3.1. Films Morphology and Composition
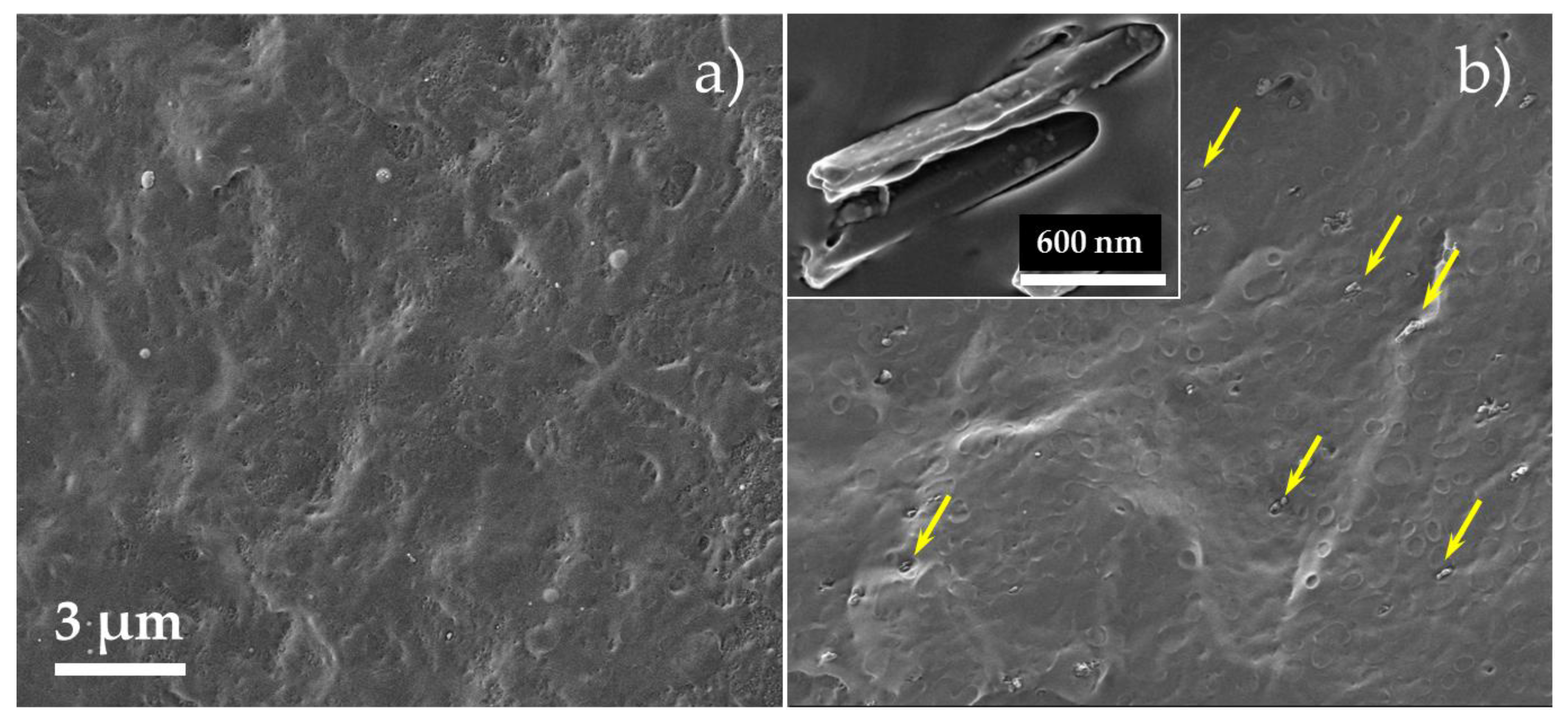
Figure 1 presents HR-SEM images of a cross-sectioned PP/(HNTs-carvacrol) film and the reference neat PP film. Micrographs of PP/carvacrol and PP/HNTs films are shown in
Figure S1 (Supplementary Materials). The HNTs are observed to be well-dispersed in the PP matrix, and aggregates or percolating networks are not detected. These results coincide well with previous studies reporting that HNTs are easily dispersible in polymers in comparison to other silicates, owing to the poor cohesion between the HNTs particles [
52]. The inset in
Figure 1b depicts a high-magnification image of two nanotubes, showing that the HNTs are coated with a thin layer of polymer, which may suggest good interactions between the PP matrix and the HNTs. The latter is further supported in the next chapter, where negative energy of mixing for PP/HNTs is calculated by molecular dynamic simulations, implying good solubility of HNTs in the PP. Yet, the few circular holes observed in
Figure 1b indicate that some of the HNTs are pulled out of the polymeric matrix during the cryofracturing process. This behavior, as well as gaps between the nanotubes and the PP matrix, were observed in several studies and were related to poor interfacial interaction between pristine HNTs and PP [
53].
