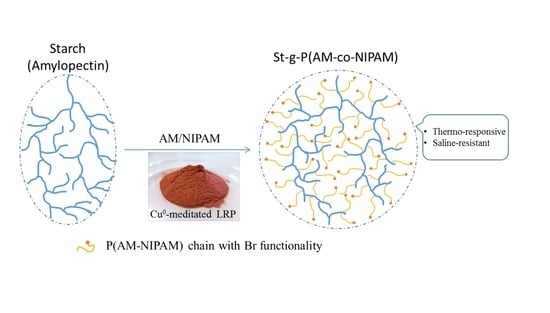
Thermo-Responsive Starch-g-(PAM-co-PNIPAM): Controlled Synthesis and Effect of Molecular Components on Solution Rheology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Starch-Based Macroinitiator (StBr)
2.4. Synthesis of St-g-(PAM-co-PNIPAM) by Aqueous Cu0-Mediated LRP
2.5. Cleaving of Graft Polymer Chains from the Starch Backbone
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kocak, G.; Tuncer, C.; Bütün, V. Ph-responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Q.; Ye, Z.; Liu, H.; Lin, Q.; Nan, K.; Li, Y.; Wang, Y.; Qi, L.; Chen, H. Copolymer brushes with temperature-triggered, reversibly switchable bactericidal and antifouling properties for biomaterial surfaces. ACS Appl. Mater. Interfaces 2016, 8, 27207–27217. [Google Scholar] [CrossRef] [PubMed]
- Zardad, A.-Z.; Choonara, Y.; du Toit, L.; Kumar, P.; Mabrouk, M.; Kondiah, P.; Pillay, V. A review of thermo- and ultrasound-responsive polymeric systems for delivery of chemotherapeutic agents. Polymers 2016, 8, 359. [Google Scholar] [CrossRef]
- Zhang, X.; Pint, C.L.; Lee, M.H.; Schubert, B.E.; Jamshidi, A.; Takei, K.; Ko, H.; Gillies, A.; Bardhan, R.; Urban, J.J.; et al. Optically- and thermally-responsive programmable materials based on carbon nanotube-hydrogel polymer composites. Nano Lett. 2011, 11, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Choi, H. Stimuli-responsive polymers and colloids under electric and magnetic fields. Polymers 2014, 6, 2803–2818. [Google Scholar] [CrossRef]
- Thevenot, J.; Oliveira, H.; Sandre, O.; Lecommandoux, S. Magnetic responsive polymer composite materials. Chem. Soc. Rev. 2013, 42, 7099–7116. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Brooks, W.L.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution: Unexpected properties from known building blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Badi, N. Non-linear peg-based thermoresponsive polymer systems. Prog. Polym. Sci. 2017, 66, 54–79. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Nie, X.; Wen, T.; Ji, Y.; Wu, X.; Zhao, Y.; Chen, C. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J. Am. Chem. Soc. 2014, 136, 7317–7326. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S. Responsive polymers for detection and sensing applications: Current status and future developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Zhang, Q.; Vancoillie, G.; Mees, M.A.; Hoogenboom, R. Thermoresponsive polymeric temperature sensors with broad sensing regimes. Polym. Chem. 2015, 6, 2396–2400. [Google Scholar] [CrossRef]
- Rotzetter, A.C.; Schumacher, C.M.; Bubenhofer, S.B.; Grass, R.N.; Gerber, L.C.; Zeltner, M.; Stark, W.J. Thermoresponsive polymer induced sweating surfaces as an efficient way to passively cool buildings. Adv. Mater. 2012, 24, 5352–5356. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Onuma, T.; Yamato, M.; Takeda, N.; Okano, T. Enhanced wettability changes by synergistic effect of micro/nanoimprinted substrates and grafted thermoresponsive polymer brushes. Macromol. Rapid Commun. 2015, 36, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Van Mastrigt, F.; Stoffelsma, T.; Wever, D.A.Z.; Picchioni, F. Thermoresponsive comb polymers as thickeners for high temperature aqueous fluids. Mater. Today Commun. 2017, 10, 34–40. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Riemsma, E.; Picchioni, F.; Broekhuis, A.A. Comb-like thermoresponsive polymeric materials: Synthesis and effect of macromolecular structure on solution properties. Polymer 2013, 54, 5456–5466. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, T.; Shao, Q.; Jiang, S.; Shen, A.Q. Thermoresponsive self-assembled nipam-zwitterion copolymers. Polym. Chem. 2015, 6, 1066–1077. [Google Scholar] [CrossRef]
- Tran, N.T.; Truong, N.P.; Gu, W.; Jia, Z.; Cooper, M.A.; Monteiro, M.J. Timed-release polymer nanoparticles. Biomacromolecules 2013, 14, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Furyk, S.; Zhang, Y.; Ortiz-Acosta, D.; Cremer, P.S.; Bergbreiter, D.E. Effects of end group polarity and molecular weight on the lower critical solution temperature of poly(n-isopropylacrylamide). J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1492–1501. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(n-isopropylacrylamide). J. Macromol. Sci. Part A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Xia, Y.; Yin, X.; Burke, N.A.D.; Stöver, H.D.H. Thermal response of narrow-disperse poly(n-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 2005, 38, 5937–5943. [Google Scholar] [CrossRef]
- Xue, N.; Qiu, X.-P.; Chen, Y.; Satoh, T.; Kakuchi, T.; Winnik, F.M. Effect of chain architecture on the phase transition of star and cyclic poly(n-isopropylacrylamide) in water. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 2059–2068. [Google Scholar] [CrossRef]
- Xia, Y.; Burke, N.A.D.; Stöver, H.D.H. End group effect on the thermal response of narrow-disperse poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 2006, 39, 2275–2283. [Google Scholar] [CrossRef]
- Ito, M.; Ishizone, T. Living anionic polymerization ofn-methoxymethyl-n-isopropylacrylamide: Synthesis of well-defined poly(N-isopropylacrylamide) having various stereoregularity. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 4832–4845. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(n-isopropylacrylamide) and copolymers: A review on recent progresses in biomedical applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Cheaburu, C.N.; