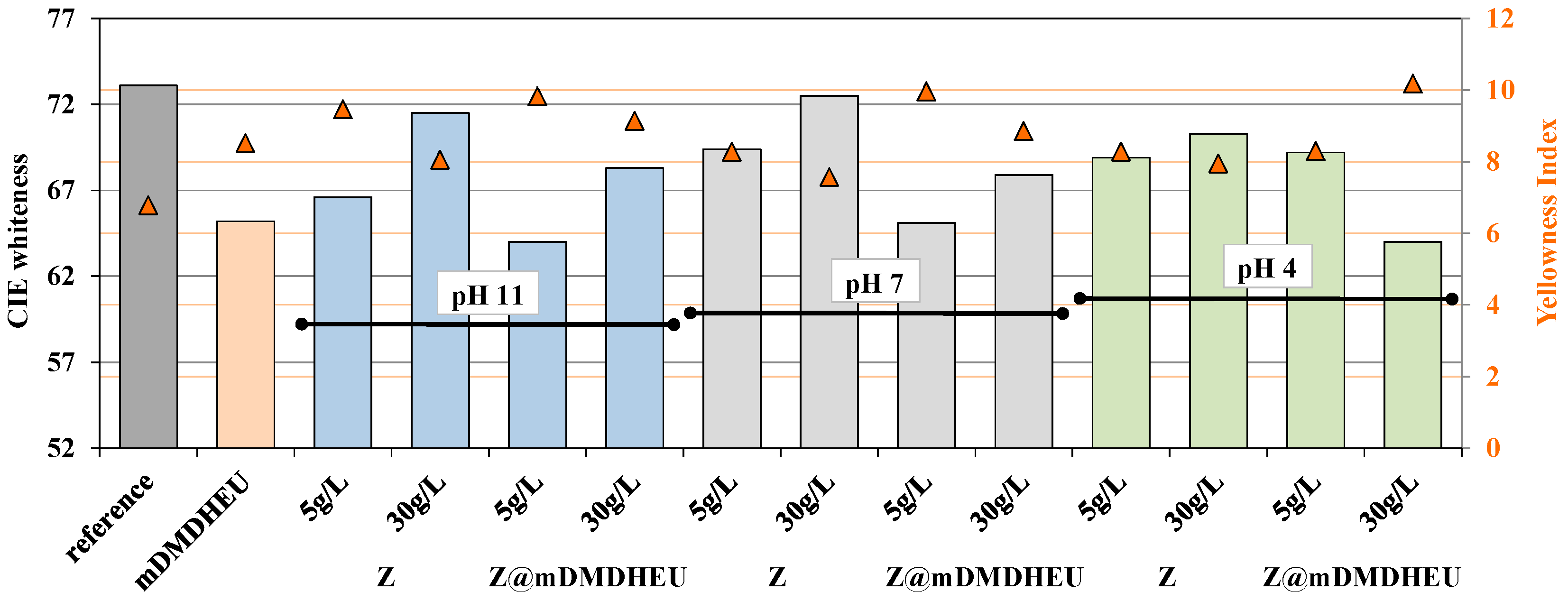
3.3. Diffuse Reflectance Spectra Profile Determination and CIE Measurement
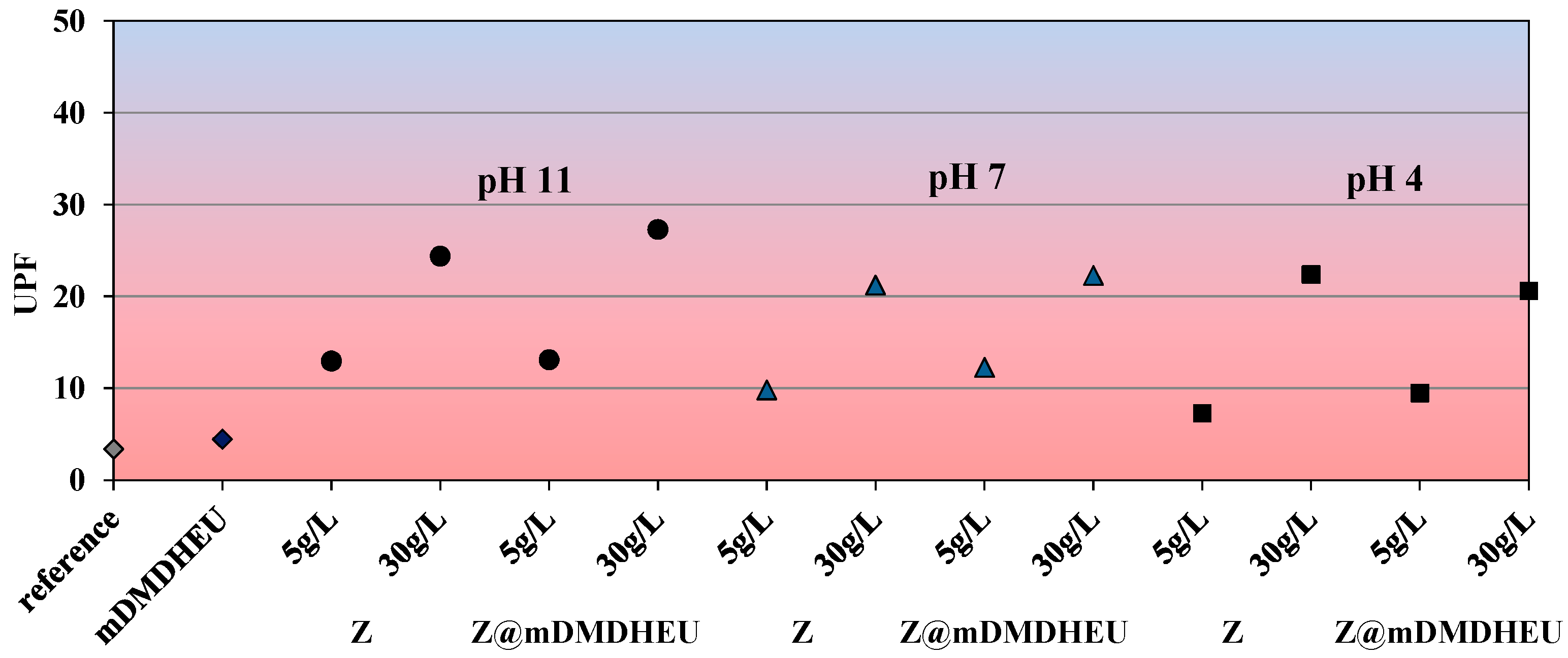
3.4. UV-Protective Properties
3.5. Mechanical Performance
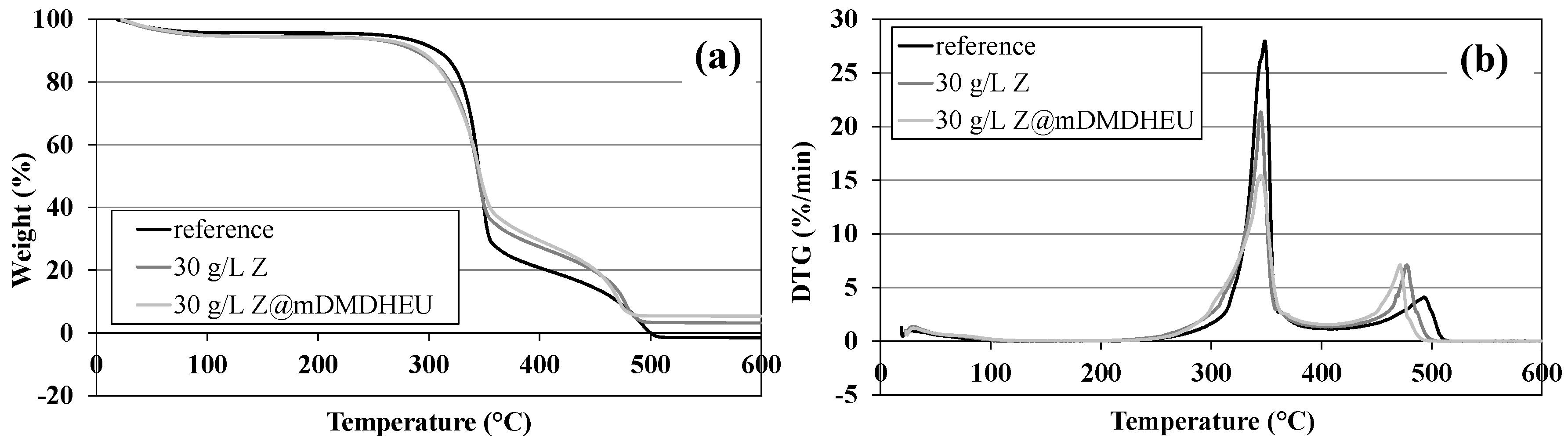
3.6. Thermal Degradation and Burning Behaviour
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fakin, D.; Veronovski, N.; Ojstršek, A.; Božič, M. Synthesis of TiO2-SiO2 colloid and its performance in reactive dyeing of cotton fabrics. Carbohydr. Polym. 2012, 8, 992–1001. [Google Scholar] [CrossRef]
- Monteiro, A.; Jarrais, B.; Rocha, I.M.; Pereira, C.; Pereira, M.F.R.; Freire, C. Efficient immobilization of montmorillonite onto cotton textiles through their functionalization with organosilanes. Appl. Clay Sci. 2014, 101, 304–314. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Youssef, M.A.; Ibrahim, H.M.; Ameen, H.A.; Salah, A.M. Multifunctional finishing of cellulose/polyester blended fabrics. Carbohydr. Polym. 2013, 97, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Montazer, M.; Alimohammadi, F.; Shamei, A.; Rahimi, M.K. In situ synthesis of nano silver on cotton using Tollens’ reagent. Carbohydr. Polym. 2012, 87, 1706–1712. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Balaji, S.; Prabhawathi, V.; Neelakandan, R.; Manoharan, P.T.; Doble, M. Effective antibacterial adhesive coating on cotton fabric using ZnO nanorods and chalcone. Carbohydr. Polym. 2010, 79, 717–723. [Google Scholar] [CrossRef]
- Veronovski, N.; Hribernik, S.; Andreozzi, P.; Sfiligoj Smole, M. Homogeneous self-cleaning coatings on cellulose materials derived from TIP/TiO2 P25. Fibers Polym. 2009, 10, 716–723. [Google Scholar]
- Tsafack, M.J.; Levalois-Grützmacher, J. Towards multifunctional surfaces using the plasma-induced graft-polymerization (PIGP) process: Flame and waterproof cotton textiles. Surf. Coat. Technol. 2007, 201, 5789–5795. [Google Scholar] [CrossRef]