The carvacrol concentration and distribution within the PP matrix are highly-important parameters, governing the antimicrobial and mechanical properties of the nanocomposites. The total carvacrol content in the films (i.e., post melt-compounding and film production) was determined by TGA, and the results are summarized in
Table 2 (the raw thermograms are presented in
Figure S2, Supplementary Materials). Carvacrol content is determined based on the mass loss of the films, occurring up to a temperature of 250 °C, ascribed to carvacrol evaporation [
34,
35]. For both PP/carvacrol and PP/(HNTs-carvacrol) films, the carvacrol concentration is found to be 3.1%
w/
w, indicating that ~80% of the initial carvacrol content is retained during the high-temperature processing steps. Previous studies have shown much lower carvacrol retention (~30–40%
w/
w) during PP/carvacrol film production by batch melt-compounding and subsequent compression molding [
16,
54]. The results obtained in present study are significantly different and may be ascribed mainly to the processing conditions, i.e., continuous vs. batch melt-compounding and film production (via cast extrusion vs. compression molding). In our case, the total calculated residence time, i.e., for both melt-compounding and cast extrusion, was 3 min. Whereas, when batch compounding was used by Ramos et al., the high-temperature processing time was notably longer (18 min) and therefore, may lead to significant losses of the volatile EO [
16].
It should be noted that the entrapment of carvacrol within HNTs (prior to melt-compounding) did not affect its content within the films, while our previous studies have demonstrated that for LDPE [
35] and polyamide [
36] matrices, carvacrol loading into HNTs was crucial to achieve a high EO content and improved film quality. Thus, the present work indicates that the carvacrol can withstand the harsh conditions (high temperature and shearing) applied during its compounding with PP, resulting in uniform and transparent carvacrol-containing PP films. Moreover, the HNTs did not affect the thermal stability of PP, as witnessed by the similar degradation profile of the nanocomposites and the neat PP (
Figure S2, Supplementary Materials).
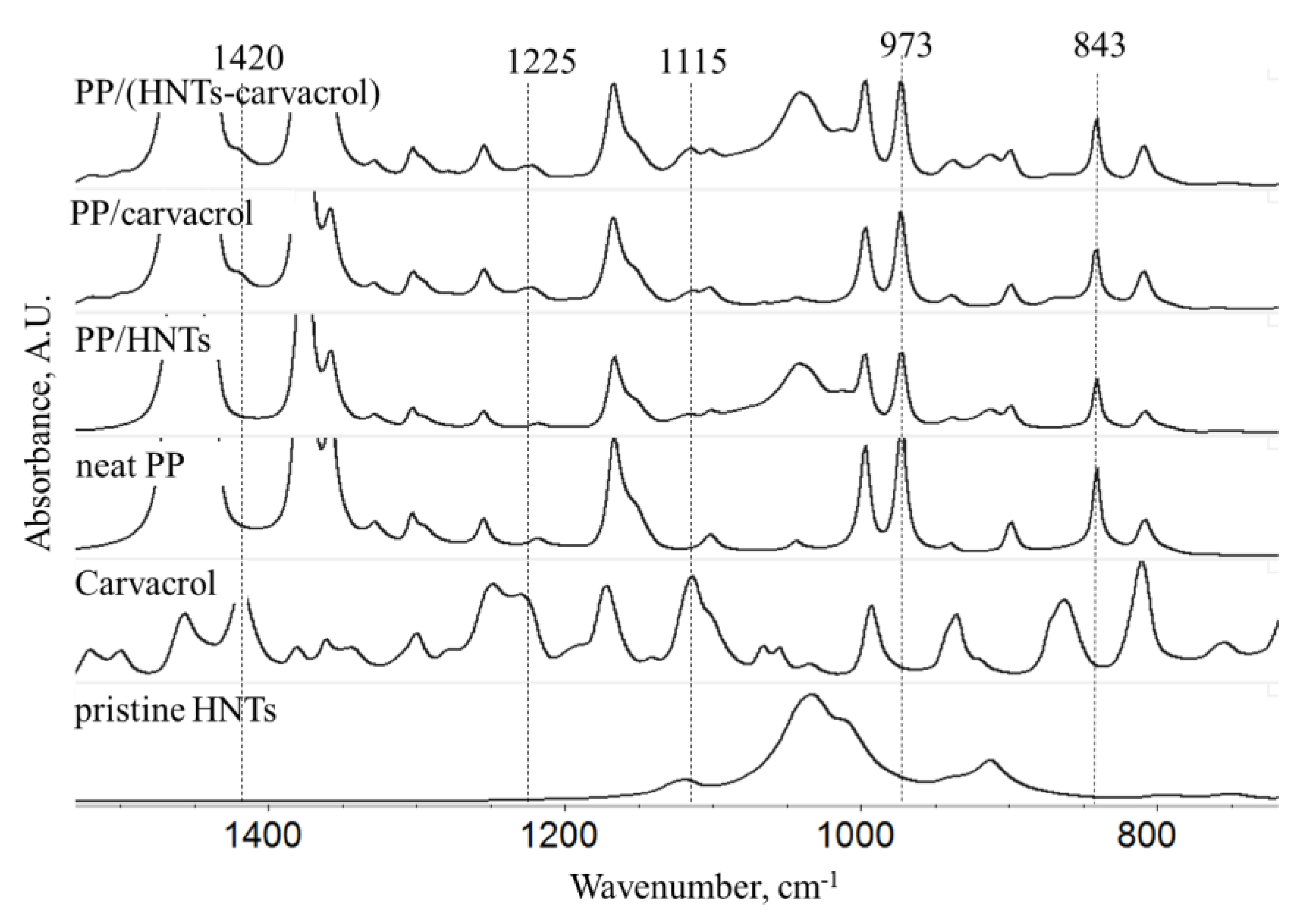
FTIR spectroscopy was used to study the composition of the films, and the results are presented in
Figure 2. The latter figure depicts only the significant region in the FTIR spectra of the studied films and the full spectra are included in
Figure S3 (Supplementary Materials). Spectra of pristine HNTs and carvacrol are also displayed for reference. The HNTs spectrum displays typical absorbance peaks at 3700–3600 cm
−1 (O–H bond stretching), at 1024 and at 750 cm
−1 (perpendicular Si–O stretching), at 900 cm
−1 (O–H deformation), and at 796 cm
−1 (and symmetric stretching of Si–O) [
55]. The small and broad band at 3550 cm
−1 belongs to adsorbed water [
56], indicating its incomplete removal during the overnight drying process. The spectrum of neat carvacrol depicts the strongest absorption in the 900–650 cm
−1 region, ascribed to the C–H out-of-plane vibrations of the aromatic ring. Overtone and combination bands, due to the C–H out-of-plane deformation vibrations, are observed between 2000 and 1660 cm
−1. The absorbance in the regions 1300–1050 cm
−1 and 850–620 cm
−1 stems from vibrational modes of the aromatic carbon-X bond. The spectrum of the neat PP exhibits characteristic strong bands near 2950 cm
