Enhanced Oxidation Resistance of Polyphenylene Sulfide Composites Based on Montmorillonite Modified by Benzimidazolium Salt
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Specimens
2.3. Characterization of Material Properties
3. Results and Discussion
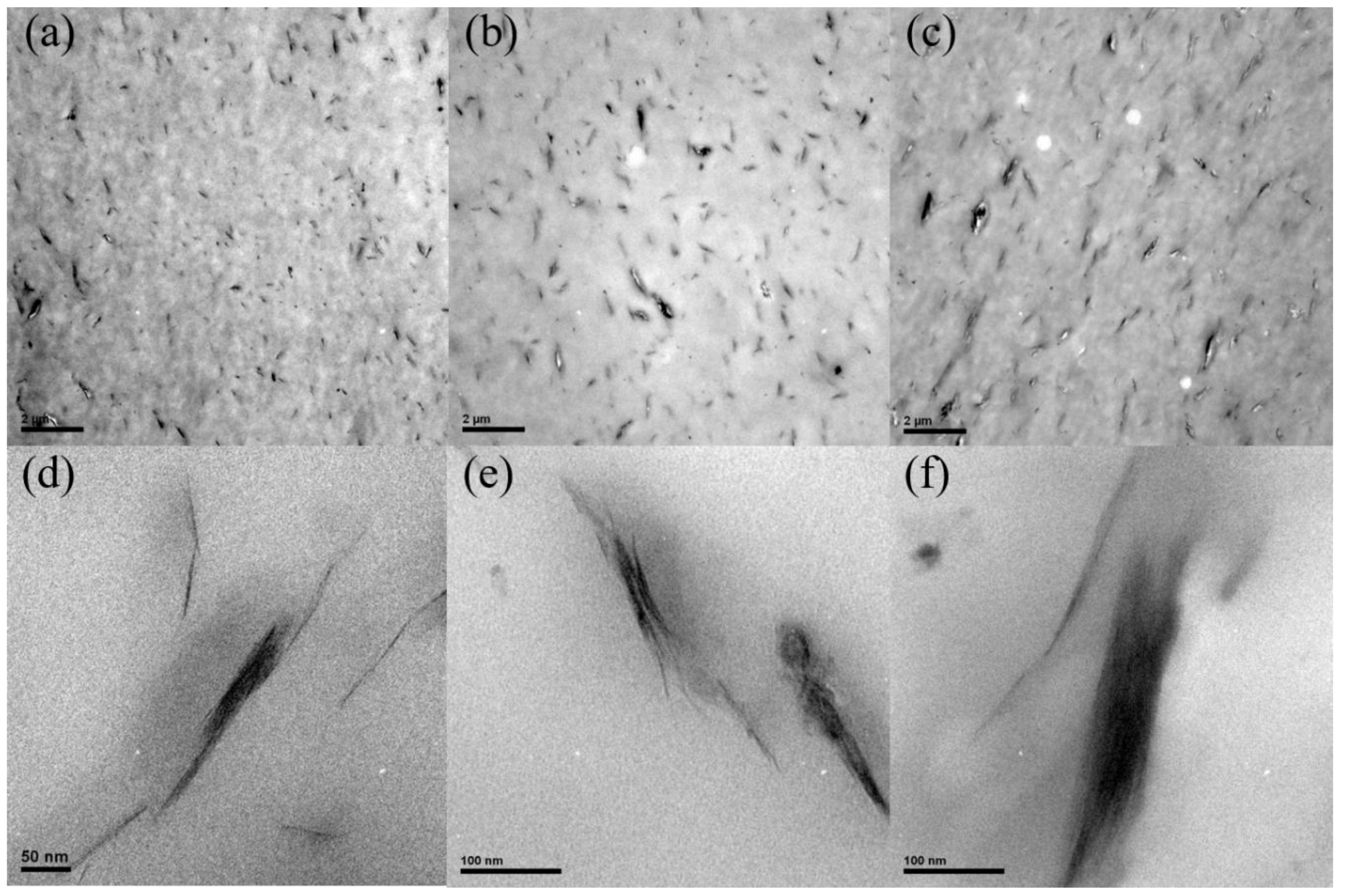
3.1. Morphology of PPSBMx Composites
3.2. Tensile Properties of PPSBMx Composites
3.3. Thermal Degradation of PPSBMx Composites
3.4. Oxidation Resistance of PPSBMx Composites
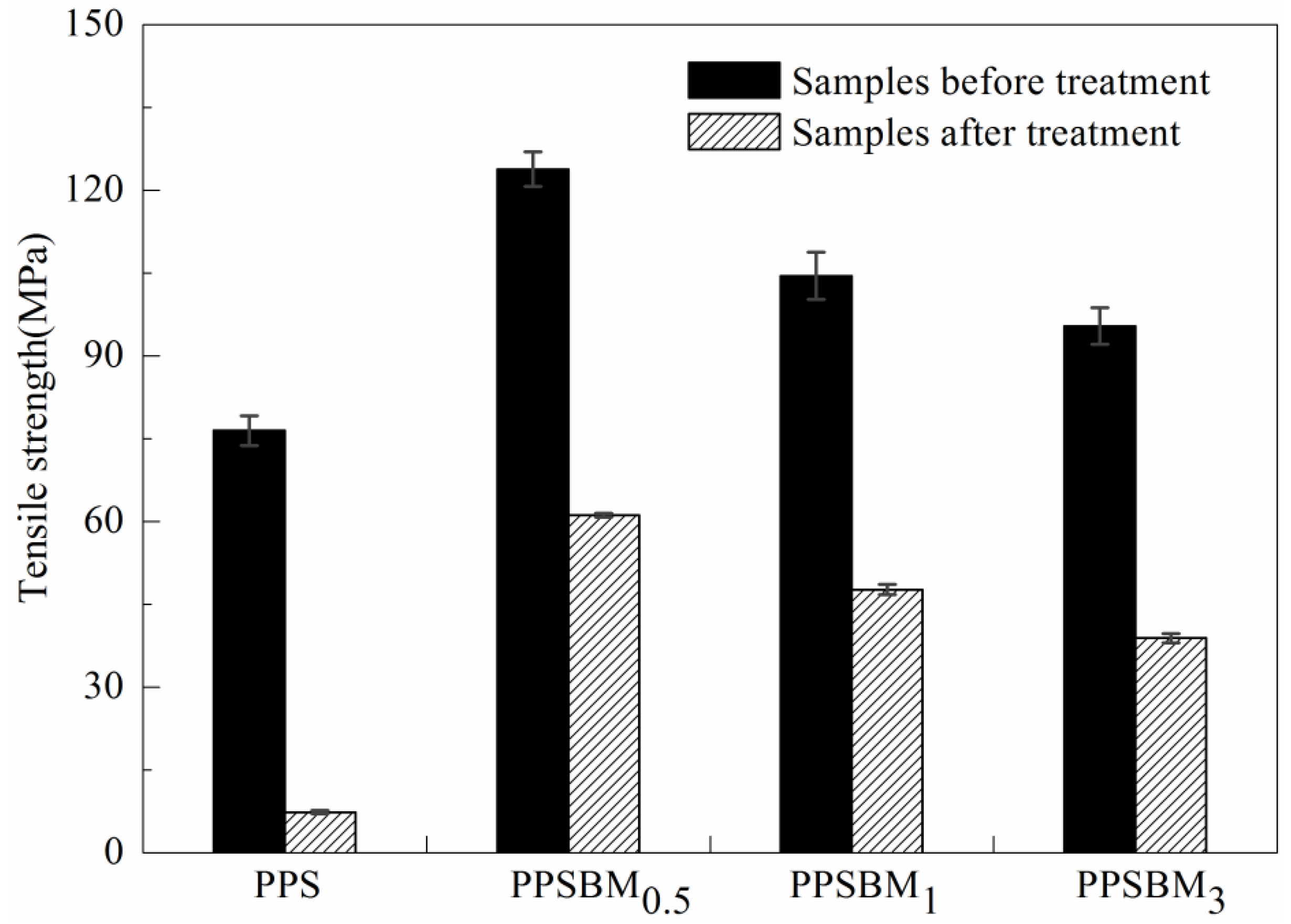
3.4.1. Tensile Strength Analysis
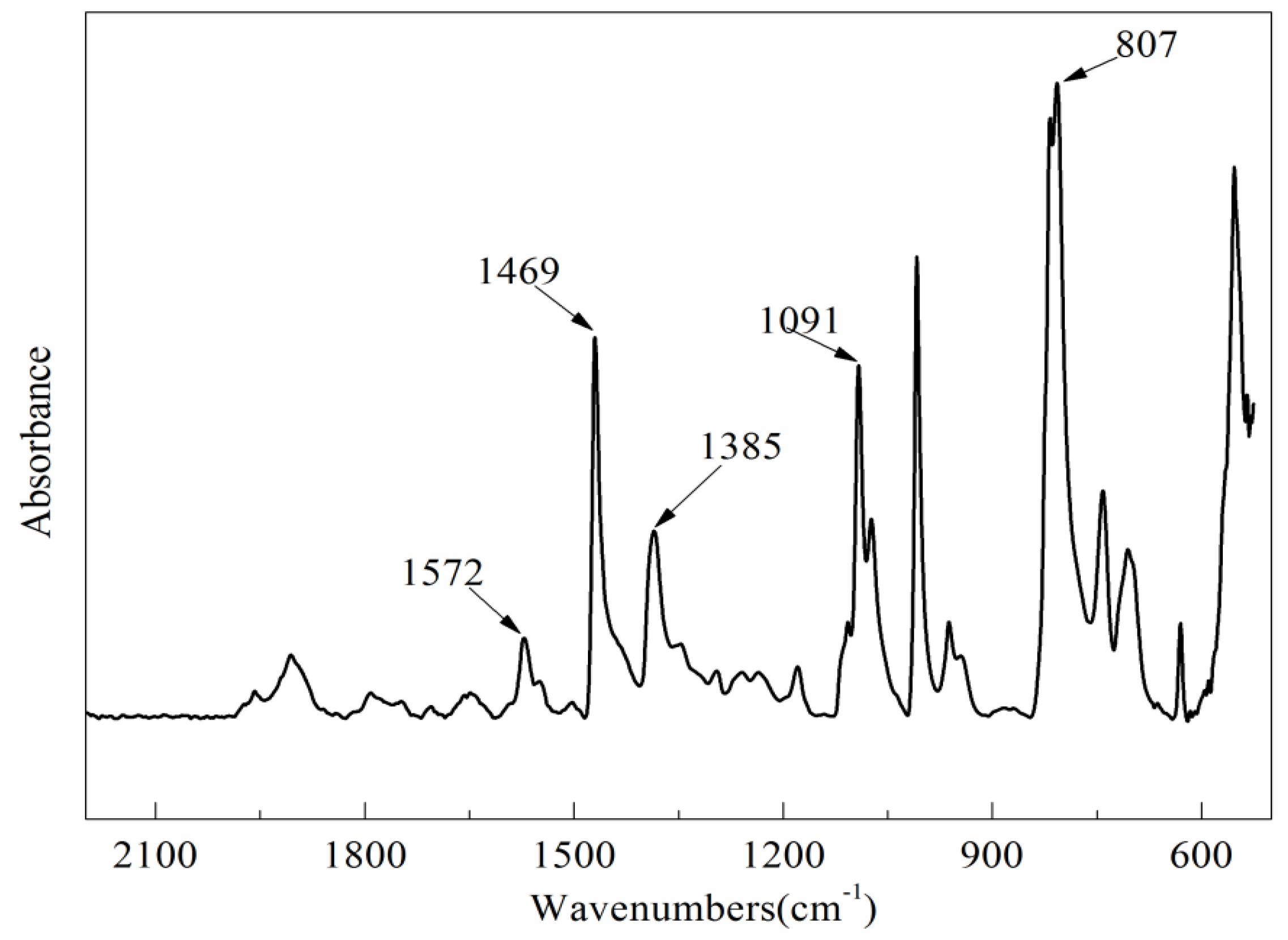
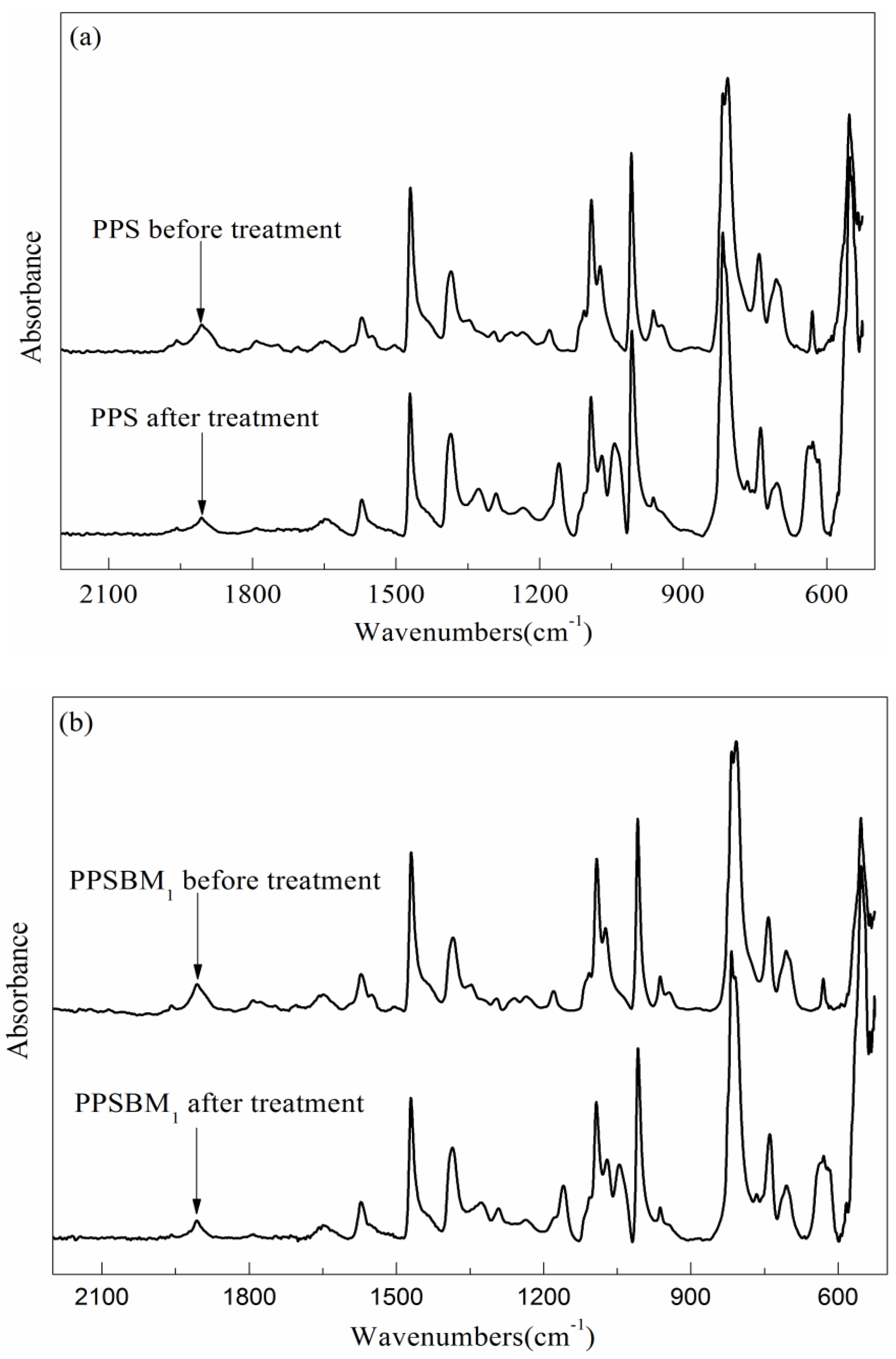
3.4.2. Chemical Functional Groups Analysis
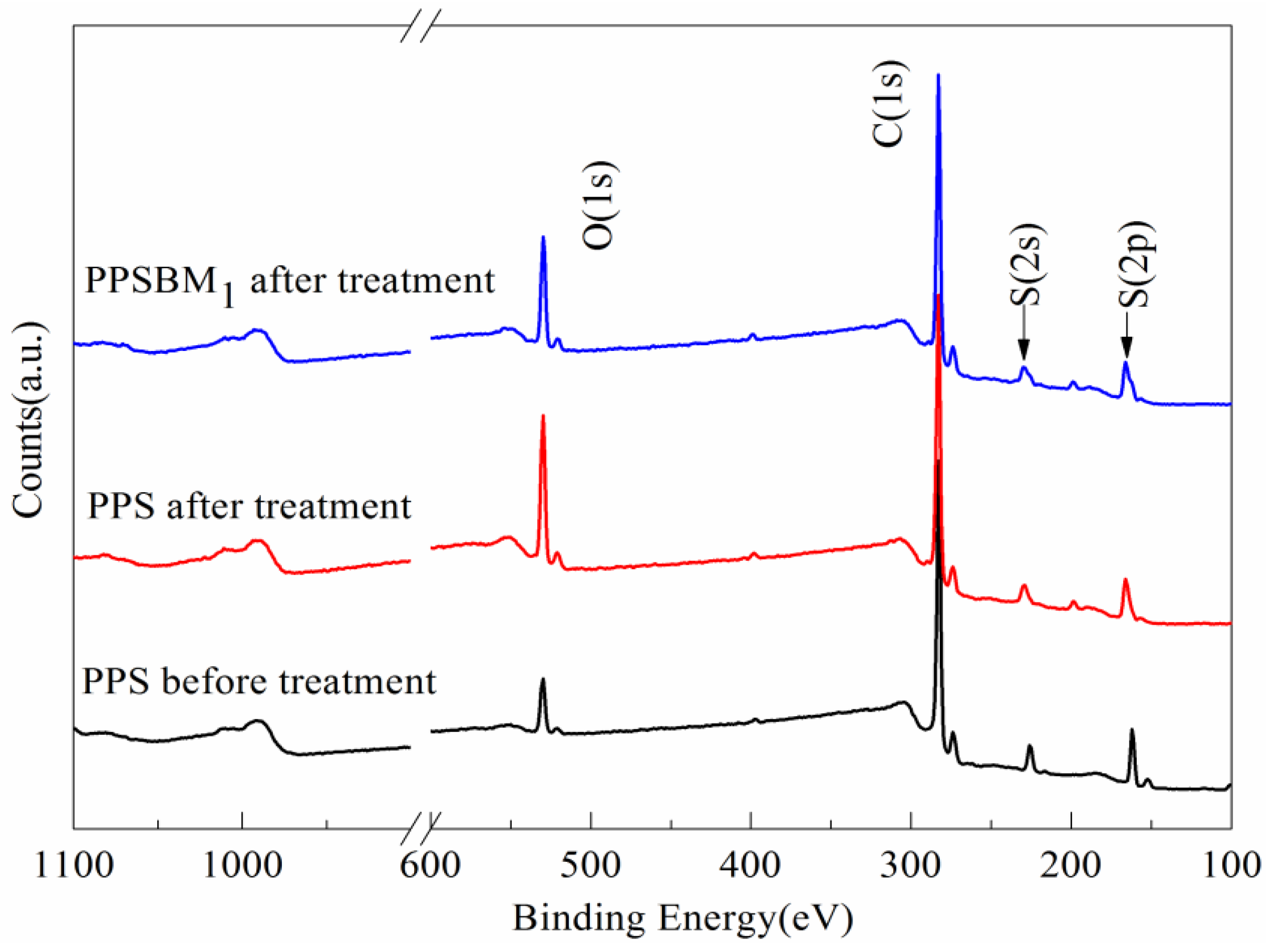
3.4.3. Surface Elements Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deng, S.L.; Cao, L.; Lin, Z.D.; Qiu, W.P.; Liang, K.Y.; Li, W. Nanidiamond as an efficient nucleating agent for polyphenylene sulfide. Thermochim. Acta 2014, 584, 51–57. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Duan, H.J.; Zhang, S.Y.; Niu, P.F.; Zhang, G.; Long, S.R.; Wang, X.J.; Yang, J. Morphology control of nanofillers in poly(phenylene sulfide): A novel method to realize the exfoliation of nanoclay by SiO2 via melt shear flow. Compos. Sci. Technol. 