A Comparative Study on Micellar and Solubilizing Behavior of Three EO-PO Based Star Block Copolymers Varying in Hydrophobicity and Their Application for the In Vitro Release of Anticancer Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods
2.1.1. Cloud Point (CP)
2.1.2. Surface Tension
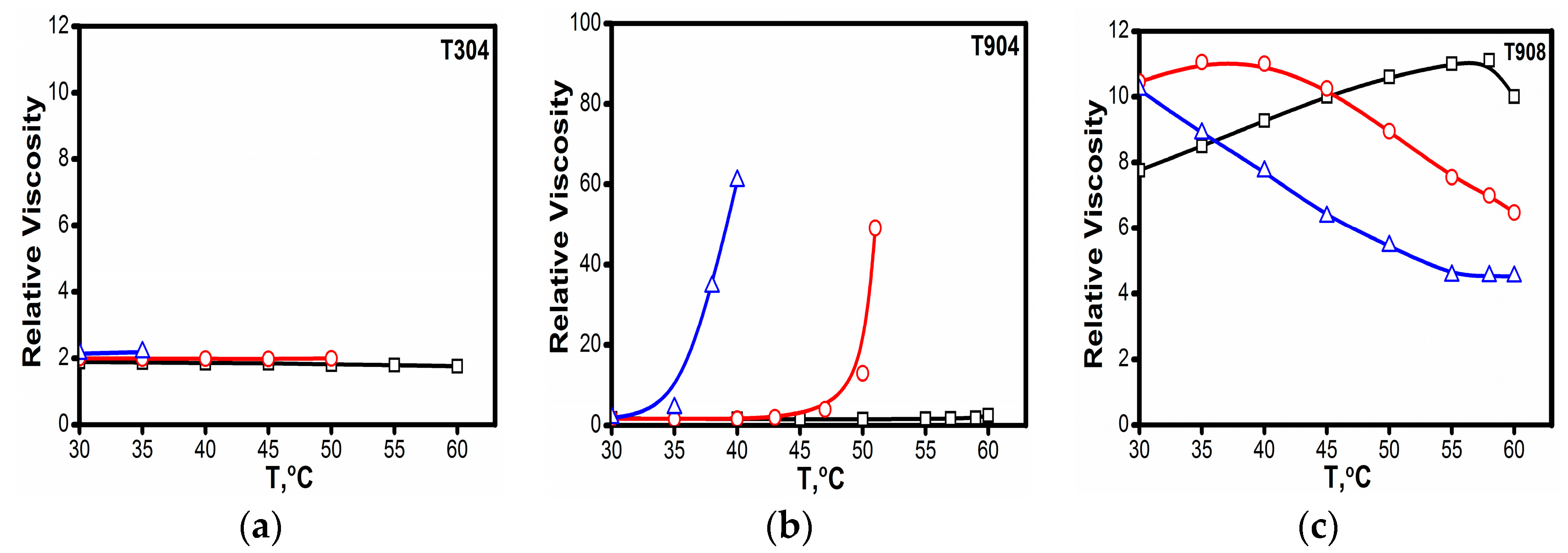
2.1.3. Viscosity
2.1.4. Nuclear Magnetic Resonance (NMR)
2.1.5. High-Sensitivity Differential Scanning Calorimetry (HSDSC)
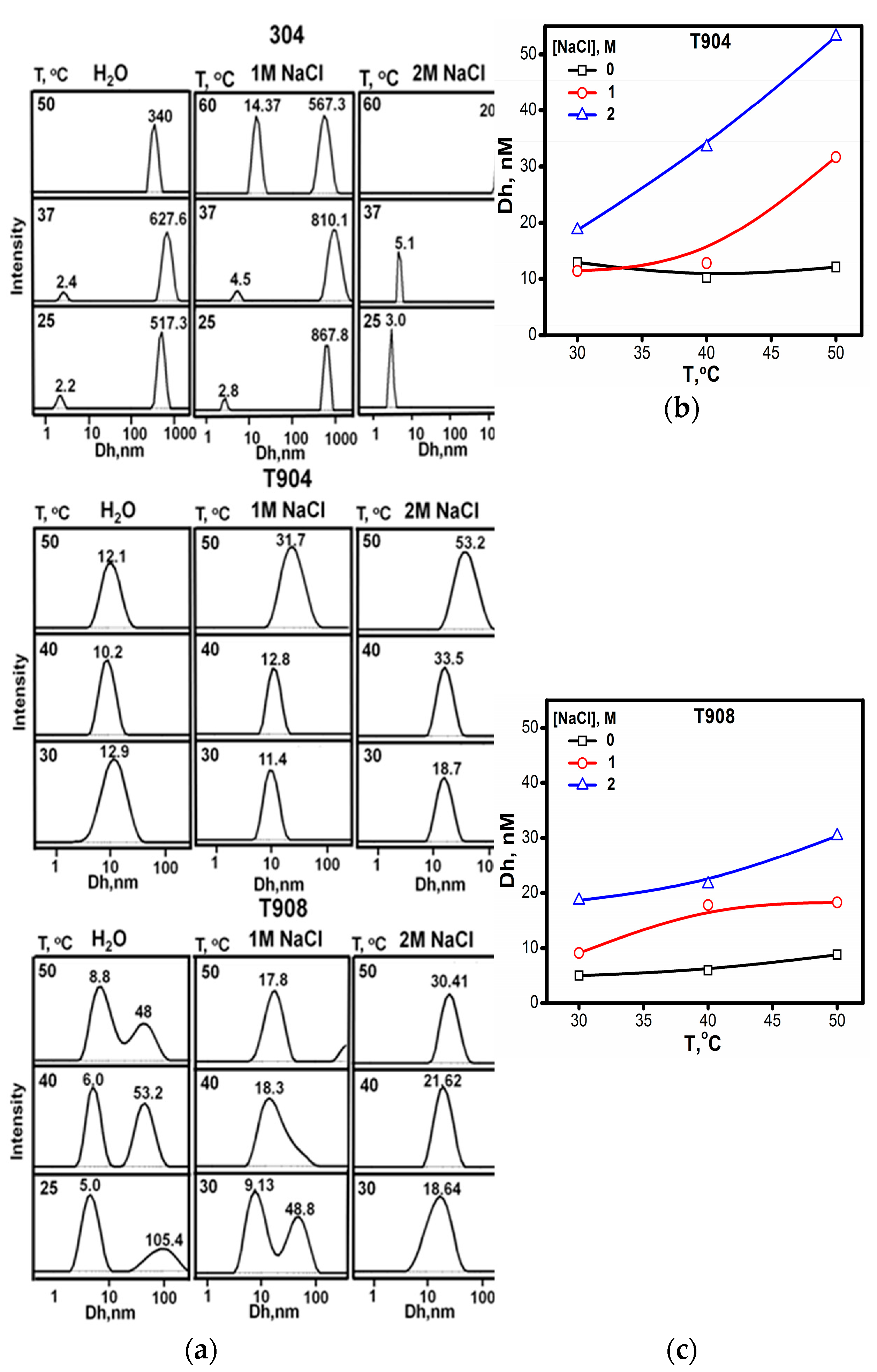
2.1.6. Dynamic Light Scattering (DLS)
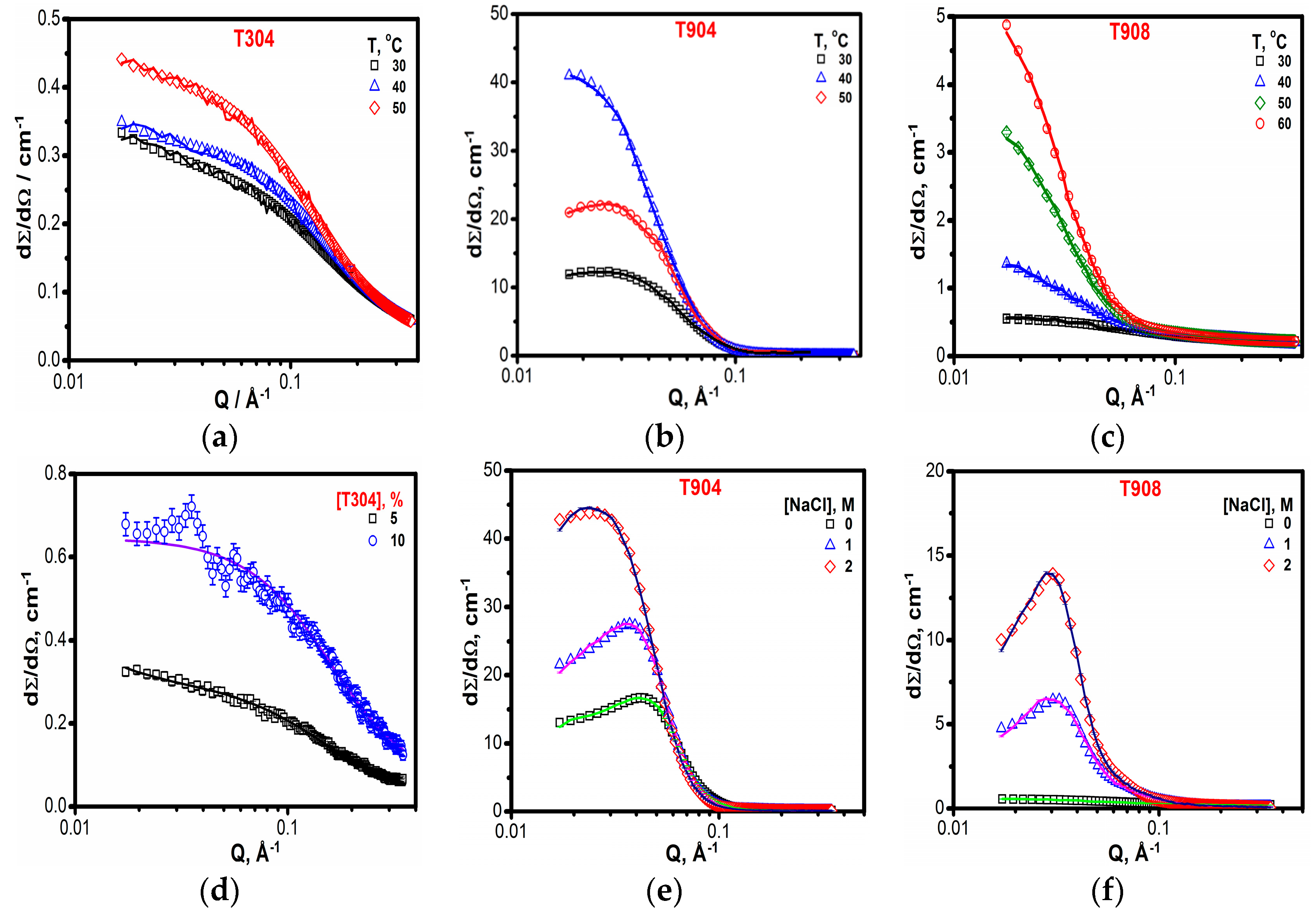
2.1.7. Small Angle Neutron Scattering (SANS)
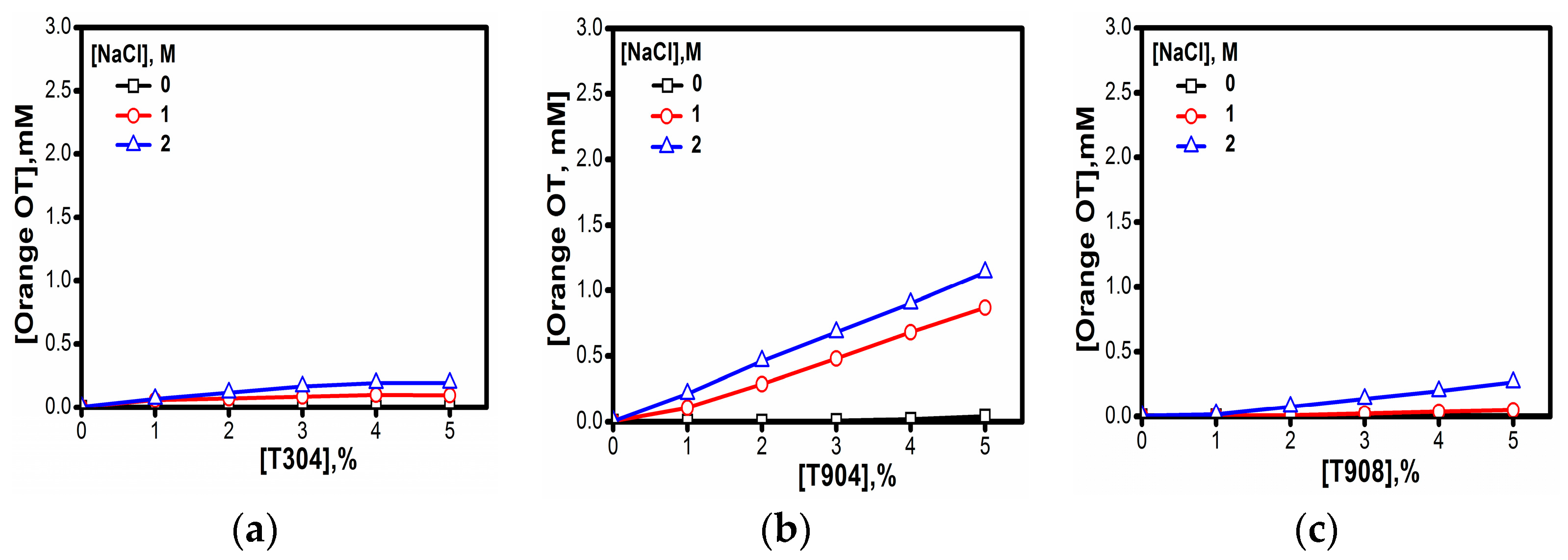
2.1.8. Solubilization
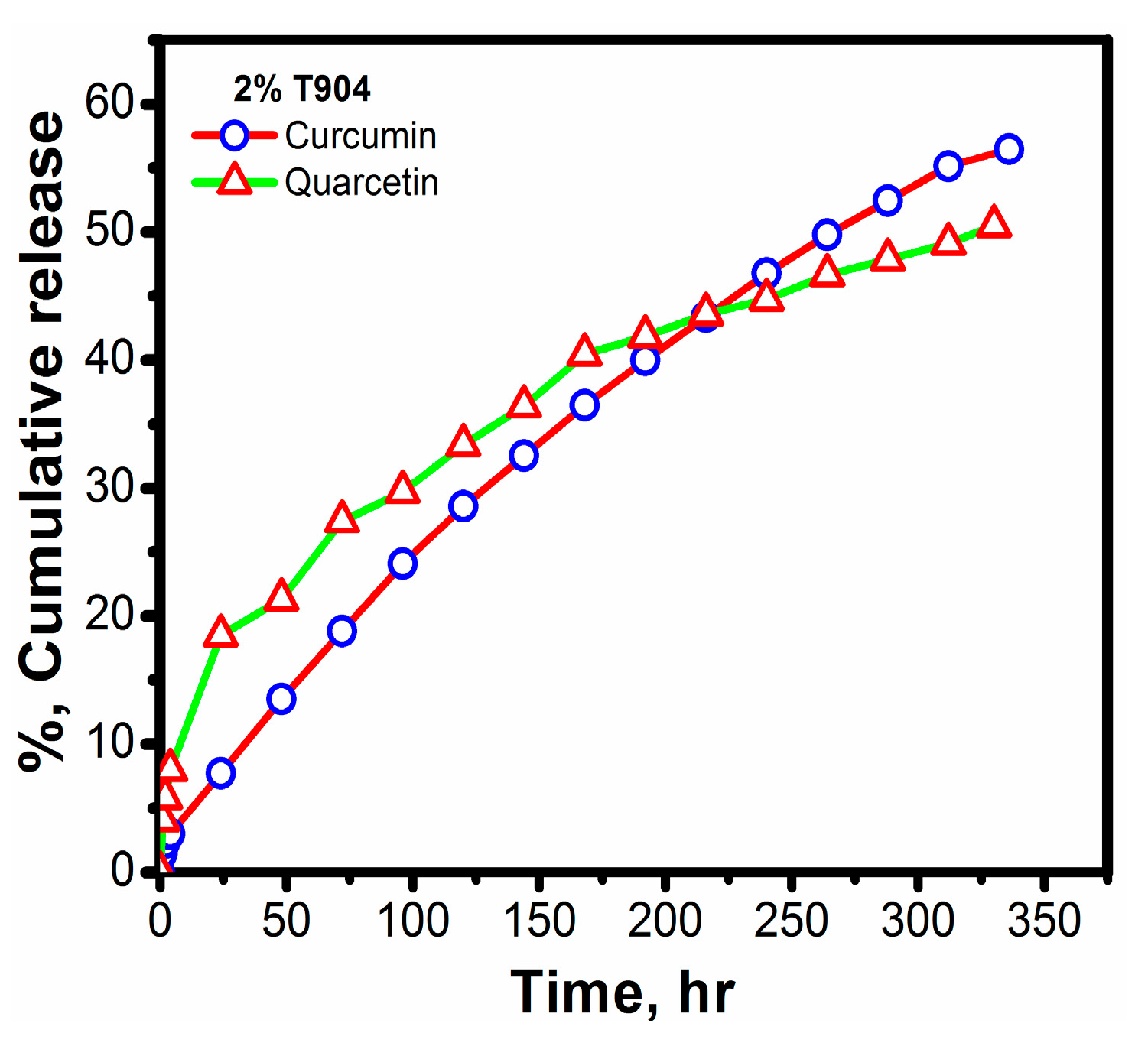
2.1.9. In Vitro Release Studies
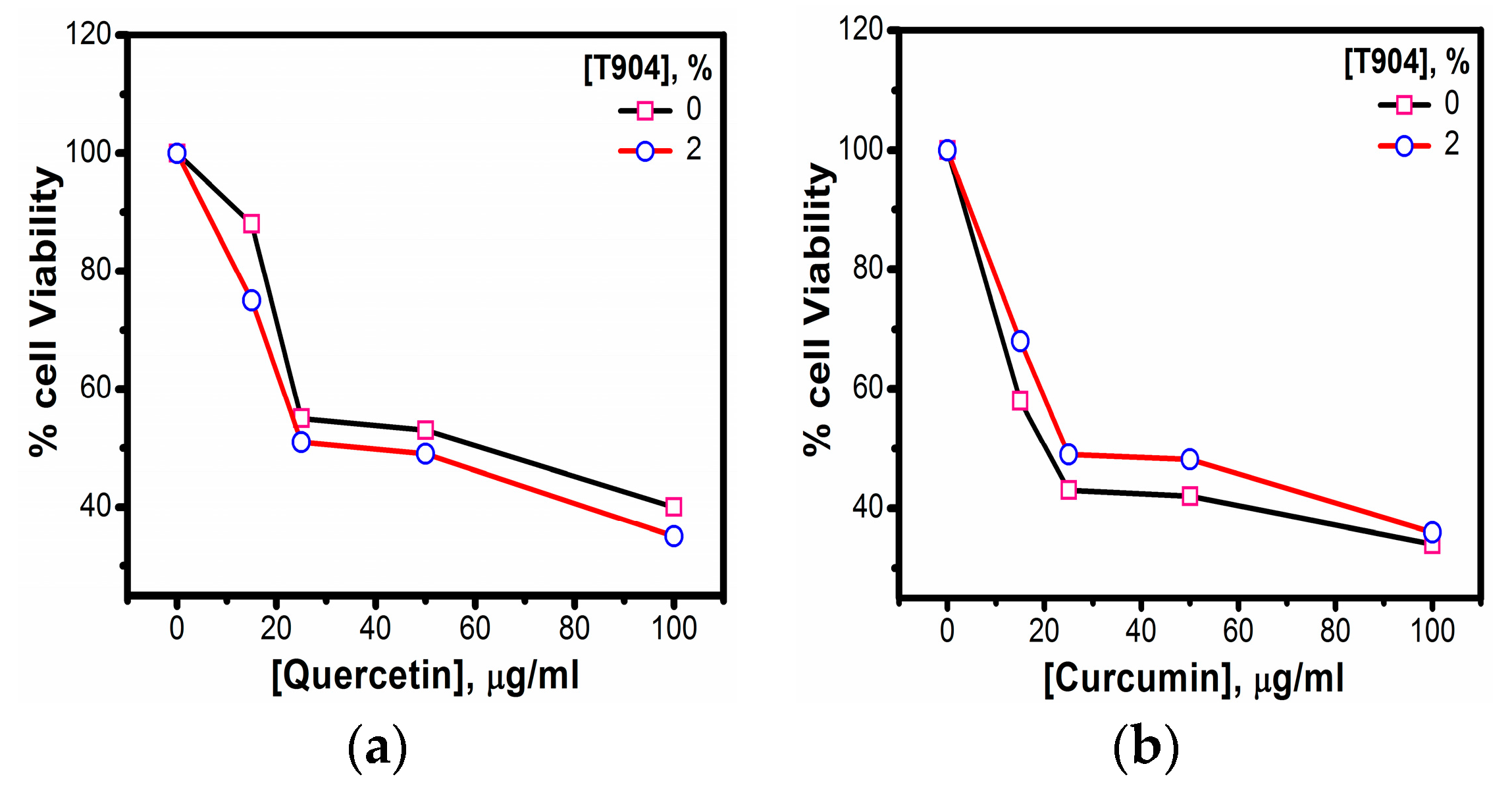
2.1.10. In Vitro Cytotoxicity Assays
3. Results
3.1. Characterization of Tetronics® Micelles and the Effect of Salt
3.1.1. Cloud Point
3.1.2. Surface Tension
3.1.3. Nuclear Magnetic Resonance (NMR)
3.1.4. HSDSC
3.1.5. Viscosity
3.1.6. DLS
3.1.7. SANS
3.1.8. Micellar Solubilization
3.1.9. In Vitro Drug Release
3.1.10. In Vitro Cytotoxicity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nakashima, K.; Bahadur, P. Aggregation of water-soluble block copolymers in aqueous solutions: Recent trends. Adv. Colloid Interface Sci. 2006, 123, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, P. Block copolymers-their microdomain formation (in solid state) and surfactant behaviour (in solution). Curr. Sci. 2001, 80, 1002–1007. [Google Scholar]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Sang, L.-C.; Coppens, M.-O. Effects of surface curvature and surface chemistry on the structure and activity of proteins adsorbed in nanopores. Phys. Chem. Chem. Phys. 2011, 13, 6689–6698. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-K.; Chang, C.-J. Fabrications and applications of stimulus-responsive polymer films and patterns on surfaces: A review. Materials 2014, 7, 805–875. [Google Scholar] [PubMed]