- Abidi, N.; Kikens, P. Chemical functionalisation of cotton fabric to impart multifunctional properties. Tekstilec 2016, 59, 156–161. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Pinto, R.J.B.; Trindade, T.; Neto, C.P. Surface modification of cellulosic fibres for multi-purpose TiO2 based nanocomposites. Compos. Sci. Technol. 2009, 69, 1051–1056. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Fan, X.R. Layer-by-layer self-assembly of TiO2 sol on wool to improve its anti-ultraviolet and anti-ageing properties. J. Sol-Gel Sci. Technol. 2012, 62, 338–343. [Google Scholar] [CrossRef]
- Xu, Z.J.; Tian, Y.L.; Liu, H.L.; Du, Z.Q. Cotton fabric finishing with TiO2/SiO2 composite hydrosol based on ionic cross-linking method. Appl. Surf. Sci. 2015, 324, 68–75. [Google Scholar] [CrossRef]
- Ojstršek, A.; Stana Kleinschek, K.; Fakin, D. Characterization of nano-sized TiO2 suspensions for functional modification of polyester fabric. Surf. Coat. Technol. 2013, 226, 68–74. [Google Scholar] [CrossRef]
- Liu, S.; Cao, X.; Li, L.; Ji, Y.; Xiao, F.S. Preformed zeolite precursor route for synthesis of mesoporous X zeolite. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 269–274. [Google Scholar] [CrossRef]
- Meng, X.; Nawaz, F.; Xiao, F.S. Templating route for synthesizing mesoporous zeolites with improved catalytic properties. Nano Today 2009, 4, 292–301. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Grancarić, A.M.; Tarbuk, A.; Kovaček, I. Nanoparticles of activated natural zeolite on textiles for protection and therapy. Chem. Ind. Chem. Eng. Q. 2009, 15, 203–210. [Google Scholar] [CrossRef]
- Grancarić, A.M.; Tarbuk, A.; McCall, D. Surface modification of polyester fabric with tribomechanically activated natural zeolite (TMAZ) nanoparticles. Polimeri 2007, 28, 219–224. [Google Scholar]
- Carran, R.S.; Ghosh, A.; Dyer, J.M. The effects of zeolite molecular sieve based surface treatments on the properties of wool fabrics. Appl. Surf. Sci. 2013, 287, 467–472. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 4th ed.; Elsevier: Amsterdam, The Nederland, 2001. [Google Scholar]