−1, 1460 cm
−1 and 1380 cm
−1, and bands of medium intensity around 1155 cm
−1 and 970 cm
−1 [
57]. The spectra of PP/HNTs, PP/carvacrol and PP/(HNTs-carvacrol) films exhibit all the major spectral elements from the individual components. Yet, some of the carvacrol-characteristic peaks, e.g., at 1180 cm
−1 and at 805 cm
−1, are masked by the PP bands.
It should be emphasized that at least five different regions of each film were studied, and the obtained spectra were highly reproducible (data not shown), indicative of their homogeneous composition, further confirming results obtained by HR-SEM (
Figure 1).
3.2. Interactions between PP, HNTs and Carvacrol by Molecular Dynamics Simulations
As the degree of compatibility between the polymer matrix and additive components greatly affects the morphology and structure of the resulting materials [
6], FTIR spectroscopy was also used to investigate interactions between the nanocomposite components. When comparing the IR spectra of the films to those of the neat components (PP, HNTs and carvacrol), no evident shifts in the characteristic peaks are observed, see
Figure 2. As the sensitivity of the FTIR may be insufficient to characterize such interactions [
58] if they exist, we have employed molecular dynamics (MD) simulations as a tool to study the interactions between the nanocomposite components.
The solubility parameters (δ) for PP, HNTs, carvacrol, and their combinations, were calculated using MD simulations and the results are summarized. In addition, we calculated the enthalpy of mixing values (Δ
Hmix) using Equation (3) [
51], and obtained values are included in
Table 3. The energy of mixing values of PP-HNTs and PP-HNTs-carvacrol are both negative and very similar, suggesting that the HNTs and carvacrol interact separately with PP, and they do not interact between themselves. To further investigate this hypothesis, we simulated the RDF for different pairs of components, and the results are presented in
Figure 3. The RDF function measures the unnormalized probability to find an atom of a certain molecule at a predefined distance from another atom placed in the same space [
51]. In this case, the probability of finding a PP carbon atom from a carbon atom on the carvacrol molecule, or from a silicon atom on the HNTs, is roughly the same, as the main interaction distance and the intensity are very similar for both PP-carvacrol and PP-HNTs pairs. On the other hand, the interaction distance of HNTs and carvacrol is much longer, implying that the HNTs do not interact with carvacrol. Therefore, carvacrol and HNTs only interact separately with PP, with no impact on each other, as was evident from the Δ
Hmix calculations (
Table 3).
It should be mentioned that the MD simulations, in this study, cannot take into consideration that carvacrol is entrapped within HNTs prior to film production. This, in turn, may somewhat reduce the predicting power of the calculations for the PP/(HNTs-carvacrol) systems. Nevertheless, the MD simulation results allow us to speculate that the interactions between carvacrol and HNTs in a PP matrix are much weaker than the interactions of carvacrol and PP. Therefore, carvacrol is stabilized during the high-temperature processing by interactions with the PP matrix, rather than by interactions with HNTs. Thus, under the conditions studied in this work, entrapment of carvacrol within HNTs does not result in a higher carvacrol content in the PP-based films.