- Singh, V.; Khullar, P.; Dave, P.N.; Kaura, A.; Bakshi, M.S.; Kaur, G. PH and thermo-responsive tetronic micelles for the synthesis of gold nanoparticles: Effect of physiochemical aspects of tetronics. Phys. Chem. Chem. Phys. 2014, 16, 4728–4739. [Google Scholar] [CrossRef] [PubMed]
- Habas, J.-P.; Pavie, E.; Perreur, C.; Lapp, A.; Peyrelasse, J. Nanostructure in block copolymer solutions: Rheology and small-angle neutron scattering. Phys. Rev. E 2004, 70, 061802. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tarrio, M.; Yañez, F.; Immesoete, K.; Alvarez-Lorenzo, C.; Concheiro, A. Pluronic and tetronic copolymers with polyglycolyzed oils as self-emulsifying drug delivery systems. AAPS PharmSciTech 2008, 9, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, E.L.; Shih, C.K.; Solchaga, L.A.; Caplan, A.I.; Mikos, A.G. Controlled release of hyaluronan oligomers from biodegradable polymeric microparticle carriers. J. Control. Release 2004, 100, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.T.; Bronich, T.K.; Kabanov, A.V. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic pluronic® block copolymers. J. Control. Release 2004, 94, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, Z.; Yüksel, N.; Baykara, T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur. J. Pharm. Biopharm. 2006, 64, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Csaba, N.; Caamaño, P.; Sánchez, A.; Domínguez, F.; Alonso, M.J. Plga: Poloxamer and plga: Poloxamine blend nanoparticles: New carriers for gene delivery. Biomacromolecules 2005, 6, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. The Physics of Block Copolymers; Oxford University Press: New York, NY, USA, 1998; Volume 19. [Google Scholar]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Zhou, Z.; Chu, B. Anomalous association behavior of an ethylene oxide/propylene oxide aba block copolymer in water. Macromolecules 1987, 20, 3089–3091. [Google Scholar] [CrossRef]
- Almgren, M.; Brown, W.; Hvidt, S. Self-aggregation and phase behavior of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymers in aqueous solution. Colloid Polym. Sci. 1995, 273, 2–15. [Google Scholar] [CrossRef]