2015, 75, 1685–1693. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Roh, H.Y.; Kim, D.; Byeon, J.; Park, J. Effect of covalent functionalization of MWCNTs on the thermal properties and non-isothermal crystallization behaviors of PPS composites. Polymers 2017, 9, 460. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Zhang, K.; Zhou, Z.; Chen, L.; Chen, Y. Preparation of radiation-resistant high-performance polyphenylene sulfide fibers with improved processing. Procedia Eng. 2012, 27, 1354–1358. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, W.; Chen, Y.; Zou, H.; Liang, M. Enhanced mechanical and thermal properties of CTAB-functionalized graphene oxide-polyphenylene sulfide composites. High Perform. Polym. 2016, 29, 889–898. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, Z.H.; Zhang, M.L.; Cheng, B.W. SiC-fixed organophilic montmorillonite hybrids for poly(phenylene sulfide) composites with enhanced oxidation resistance. RSC Adv. 2017, 7, 46678–46689. [Google Scholar] [CrossRef]
- Tanthapanichakoon, W.; Furuuchi, M.; Nitta, K.-H.; Hata, M.; Endoh, S. Degradation of semi-crystalline PPS bag-filter materials by NO and O2 at high temperature. Polym. Degrad. Stab. 2006, 91, 1637–1644. [Google Scholar] [CrossRef]