- Dehabadi, V.A.; Buschmann, H.J.; Gutmann, J.S. Durable press finishing of cotton fabrics: An overview. Text. Res. J. 2013, 83, 1974–1995. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; El-Batal, H. A novel approach for adding smart functionalities to cellulosic fabrics. Carbohydr. Polym. 2012, 87, 744–751. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Zairy, M.R.; Eid, B.M.; El-Zairy, E.M.R.; Emam, E.M. New finishing possibilities for producing durable multifunctional cotton/wool and viscose/wool blended fabrics. Carbohydr. Polym. 2015, 119, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.K.; Kanwith, C.W. Low-Stress mechanical properties of cotton fabric treated with titanium dioxide-catalyzed wrinkle-resistant finishing. J. Nat. Fibers 2016, 13, 451–457. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Salagre, P. Comparison of dealumination of zeolites beta, mordenite and ZSM-5 by treatment with acid under microwave irradiation. Microporous Mesoporous Mater. 2011, 144, 162–170. [Google Scholar] [CrossRef]
- Palme, A.; Theliander, H.; Brelid, H. Acid hydrolysis of cellulosic fibres: Comparison of bleached Kraft pulp, dissolving pulps and cotton textile cellulose. Carbohydr. Polym. 2016, 136, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Gargoubi, S.; Tolouei, R.; Chevalier, P.; Levesque, L.; Ladhari, N.; Boudokhane, C.; Mantovani, D. Enhancing the functionality of cotton fabric by physical and chemical pre-treatments: A comparative study. Carbohydr. Polym. 2016, 147, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yang, C.Q.; Wang, S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydr. Polym. 2012, 87, 491–499. [Google Scholar] [CrossRef]
- Mohsin, M.; Ramzan, N.; Ahmad, S.; Farooq, U.; Rasheed, A.; Ahsan, M. Softener impact on environment friendly low and zero formaldehyde cross-linker performance for cotton. Ind. Text. 2014, 65, 134–138. [Google Scholar]
- Alimohammadi, F.; Gashti, M.P.; Shamei, A. A novel method for coating of carbon nanotube on cellulose fiber using 1,2,3,4-butanetetracarboxylic acid as a cross-linking agent. Prog. Org. Coat. 2012, 74, 470–478. [Google Scholar] [CrossRef]