- Liu, T.; Xu, G.; Gong, H.; Pang, J.; He, F. Effect of alcohols on aggregation behaviors of branched block polyether tetronic 1107 at an air/liquid surface. Langmuir 2011, 27, 9253–9260. [Google Scholar] [CrossRef] [PubMed]
- Nivaggioli, T.; Tsao, B.; Alexandridis, P.; Hatton, T.A. Microviscosity in pluronic and tetronic poly(ethylene oxide) poly(propylene oxide) block-copolymer micelles. Langmuir 1995, 11, 119–126. [Google Scholar] [CrossRef]
- Ganguly, R.; Kadam, Y.; Choudhury, N.; Aswal, V.; Bahadur, P. Growth and interaction of the tetronic 904 micelles in aqueous alkaline solutions. J. Phys. Chem. B 2011, 115, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lopez, J.; Alvarez-Lorenzo, C.; Taboada, P.; Sosnik, A.; Sandez-Macho, I.; Concheiro, A. Self-associative behavior and drug-solubilizing ability of poloxamine (tetronic) block copolymers. Langmuir 2008, 24, 10688–10697. [Google Scholar] [CrossRef] [PubMed]
- Goy-López, S.; Taboada, P.; Cambón, A.; Juárez, J.; Alvarez-Lorenzo, C.; Concheiro, A.; Mosquera, V.C. Modulation of size and shape of au nanoparticles using amino-x-shaped poly(ethylene oxide)—Poly(propylene oxide) block copolymers. J. Phys. Chem. B 2009, 114, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chowdhry, B.Z.; Leharne, S.A. Surface activity of poloxamines at the interfaces between air-water and hexane-water. Colloids Surf. A 2003, 212, 9–17. [Google Scholar] [CrossRef]
- Dong, J.; Armstrong, J.; Chowdhry, B.Z.; Leharne, S.A. Thermodynamic modelling of the effect of pH upon aggregation transitions in aqueous solutions of the poloxamine, T701. Thermochim. Acta 2004, 417, 201–206. [Google Scholar] [CrossRef]
- Dong, J.; Chowdhry, B.Z.; Leharne, S.A. Solubilisation of polyaromatic hydrocarbons in aqueous solutions of poloxamine t803. Colloids Surf. A 2004, 246, 91–98. [Google Scholar] [CrossRef]
- Armstrong, J.K.; Chowdhry, B.Z.; Snowden, M.J.; Dong, J.; Leharne, S.A. The effect of ph and concentration upon aggregation transitions in aqueous solutions of poloxamine T701. Int. J. Pharm. 2001, 229, 57–66. [Google Scholar] [CrossRef]
- Tirnaksiz, F.; Kalsin, O. A topical w/o/w multiple emulsions prepared with tetronic 908 as a hydrophilic surfactant: Formulation, characterization and release study. J. Pharm. Pharm. Sci. 2004, 8, 299–315. [Google Scholar]