- Wan, J.X.; Qin, Y.F.; Li, S.B.; Wang, X.H. Studies on Preparation and Characterization of Anti-oxidizing polyphenylene sulfide. Adv. Mater. Res. 2011, 332–334, 1045–1048. [Google Scholar] [CrossRef]
- Sugama, T. Polyphenylene sulfide/montomorillonite clay nanocomposite coatings: Their efficacy in protecting steel against corrosion. Mater. Lett. 2006, 60, 2700–2706. [Google Scholar] [CrossRef]
- Sugama, T. Antioxidants for retarding hydrothermal oxidation of polyphenylene sulfide coating in geothermal environments. Mater. Lett. 2000, 43, 4282–4290. [Google Scholar] [CrossRef]
- Sheng, X.Q.; Zhang, R.P.; Niu, M.; Yang, H.; Dai, J.M. Preparation of SiO2/PPS fiber and study of its heat-resistant properties. Adv. Mater. Res. 2011, 287–290, 2590–2597. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Yan, B. Study on photo stability of blending modified polyphenylene sulfide fiber. China Syn. Fiber Ind. 2008, 32, 8–11. [Google Scholar]
- Sugama, T.; Gawlik, K. Self-repairing poly (phenylene sulfide) coatings in hydrothermal environments at 200 °C. Mater. Lett. 2003, 57, 4282–4290. [Google Scholar] [CrossRef]
- Oliveira, M.C.L.; Sayeg, I.J.; Ett, G.; Antunes, R.A. Corrosion behavior of polyphenylene sulfide-carbon black-graphite composites for bipolar plates of polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2014, 39, 16405–16418. [Google Scholar] [CrossRef]
- Shi, L.T.; Hu, J.; Lin, X.D.; Fang, L.; Wu, F. A robust superhydrophobic PPS-PTFE/SiO2 composite coating on AZ31 Mg alloy with excellent wear and corrosion resistance properties. J. Alloy. Compd. 2017, 721, 157–163. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, B.Y.; Liu, Q.S.; Lu, Y.Q.; Xing, J. Study on PPS non-woven filter material impregnated by nano SiO2/PTFE emulsion to improve its performance. J. Tex. Res. 2013, 34, 77–81. [Google Scholar]
- Achaby, M.E.; Ennajih, H.; Arrakhiz, F.Z.; Kadib, A.E.; Bouhfid, R. Modification of montmorillonite by novel geminal benzimidazolium surfactant and its use for the preparation of polymer organoclay nanocomposite. Compos. Part B Eng. 2013, 51, 210–317. [Google Scholar] [CrossRef]
- Costache, M.C.; Heidecker, M.J.; Manias, E.; Camino, G.; Frache, A. The influence of carbon nanotubes, organically modified montmorillonites and layered double hydroxides on the thermal degradation and fire retardancy of polyethylene, ethylene-vinylacetate copolymer and polystyrene. Polymer 2007, 48, 6532–6545. [Google Scholar] [CrossRef]
- Martino, L.; Guigo, N.; Berkei, J.G.; Sbirrazzuoli, N. Influence of organically modified montmorillonite and sepiolite clays on the physical properties of bio-based poly(ethylene 2,5-furandicarboxylate). Compos. Part B Eng. 2017, 110, 96–105. [Google Scholar] [CrossRef]
- Shah, K.J.; Shukla, A.D.; Shah, D.O.; Imae, T. Effect of organic modifiers on dispersion of organoclay in polymer composites to improve mechanical properties. Polymer 2016, 97, 525–532. [Google Scholar] [CrossRef]
- Zou, H.; Xu, W.; Zhang, Q.; Fu, Q. Effect of Alkylammonium salt on the dispersion and properties of poly(p-phenylene sulfide)/clay nanocomposites via melt intercalation. J. Appl. Polym. Sci. 2006, 99, 1724–1731. [Google Scholar] [CrossRef]
- Xing, J.; Deng, B.Y.; Liu, Q.S. Effect of benzimidazolium salt on dispersion and properties of polyphenylene sulfide/organic clay nanocomposites via melt intercalation. Fiber Polym. 2015, 16, 1220–1229. [Google Scholar] [CrossRef]
- Winyu, T.; Hata, M.; Nitta, K.H.; Furuuchi, M.; Otani, Y. Mechanical degradation of fliter polymer materials: Polyphenylene sulfide. Polym. Degrad. Stab. 2006, 91, 2614–2621. [Google Scholar]
- Usuki, A.; Hasegawa, N.; Kato, M.; Kobayasgi, S. Polymer-clay nanocomposies, Inorganic Polymeric Nanocomposites and Membranes. Adv. Polym. Sci. 2005, 179, 135–195. [Google Scholar]
- Leswzynska, A.; Njugumna, J.; Pielichowski, K.; Banerjee, J.R. Polymer/montmorillonite nanocomposites with improved thermal properties. Part II. Thermal stability of montmorillonite nanocomposites based on different polymeric matrixes. Thermochim. Acta 2007, 454, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.B. Flame retarded polymer layered silicate nanocomposites: a review of conmmerical and open literature systems. Polym. Adv. Technol. 2006, 17, 206–217. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Gu, J.W.; Xie, C.; Li, H.L.; Dang, J.; Geng, W. Thermal percolation behavior of graphene nanoplatelets/polyphenylene sulfide thermal conductivity composites. Polym. Compos. 2014, 35, 1087–1092. [Google Scholar] [CrossRef]
- Gu, J.W.; Meng, X.D.; Tang, Y.S.; Li, Y.; Zhuang, Q. Hexagonal boron nitride/polymethyl-vinyl siloxane rubber dielectric thermally conductive composites with ideal thermal stabilities. Compos. Part A Appl. Sci. Manuf. 2017, 92, 27–32. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoliu, G.; Park, S.; Stoller, M. Chemical analysis of graphene oxide films after heat and chemical treatment by X-ray photoelectrona and micro-raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin Elmer Corp: Waltham, MA, USA, 1979. [Google Scholar]
- Lian, D.D.; Dai, J.M.; Zhang, R.P.; Niu, M.; Huang, Y. Enhancing the resistance against oxidation of polyphenylene sulphide fiber via incporation of nano TiO2–SiO2 and its mechanistic analysis. Polym. Degrad. Stab. 2016, 129, 77–86. [Google Scholar] [CrossRef]



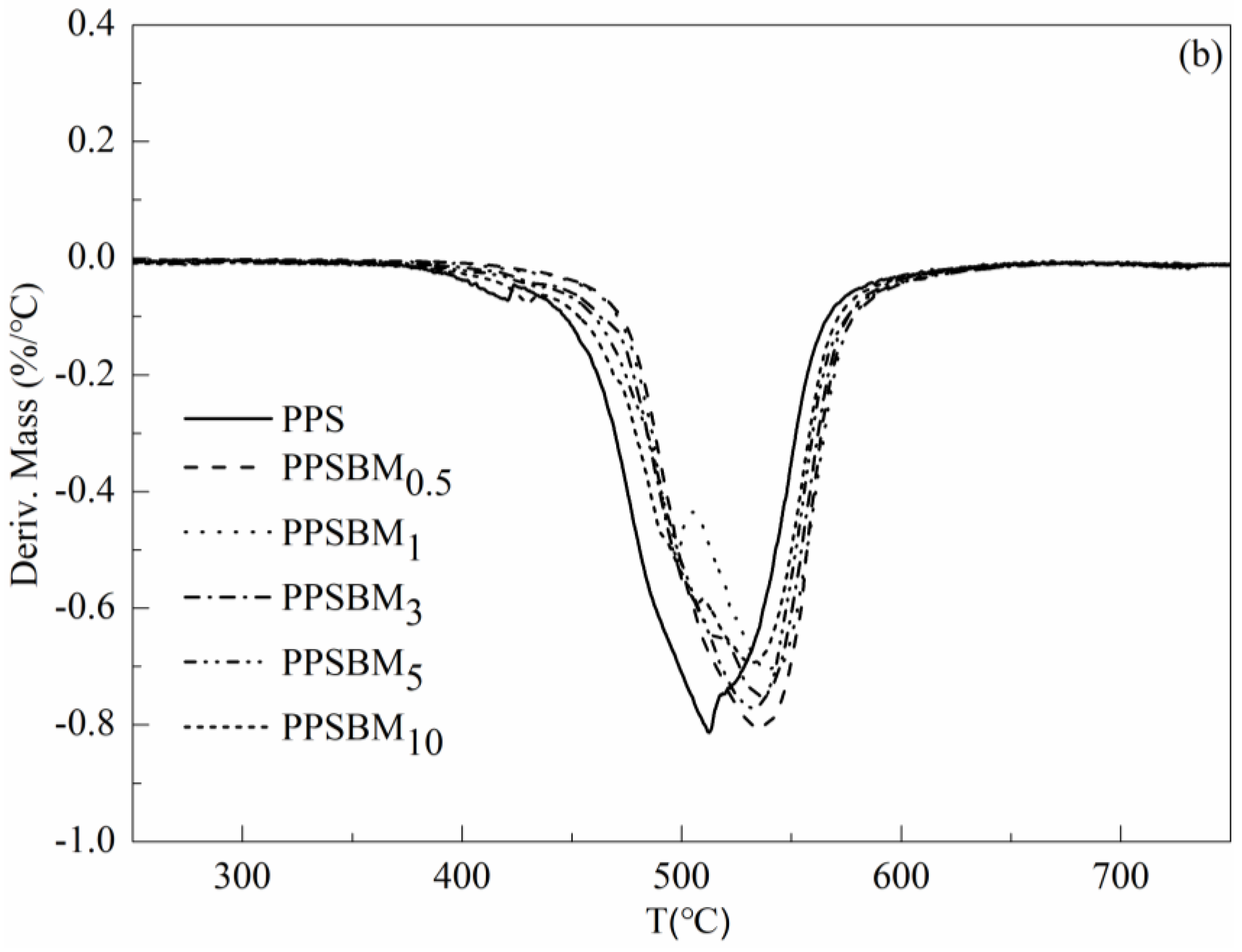

| Samples | T5% (°C) | T15% (°C) | T30% (°C) | T50% (°C) | THRI (°C) | Tmax (°C) |
|---|---|---|---|---|---|---|
| PPS | 452.2 | 484.7 | 507.0 | 533.9 | 237.7 | 512.3 |
| PPSBM0.5 | 484.5 | 508.9 | 528.1 | 554.4 | 250.2 | 536.5 |
| PPSBM1 | 482.9 | 507.1 | 533.5 | 570.1 | 251.5 | 537.8 |
| PPSBM3 | 470.9 | 501.1 | 524.8 | 553.6 | 246.6 | 536.6 |
| PPSBM5 | 463.5 | 498.7 | 522.6 | 550.6 | 244.5 | 532.5 |
| PPSBM10 | 450.6 | 492.1 | 518.4 | 549.1 | 240.7 | 532.1 |
| Wavenumber (cm−1) | Relative Absorbance | |||
|---|---|---|---|---|
| PPS before Treatment | PPS after Treatment | PPSBM1 before Treatment | PPSBM1 after Treatment | |