3.3. Polypropylene Crystalline and Amorphous Phases (DSC, FTIR, WAXS)
The degree of crystallinity as well as the crystalline structure of semi-crystalline polymers plays a key role in determining their resulting properties [
59,
60]. HNTs were shown to influence the crystallization process of different semi-crystalline polymers [
23]. In PP/HNTs nanocomposites, in which the HNTs content was >5%
w/
w, it was shown that the nanotubes can act as a nucleating agent for the alpha and beta crystalline phases, i.e. α-iPP and β-iPP [
61], resulting in an increased PP crystallinity [
62]. The addition of volatile compounds, such as carvacrol, was reported to either increase crystallinity by promoting diffusional mobility of the polymer chains [
22] or to decrease crystallinity owing to interactions of carvacrol with the polymer matrix [
16]. Herein, we have used DSC to characterize the effects of HNTs and carvacrol on the PP crystalline characteristics. Thus, the thermal behavior of the PP/(HNTs-carvacrol) nanocomposite was studied and compared to that of neat PP, PP/carvacrol and PP/HNTs films. The latter films were investigated in an attempt to elucidate the effect of the individual components, i.e., HNTs and carvacrol.
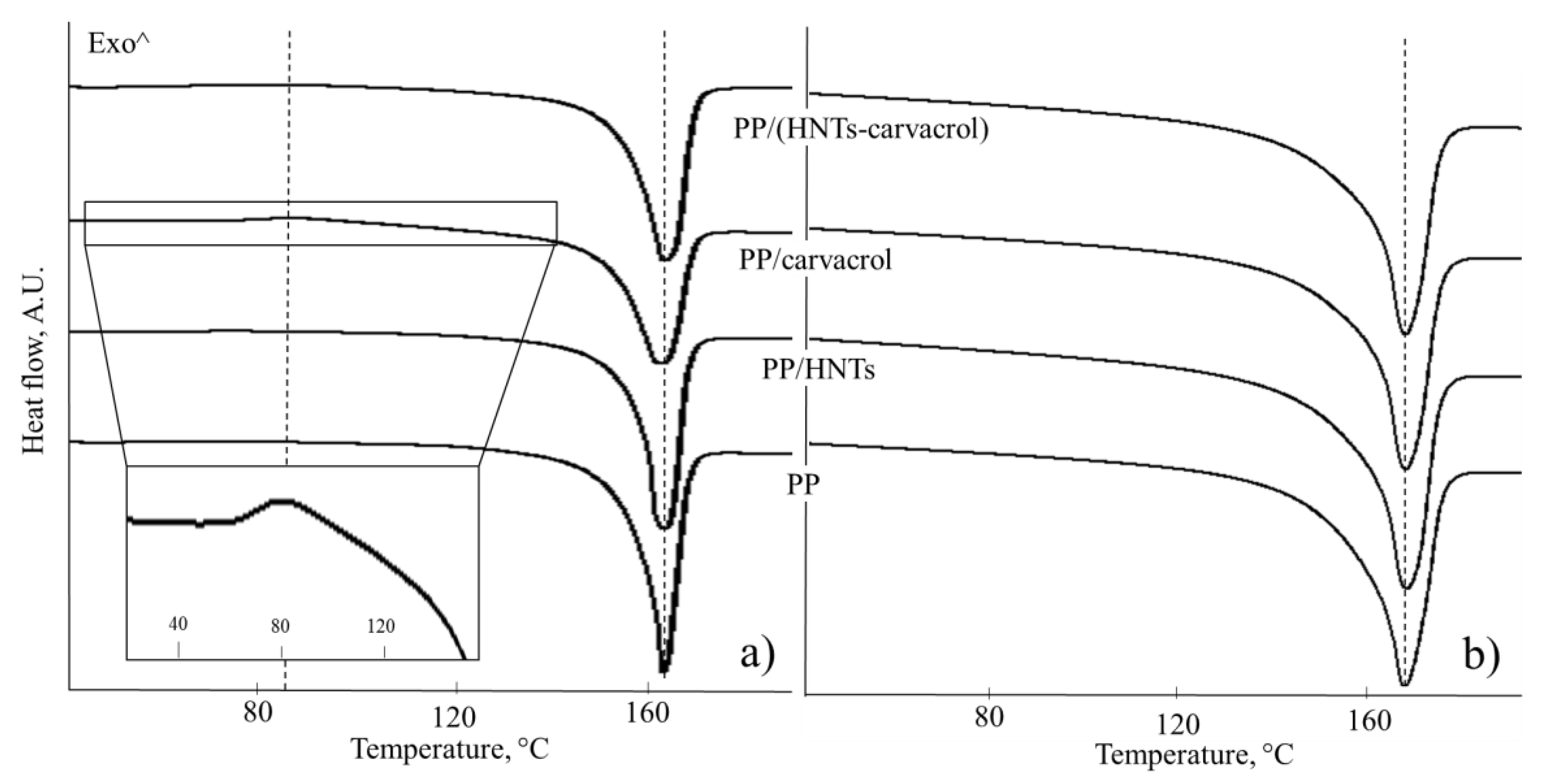
Figure 4 presents the DSC curves (first and second heating cycles) for the studied films and
Table 4 summarizes the thermal characteristics obtained.
The DSC curve of PP/carvacrol film features a clear exothermal peak around 90 °C during the first heating (
Figure 4a, inset), which is assigned to the conversion of a mesomorphic crystalline form to an α–form, upon heating [
63]. This solid–solid transition is very weak and almost undetectable for the PP/(HNTs-carvacrol) films, and is not observed for the neat PP and PP/HNTs films during the first heating, or in any of the studied films upon the second heating. The mesomorphic phase, which is also referred to as paracrystalline or smectic, is a solid phase, where the polymer chains have undergone conformational ordering, from random coils to 3/1 helices, but the helices are not densely packed. As a result, it is characterized by small-ordered clusters of parallel chains in the direction of the chain axes and lateral disorder [
64]. Thus, being highly-ordered in only one direction, it has molecular ordering between amorphous and crystalline phases. The mesomorphic phase may be detected in iPP that was rapidly quenched from the melt, and is readily converted into the more stable α–form, by annealing at elevated temperatures [
65]. In our case, the formation of the mesomorphic phase is facilitated by the addition of carvacrol to the neat PP.
The endothermal peaks in
Figure 4 at around 167 °C are attributed to the melting of α-crystals of the PP [
61].
Figure 4a shows that the fusion peaks of PP (first heating cycle) for the carvacrol-containing films (both PP/carvacrol and PP/(HNTs-carvacrol)) are slightly broader than those for the neat PP and PP/HNTs films, suggesting that carvacrol induces heterogeneity in the PP crystalline structure. The degree of crystallinity, calculated using Equation (1), for the first heating cycle is similar for all studied films. However, the endothermal event of PP melting, between 130 and 170 °C (
Figure 4a), probably masks the endothermal event, which is ascribed to the evaporation of carvacrol from the films. This suggestion is supported by the TGA thermograms (see
Figure S2), where carvacrol is lost from the PP films in this temperature range. Thus, the crystallinity calculated for the first heating cycle, for the carvacrol-containing PP films, is an overestimation and does not reflect the actual crystallinity in the as-produced PP/carvacrol and PP/(HNTs-carvacrol) films. In contrast, the degree of crystallinity calculated from the second heating step is more accurate, as most of the carvacrol is lost during the first heating, and these values are found to be similar for all studied films, see
Table 3. Moreover, the shape of these melting peaks is similar for all studied systems (
Figure 4b) as well as the melting temperature values (~167 °C), which are comparable. Thus, these results indicate that the effect of HNTs (at low concentrations of 2%
w/
w) on PP crystallinity is minor and in agreement with previous studies [
61], where HNTs were found to influence the PP crystalline structure at concentrations higher than 5%
w/
w.
As the overlapping exothermal events of the PP melting and carvacrol evaporation, during the first heating step, prevent accurate determination of the degree of crystallinity in the as-produced PP/carvacrol and PP/(HNTs-carvacrol) films, we have employed both IR spectroscopy and WAXS studies. These techniques are non-destructive and are performed at a room temperature, eliminating artefacts caused by specimen instability, as in the case of DSC, where heating triggers fast carvacrol release from the studied specimen during the measurement [
66].
From the IR spectra of the films, presented in
Figure 2, we have extracted the absorbance values at 841 and 973 cm
−1 and calculated the ratio between these bands (A
841/A
973). The latter ratio was shown to reflect the degree of crystallinity of PP films [
67].
Table 4 summarizes the calculated A
841/A
973 ratio for all films. The PP/HNTs nanocomposites exhibited the highest A
841/A
973 value, greater than those of neat PP films, suggesting a nucleating effect of the HNTs. The lowest A
841/A
973 value was obtained for the PP/carvacrol films, indicating that the addition of carvacrol to the neat PP reduces the PP crystallinity, in comparison to neat PP. The similar values for the A
841/A
973 ratio, determined for both neat PP and PP/(HNTs-carvacrol) systems, suggest that the films exhibit a similar degree of crystallinity, while their crystalline structures may differ. To further elucidate the effects of carvacrol and HNTs on the degree of crystallinity and on the crystalline structure of PP, and to validate the results obtained by the DSC and IR spectroscopy, the films were studied by WAXS (
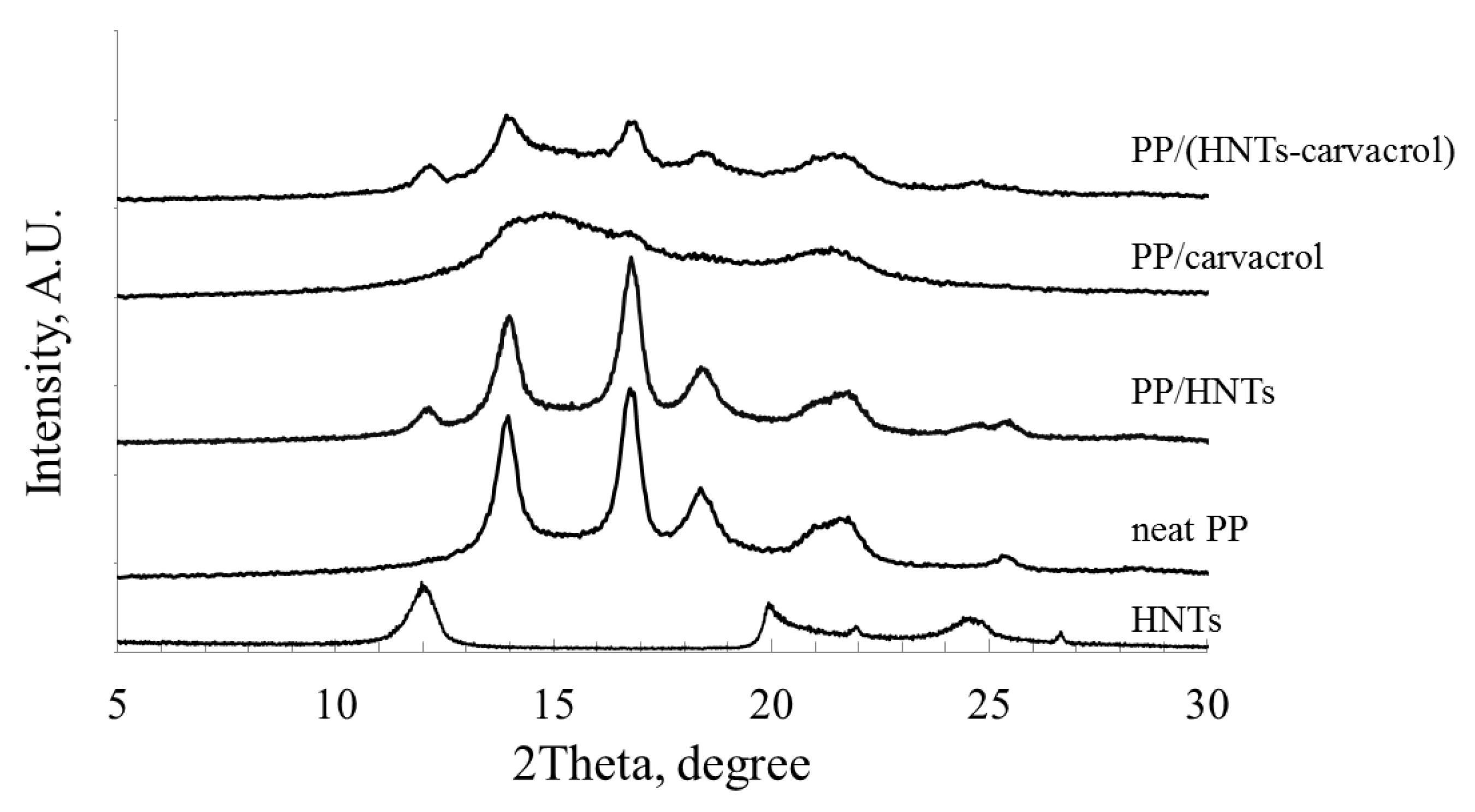
Figure 5).
The WAXS diffractogram for the neat PP exhibits characteristic peaks at 2Θ of 14.0° (6.3 Å, 110 plane), 16.9° (5.2 Å, 040 plane), 18.5° (4.7 Å, 130 plane) and 21.7° (4.1 Å, 111 plane), in agreement with previous studies [
61]. The PP/HNTs diffractogram combines all the major PP-related peaks with the HNTs characteristic peaks, at 2Θ of 12.0° (7.4 Å, 001 plane), and 24.5° (3.6 Å, 002 plane) [
23]. In contrast, in the PP/carvacrol system, the PP-characteristic peaks completely disappear, and only broad and diffuse halos (at 2Θ of 15° and 22°) are observed. This diffractogram was previously shown to characterize the mesomorphic form of iPP homopolymer [
65]. Thus, the WAXS analysis for the PP/carvacrol films confirms the DSC result (
Figure 4, inset), where transition of the mesomorphic crystalline form to an α–form upon heating is observed. In the PP/(HNTs-carvacrol) nanocomposites, the major diffraction features of both PP and HNTs are superimposed onto the mesophase halos (
Figure 5).
The bands from the second order of diffraction are usually observed in highly-ordered samples only, while they tend to disappear in poorly-ordered polymers. The diffractograms of the neat PP and PP/HNTs films feature a broad band at ~28°, ascribed to the second order diffraction from the 110 plane of the PP (corresponding to 2Θ of 14°). This band is barely observable for the PP/(HNTs-carvacrol) system, and cannot detected for the PP/carvacrol films, thus indicating that the degree of molecular order in the PP/carvacrol films is significantly lower than in PP/(HNTs-carvacrol) nanocomposites.