- Fernandez-Tarrio, M.; Alvarez-Lorenzo, C.; Concheiro, A. Calorimetric approach to tetronic/water interactions. J. Therm. Anal. Calorim. 2007, 87, 171–178. [Google Scholar] [CrossRef]
- Xin, X.; Xu, G.; Zhang, Z.; Chen, Y.; Wang, F. Aggregation behavior of star-like peo–ppo–peo block copolymer in aqueous solution. Eur. Polym. J. 2007, 43, 3106–3111. [Google Scholar] [CrossRef]
- Kadam, Y.; Singh, K.; Marangoni, D.; Ma, J.; Aswal, V.; Bahadur, P. Induced micellization and micellar transitions in aqueous solutions of non-linear block copolymer Tetronic® T904. J. Colloid Interface Sci. 2010, 351, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Branca, C.; Magazu, S.; Migliardo, F. Star polymer/water solutions: New experimental findings. Condens. Matter Phys. 2008, 5, 275–284. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Y.; Dabros, T.; Hamza, H. Effect of EO and PO positions in nonionic surfactants on surfactant properties and demulsification performance. Colloids Surf. A 2005, 252, 79–85. [Google Scholar] [CrossRef]
- Mansur, C.R.; Barboza, S.P.; González, G.; Lucas, E.F. Pluronic× tetronic polyols: Study of their properties and performance in the destabilization of emulsions formed in the petroleum industry. J. Colloid Interface Sci. 2004, 271, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Tonge, S.; Jones, L.; Goodall, S.; Tighe, B. The ex vivo wettability of soft contact lenses. Curr. Eye Res. 2001, 23, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, L.N.; Bayer, S.; Gepr, S.; Glasier, M.-A.; Lorentz, H.; Senchyna, M.; Jones, L. Rewetting drops containing surface active agents improve the clinical performance of silicone hydrogel contact lenses. Optom. Vis. Sci. 2006, 83, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Cappel, M.J.; Kreuter, J. Effect of nonionic surfactants on transdermal drug delivery: Ii. Poloxamer and poloxamine surfactants. Int. J. Pharm. 1991, 69, 155–167. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Trends Biotechnol. 2000, 18, 412–420. [Google Scholar] [CrossRef]
- Sosnik, A.; Sefton, M.V. Poloxamine hydrogels with a quaternary ammonium modification to improve cell attachment. J. Biomed. Mater. Res. Part A 2005, 75, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Leung, B.; McGuigan, A.P.; Sefton, M.V. Collagen/poloxamine hydrogels: Cytocompatibility of embedded hepg2 cells and surface-attached endothelial cells. Tissue Eng. 2005, 11, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- De Lisi, R.; Giammona, G.; Lazzara, G.; Milioto, S. Copolymers sensitive to temperature and pH in water and in water+ oil mixtures: A dsc, itc and volumetric study. J. Colloid Interface Sci. 2011, 354, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Isasi, J.R. Non-covalent hydrogels of cyclodextrins and poloxamines for the controlled release of proteins. Carbohydr. Polym. 2014, 102, 674–681. [Google Scholar] [CrossRef] [PubMed]
- González-Gaitano, G.; Müller, C.L.; Radulescu, A.; Dreiss, C.C.A. Modulating the self-assembly of amphiphilic x-shaped block copolymers with cyclodextrins: Structure and mechanisms. Langmuir 2015, 31, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Poellmann, M.J.; Sosnick, T.R.; Meredith, S.C.; Lee, R.C. The pentablock amphiphilic copolymer T1107 prevents aggregation of denatured and reduced lysozyme. Macromol. Biosci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.A.; Bharatiya, B.; Casas, M.; Lage, E.V.; Sandez-Macho, I.; Pal, H.; Bahadur, P. A multitechnique approach on adsorption, self-assembly and quercetin solubilization by Tetronics® micelles in aqueous solutions modulated by glycine. Colloids Surf. B 2016, 148, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.A.; Lee, C.-F.; Chen, L.-J.; Aswal, V.K.; Bahadur, P. Glycine elicited self-assembly of amphiphilic star block copolymers with contradistinct hydrophobicities. Colloids Surf. A 2016, 506, 234–244. [Google Scholar] [CrossRef]
- Pillai, S.A.; Lee, C.-F.; Ray, D.; Aswal, V.K.; Pal, H.; Chen, L.-J.; Bahadur, P. Microstructure of copolymeric micelles modulated by ionic liquids: Investigating the role of the anion and cation. RSC Adv. 2016, 6, 87299–87313. [Google Scholar] [CrossRef]
- Pillai, S.A.; Sheth, U.; Bahadur, A.; Aswal, V.K.; Bahadur, P. Salt induced micellar growth in aqueous solutions of a star block copolymer tetronic® 1304: Investigating the role in solubilizing, release and cytotoxicity of model drugs. J. Mol. Liq. 2016, 224, 303–310. [Google Scholar] [CrossRef]
- Pillai, S.A.; Lee, C.-F.; Chen, L.-J.; Bahadur, P.; Aswal, V.K.; Bahadur, P. Thermal and scattering studies of tetronic® 1304 micelles in the presence of industrially important glycols, their oligomers, cellosolves, carbitols, ethers and esters. Colloids Surf. A 2016, 506, 576–585. [Google Scholar] [CrossRef]
- Patidar, P.; Pillai, S.A.; Sheth, U.; Bahadur, P.; Bahadur, A. Glucose triggered enhanced solubilisation, release and cytotoxicity of poorly water soluble anti-cancer drugs from T1307 micelles. J. Biotechnol. 2017, 254, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Patidar, P.; Pillai, S.A.; Bahadur, P.; Bahadur, A. Tuning the self-assembly of EO-PO block copolymers and quercetin solubilization in the presence of some common pharmacuetical excipients: A comparative study on a linear triblock and a starblock copolymer. J. Mol. Liq. 2017, 241, 511–519. [Google Scholar] [CrossRef]
- Chen, S.-H.; Lin, T.-L.; Price, D. Methods of Experimental Physics; Academic Press: New York, NY, USA, 1987; Volume 23, p. 489. [Google Scholar]
- Percus, J.K.; Yevick, G.J. Analysis of classical statistical mechanics by means of collective coordinates. Phys. Rev. 1958, 110. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F. Differential scanning calorimetry investigation of the effect of salts on aqueous solution properties of an amphiphilic block copolymer (poloxamer). Langmuir 1997, 13, 6074–6082. [Google Scholar] [CrossRef]
- Jain, N.; George, A.; Bahadur, P. Effect of salt on the micellization of pluronic P65 in aqueous solution. Colloids Surf. A 1999, 157, 275–283. [Google Scholar] [CrossRef]
- Pandit, N.; Trygstad, T.; Croy, S.; Bohorquez, M.; Koch, C. Effect of salts on the micellization, clouding, and solubilization behavior of pluronic f127 solutions. J. Colloid Interface Sci. 2000, 222, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Galera-Gomez, P. Clouding of triton x-114: The effect of added electrolytes on the cloud point of triton x-114 in the presence of ionic surfactants. Colloids Surf. A 1995, 104, 307–312. [Google Scholar] [CrossRef]