- Yuan, H.; Xing, W.; Zhang, P.; Song, L.; Hu, Y. Functionalization of cotton with UV-cured flame retardant coatings. Ind. Eng. Chem. Res. 2012, 51, 5394–5401. [Google Scholar] [CrossRef]




| Sample | Elongation at Break (%) | Tensile Strength (N) | Breaking Tenacity (cN/tex) | CRA (°) | Q (%) |
|---|---|---|---|---|---|
| Reference warp weft | 8.65 ± 0.37 14.31 ± 0.38 | 421 ± 22 54 ± 18 | 441 ± 22 371 ± 19 | 63.1 63.1 | 17.1 17.1 |
| mDMDHEU warp weft | 8.12 ± 0.27 13.26 ± 0.28 | 409 ± 27 322 ± 28 | 397 ± 29 341 ± 31 | 93.2 82.4 | 41.3 36.4 |
| 5 g/L Z warp weft | 9.24 ± 0.71 13.80 ± 2.20 | 495 ± 33 389 ± 15 | 518 ± 34 407 ± 16 | 56.7 54.6 | 15.9 14.3 |
| 5 g/L Z@mDMDHEU warp weft | 7.49 ± 0.59 10.19 ± 1.60 | 435 ± 18 363 ± 28 | 456 ± 18 380 ± 29 | 91.3 89.4 | 36.3 34.1 |
| 30 g/L Z warp weft | 8.51 ± 0.26 11.91 ± 1.08 | 504 ± 36 414 ± 17 | 528 ± 37 434 ± 18 | 51.3 52.5 | 13.3 15.1 |
| 30 g/L Z@mDMDHEU warp weft | 8.31 ± 0.68 10.30 ± 0.81 | 501 ± 28 372 ± 33 | 524 ± 29 389 ± 34 | 66.8 61.7 | 16.4 13.8 |
| Sample | T of Exothermic Peaks (°C) | Weight Loss (%) | Residual at 600 °C (%) | |||
|---|---|---|---|---|---|---|
| 1st | 2nd | <220 °C | 220–400 °C | 400–530 °C | ||
| Reference | 349 | 494 | 4.2 | 75.1 | 22.2 | - |
| 30 g/L Z | 345 | 477 | 5.3 | 67.2 | 24.1 | 3.1 |
| 30 g/L Z@mDMDHEU | 345 | 471 | 5.4 | 65.2 | 23.9 | 5.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojstršek, A.; Hribernik, S.; Fakin, D. Thermal, Mechanical and Optical Features of Aluminosilicate-Coated Cotton Textiles via the Crosslinking Method. Polymers 2018, 10, 57. https://doi.org/10.3390/polym10010057
Ojstršek A, Hribernik S, Fakin D. Thermal, Mechanical and Optical Features of Aluminosilicate-Coated Cotton Textiles via the Crosslinking Method. Polymers. 2018; 10(1):57. https://doi.org/10.3390/polym10010057
Chicago/Turabian StyleOjstršek, Alenka, Silvo Hribernik, and Darinka Fakin. 2018. "Thermal, Mechanical and Optical Features of Aluminosilicate-Coated Cotton Textiles via the Crosslinking Method" Polymers 10, no. 1: 57. https://doi.org/10.3390/polym10010057