| 1572 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1178 | 0.31 | 1.97 | 0.27 | 1.76 |
| 1091 | 3.62 | 2.52 | 3.71 | 2.89 |
| 1075 | 1.32 | 0.79 | 0.99 | 0.16 |
| 1044 | - | 2.46 | - | 2.40 |
| 807 | 9.92 | 9.27 | 11.07 | 9.57 |
| Samples | Relative Contents (%) | ||
|---|---|---|---|
| C–S | –SO– | –SO2– | |
| PPS before treatment | 67.4 | 32.6 | - |
| PPS after treatment | 14.1 | 29.6 | 56.3 |
| PPSBM1 after treatment | 18.8 | 15.6 | 65.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, J.; Xu, Z.; Deng, B. Enhanced Oxidation Resistance of Polyphenylene Sulfide Composites Based on Montmorillonite Modified by Benzimidazolium Salt. Polymers 2018, 10, 83. https://doi.org/10.3390/polym10010083
Xing J, Xu Z, Deng B. Enhanced Oxidation Resistance of Polyphenylene Sulfide Composites Based on Montmorillonite Modified by Benzimidazolium Salt. Polymers. 2018; 10(1):83. https://doi.org/10.3390/polym10010083
Chicago/Turabian StyleXing, Jian, Zhenzhen Xu, and Bingyao Deng. 2018. "Enhanced Oxidation Resistance of Polyphenylene Sulfide Composites Based on Montmorillonite Modified by Benzimidazolium Salt" Polymers 10, no. 1: 83. https://doi.org/10.3390/polym10010083