- Bahadur, A.; Cabana-Montenegro, S.; Aswal, V.K.; Lage, E.V.; Sandez-Macho, I.; Concheiro, A.; Alvarez-Lorenzo, C.; Bahadur, P. Nacl-triggered self-assembly of hydrophilic poloxamine block copolymers. Int. J. Pharm. 2015, 494, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Alexandridis, P.; Nivaggioli, T.; Hatton, T.A. Temperature effects on structural properties of pluronic P104 and f108 peo-ppo-peo block copolymer solutions. Langmuir 1995, 11, 1468–1476. [Google Scholar] [CrossRef]
- Parekh, P.; Singh, K.; Marangoni, D.; Bahadur, P. Micellization and solubilization of a model hydrophobic drug nimesulide in aqueous salt solutions of tetronic® T904. Colloids Surf. B 2011, 83, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Tanner, P.; Baumann, P.; Enea, R.; Onaca, O.; Palivan, C.; Meier, W. Polymeric vesicles: From drug carriers to nanoreactors and artificial organelles. Acc. Chem. Res. 2011, 44, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine 2010, 6, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The epr effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]

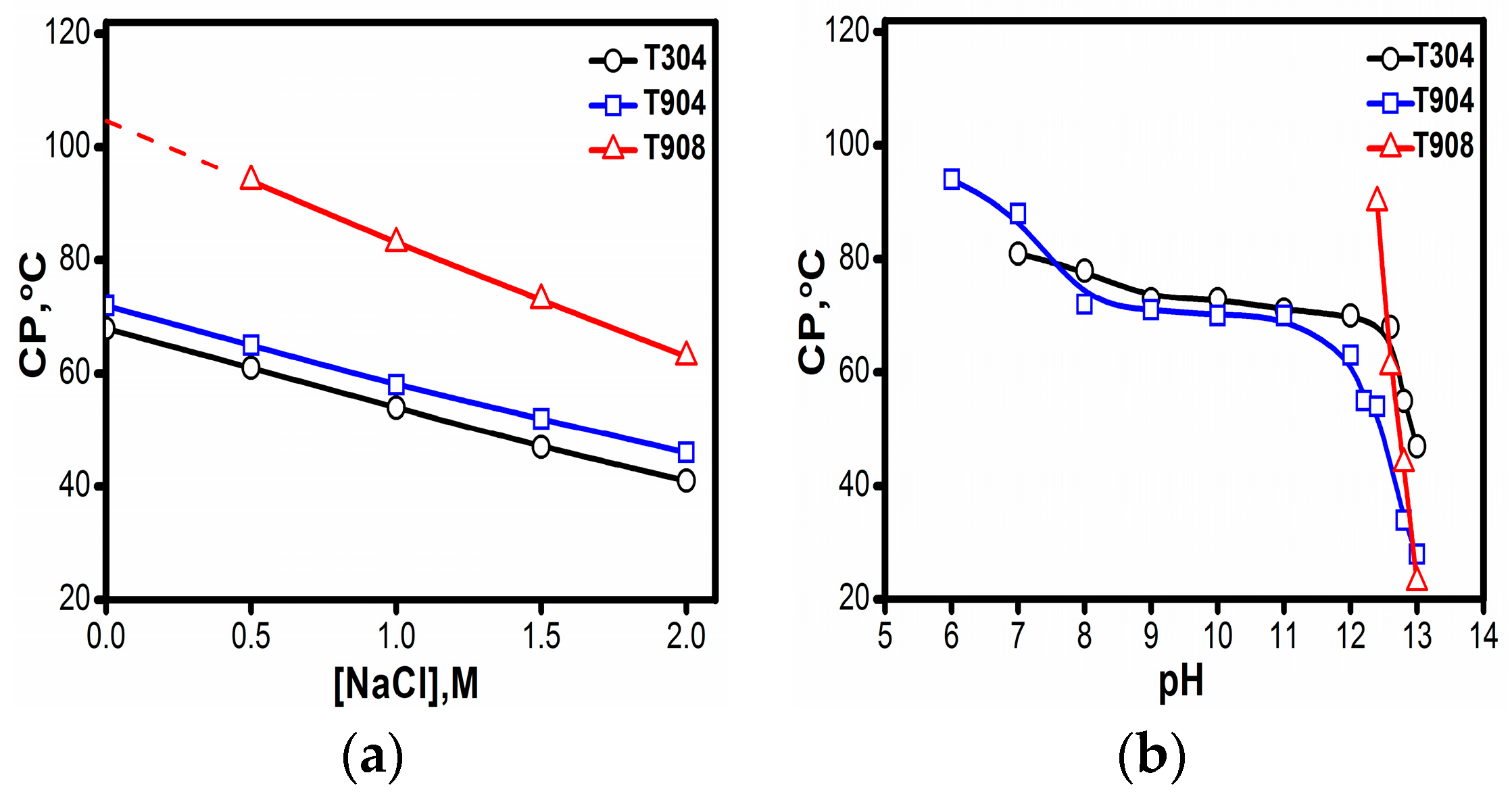
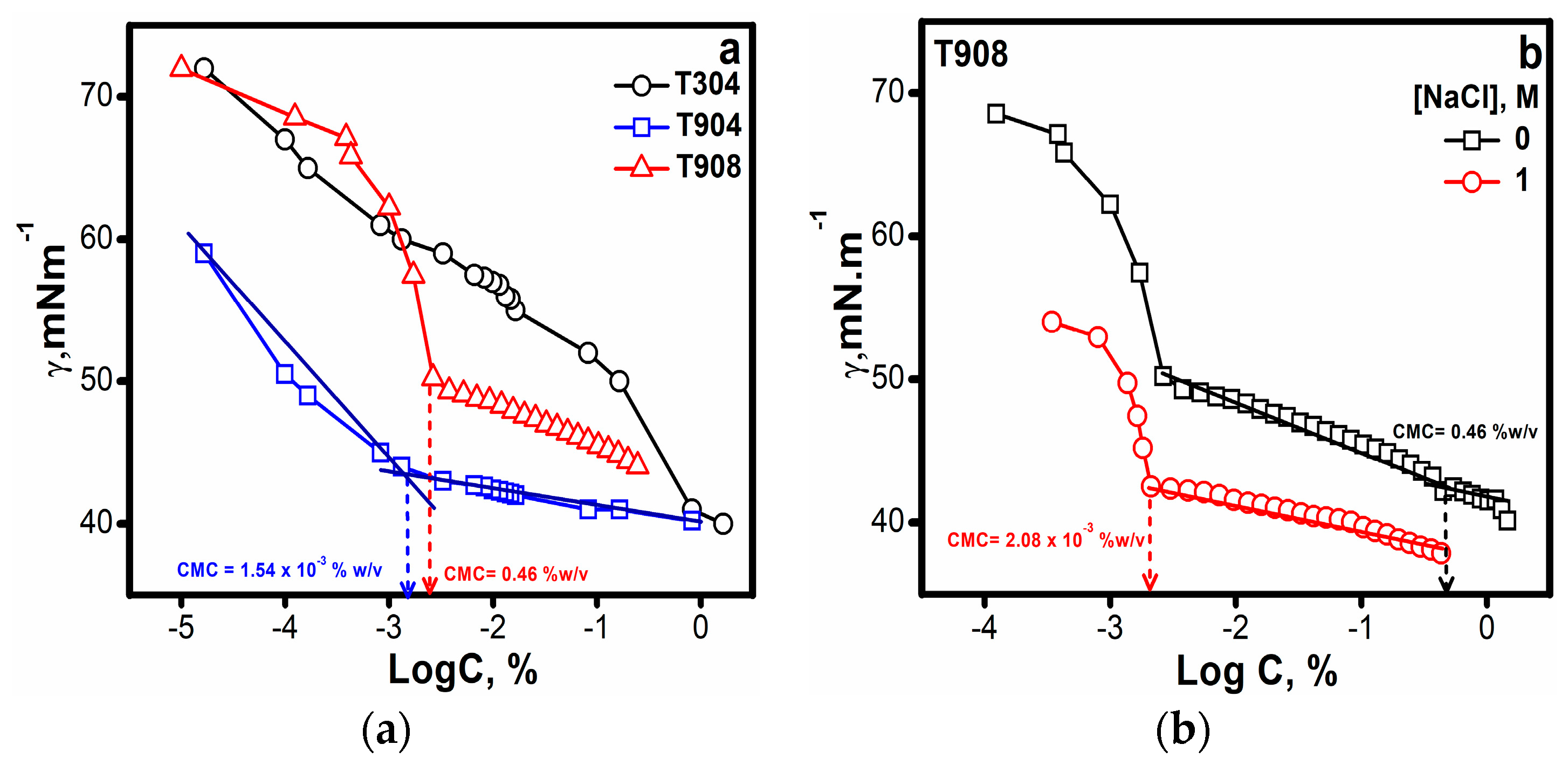
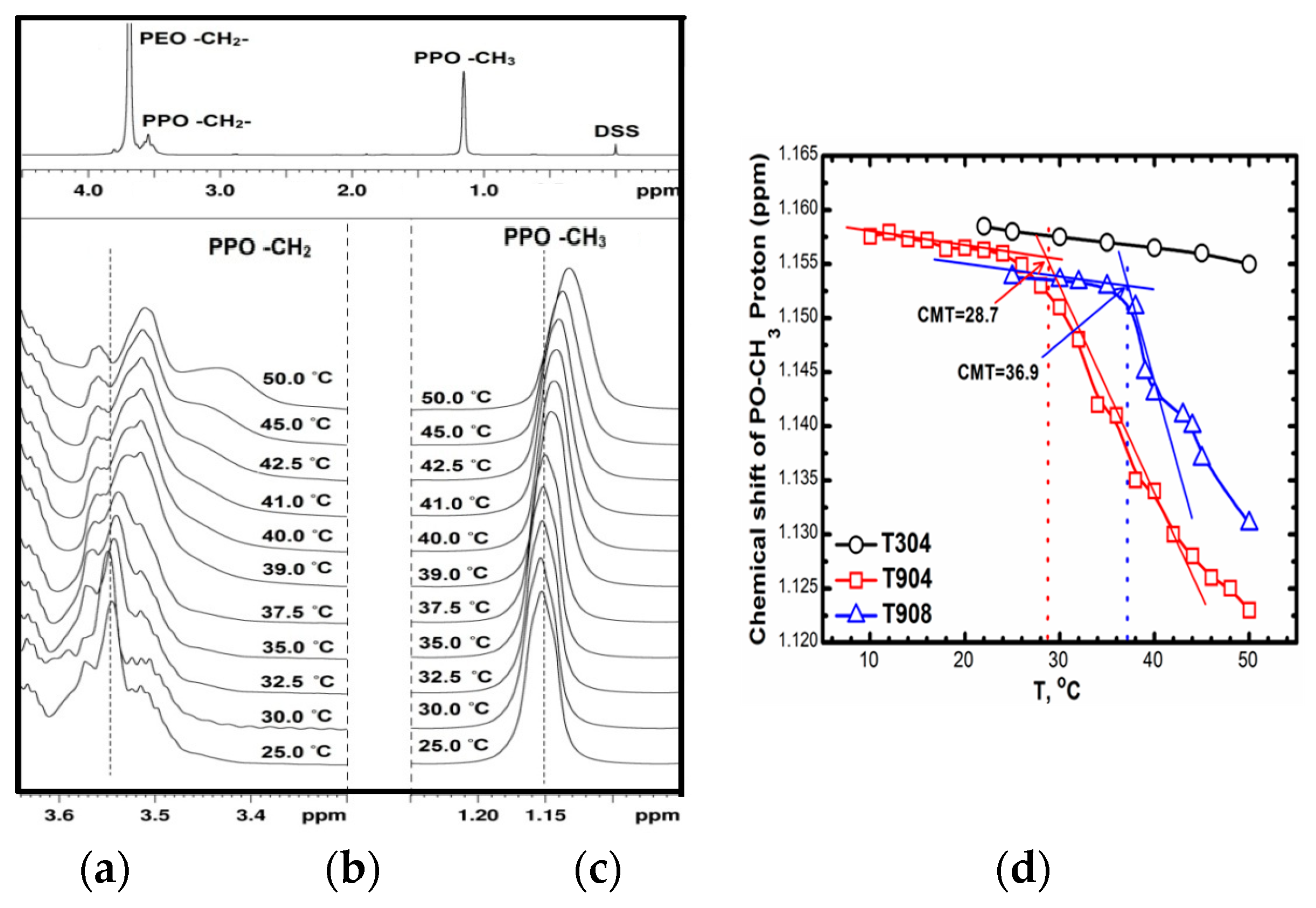

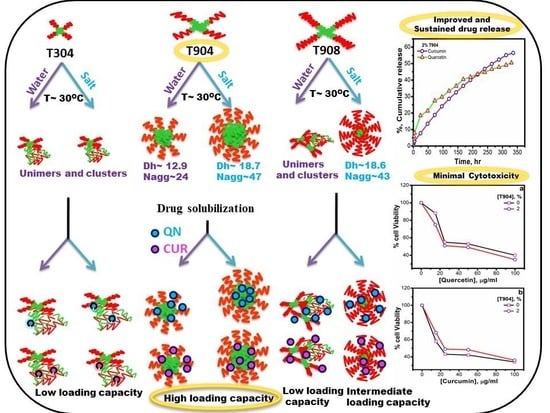
| Tetronic® | Mw a | NEO | NPO | HLB a | CP a (°C) | pKa1 a | pKa2 a |
|---|---|---|---|---|---|---|---|
| T304 | 1650 | 3.7 | 4.3 | 12-18 | 72 | 4.3 | 8.1 |
| T904 | 6700 | 15 | 17 | 12-18 | 78 | 4.0 | 7.8 |
| T908 | 25,000 | 114 | 21 | >24 | >100 | 5.2 | 7.9 |
| [NaCl], M | Tonset (°C) | Tinf (°C) | Tm (°C) | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (kJ/mol·K) |
|---|---|---|---|---|---|---|
| 0 | 32.00 | 32.06 | 37.82 | 133.38 | −26.31 | 0.51 |
| 1 | 19.03 | 19.73 | 24.83 | 178.45 | −25.25 | 0.68 |
| 2 | a | a | 20.26 | a | −24.80 | a |
| Tetronic® | [NaCl], M | Temperature, °C | Rc, Å | Rhs, Å | Rg, Å | Nagg |
|---|---|---|---|---|---|---|