The degree of crystallinity for all films was determined from the WAXS diffractograms after their careful deconvolution, and the results are summarized in
Table 4. The WAXS-based degrees of crystallinity values are in excellent agreement with the IR studies, where the lowest crystallinity is observed for the PP/carvacrol system. The PP/HNTs film crystallinity is slightly higher than that of neat PP, ascribed to the HNTs nucleating effect, and the PP/(HNTs-carvacrol) nanocomposites demonstrate crystallinity similar to the neat PP films. It may be suggested that in the PP/carvacrol films, carvacrol interference with the PP crystalline structure may lead to the formation of a disordered mesomorphic solid state, while the loading of carvacrol upon HNTs helps to preserve a stable and ordered crystalline structure.
The effects of the HNTs and carvacrol on the PP amorphous phase in the nanocomposite films were studied by DSC, and the glass transition temperature (
Tg) values are summarized in (
Table 4). Note that to measure the
Tg, the films were quenched, and a high heating rate of 50 °C·min
−1 was used. Previous work by Persico et al. [
22] has demonstrated the plasticization effect of carvacrol on LDPE matrices. However, the effects of carvacrol and/or HNTs on the PP amorphous phase were not studied, to the best of our knowledge. The displayed
Tg values for both PP/carvacrol and PP/(HNTs-carvacrol) films are significantly lower (by 7 °C and 6 °C, respectively) in comparison to neat PP. These results reveal the carvacrol plasticization effect on the PP matrix. It is worth mentioning that neat PP and PP/HNTs films exhibit a similar
Tg (0 °C) [
63]. This result indicates that for the studied concentration, HNTs presence does not affect the amorphous phase characteristics.
3.4. Mechanical Properties
The structure and orientation of the crystalline and amorphous phases greatly affect the mechanical properties of the polymer [
59]. To elucidate the effects of carvacrol and HNTs on the mechanical properties of the cast films, tensile tests were performed, and
Table 5 summarizes the results of these studies for all films in both machine and transverse directions.
The addition of HNTs to the PP matrix was not found to affect its mechanical properties. Minor, statistically insignificant, changes in the tensile stress at break (TS) were observed, aligning with our DSC and WAXS results, and with other reports [
42], where HNTs had no or only a small influence on the crystallinity and mechanical properties of the PP. The addition of carvacrol to the PP matrix resulted in a profound decrease in the yield stress (YS) and an increase in the elongation at break (EB) values, in both directions. This behavior is attributed to the plasticizing effect of carvacrol, in agreement with the DSC results described in the previous section, see
Table 4. The latter revealed that the carvacrol-containing PP films exhibit lower
Tg values (by ~7 °C) in comparison to neat PP. Our results are also consistent with earlier studies, where carvacrol was reported to increase EB in PP [
16] and LDPE [
22,
68]. Furthermore, carvacrol addition also reduced the characteristic anisotropy in the mechanical properties of PP cast films [
59], specifically, the values of EB. This observation coincides with the WAXS results (
Figure 5), in which the PP/carvacrol films displayed the lowest degree of crystalline order.
The PP/(HNTs-carvacrol) nanocomposites exhibited inferior mechanical properties, in terms of YS and TS values, in comparison to the neat PP and PP/HNTs films. This behavior is ascribed to the plasticizing effect of the “free” carvacrol (carvacrol fraction which is dissolved in the amorphous phase and is not entrapped within the HNTs). Yet, these values are higher than those measured for the PP/carvacrol system, which can be ascribed to complex role of the HNTs in the nanocomposites. First, the HNTs serve as nanocarriers of carvacrol—its concentration in the PP matrix is effectively lower than in the corresponding PP/carvacrol films [
69]. Second, the HNTs act as a nucleating agent in the nanocomposites, leading to a higher crystallinity. The degree of crystallinity of the PP/(HNTs-carvacrol) system is comparable to that of neat PP and PP/HNTs films (see
Table 4) resulting in improved YS and TS values. Noteworthy are the superior EB values, in both machine and transverse directions, exhibited by the nanocomposites and the isotropic behavior of the films.
3.5. Release Studies and Antimicrobial Activity
The release kinetics of the volatile antimicrobial agent from the film is crucial for determining its antimicrobial performance and potential applicability as a packaging material. Moreover, the ability to control the release kinetics is desired for tailoring the material’s properties for a specific application or condition [
6]. TGA was used to characterize the carvacrol release from the different films, by monitoring their weight loss over time, at a constant temperature (60 °C) [
35,
70], and
Table 6 presents the calculated diffusion coefficient values. The results show that the diffusivity of carvacrol in the PP/(HNTs-carvacrol) films is lower by ~30%, in comparison to the corresponding PP/carvacrol system, leading to sustained release of the carvacrol from the nanocomposite. The latter is ascribed to the role of HNTs as: (i) nanocarriers, where the release of carvacrol from the PP/(HNTs-carvacrol) films is determined not only by the diffusion coefficient of carvacrol in the polymer matrix, but also by its out-diffusion from HNTs’s lumen and its desorption rate from HNTs’s surface [
71]; (ii) nano-scale fillers, forming a tortuous diffusion path in the PP matrix [
72,
73]; (iii) a nucleating agent, increasing the crystallinity and facilitating the formation of more ordered crystallites (in comparison to the PP/carvacrol system).