| 5% T304 | 0 | 30 | - | - | 10.6 | - |
| 10% T304 | 0 | 30 | - | - | 11.8 | - |
| 10% T304 | 0 | 35 | - | - | 11.1 | - |
| 10% T304 | 0 | 50 | - | - | 13.0 | - |
| 10% T908 | 0 | 30 | - | - | 25.8 | - |
| 10% T908 | 0 | 40 | 39.9 | - | - | 7 |
| 10% T908 | 0 | 50 | 57.2 | - | - | 10 |
| 10% T908 | 0 | 60 | 60.1 | - | - | 13 |
| 5% T904 * | 0 | 30 | 25.0 | 52.2 | - | 10 |
| 5% T904 * | 0 | 40 | 29.9 | 52.2 | - | 18 |
| 5% T904 * | 0 | 50 | 33.0 | 129.0 | - | 23 |
| 10% T904 | 0 | 30 | 34.7 | 51.6 | - | 24 |
| 10% T904 | 1 M | 30 | 39.9 | 59.6 | - | 36 |
| 10% T904 | 2 M | 30 | 43.61 | 65.7 | - | 47 |
| 10% T908 | 1 M | 30 | 25.36 | 81.6 | - | 8 |
| 10% T908 | 2 M | 30 | 44.3 | 84.9 | - | 43 |
| Drug | Mathematical Models for Drug Release Kinetics | |||||
|---|---|---|---|---|---|---|
| Zero Order | First Order | Higuchi | ||||
| k0 (M. h−1) | R2 | k1 (h−1) | R2 | kH (M. h−1/2) | R2 | |
| curcumin | 0.169 | 0.99 | 0.004 | 0.76 | 3.321 | 0.98 |
| quercetin | 0.131 | 0.90 | 0.002 | 0.69 | 2.69 | 0.99 |
| Solvent | IC50, μg/mL | |
|---|---|---|
| Curcumin | Quercetin | |
| H2O | 18.60 | 55.2 |
| T904 | 45.8 | 48.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyas, B.; Pillai, S.A.; Bahadur, A.; Bahadur, P. A Comparative Study on Micellar and Solubilizing Behavior of Three EO-PO Based Star Block Copolymers Varying in Hydrophobicity and Their Application for the In Vitro Release of Anticancer Drugs. Polymers 2018, 10, 76. https://doi.org/10.3390/polym10010076
Vyas B, Pillai SA, Bahadur A, Bahadur P. A Comparative Study on Micellar and Solubilizing Behavior of Three EO-PO Based Star Block Copolymers Varying in Hydrophobicity and Their Application for the In Vitro Release of Anticancer Drugs. Polymers. 2018; 10(1):76. https://doi.org/10.3390/polym10010076
Chicago/Turabian StyleVyas, Bijal, Sadafara A. Pillai, Anita Bahadur, and Pratap Bahadur. 2018. "A Comparative Study on Micellar and Solubilizing Behavior of Three EO-PO Based Star Block Copolymers Varying in Hydrophobicity and Their Application for the In Vitro Release of Anticancer Drugs" Polymers 10, no. 1: 76. https://doi.org/10.3390/polym10010076