To evaluate the biofunctionality of the carvacrol in the films, in terms of its antimicrobial activity, we studied the effect of the carvacrol-containing films on the growth of common bacterial and fungal microorganisms. The gram-negative
Escherichia coli (
E. coli) was chosen as a model bacterial species, as some of its strains are widely-spread foodborne pathogens. The model fungus is the phytopathogenic
Alternaria alternata (
A. alternate), which was chosen owing to its clinical relevance and its significant implications for food spoilage as well as food and feed safety [
74].
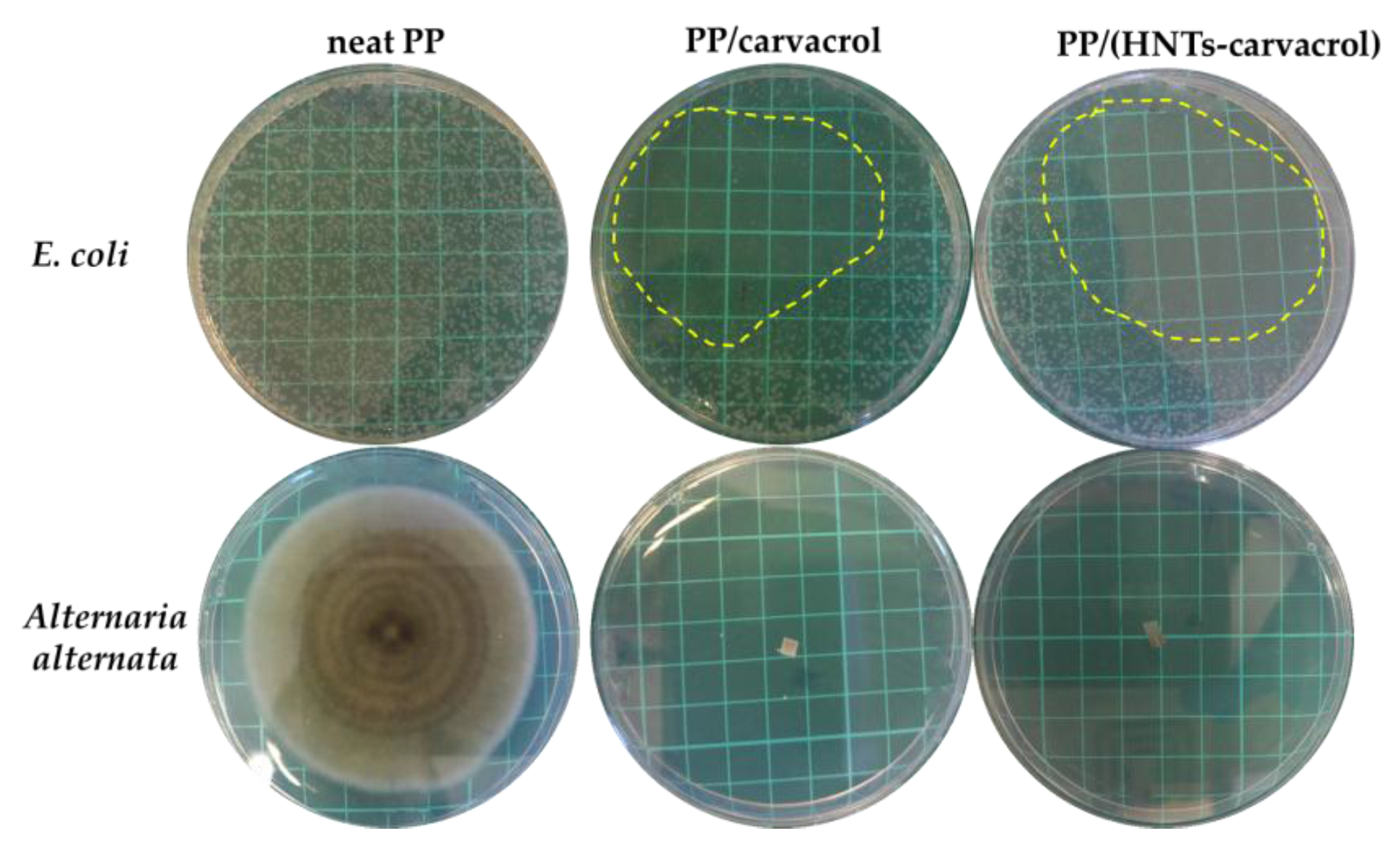
Figure 6 depicts characteristic images of Petri dishes after the exposure of the two model microorganisms to the different films. Both PP/carvacrol and PP/(HNTs-carvacrol) films exhibit a profound inhibition of
E. coli growth, as most of the dish area is observed to be colony-free, see
Figure 6 (upper panel). The carvacrol-containing films also display a strong antifungal activity, completely arresting hyphal growth and sporulation of
A. alternata. Whereas, for the reference neat PP and PP/HNTs (data not shown) films, no effect on bacterial and fungal development is observed. Thus, the high antimicrobial activity of carvacrol, which is related to its ability to damage the integrity of the cell membrane [
75] and interfere with the metabolic activity of the cell [
76], is retained in the films, despite their processing.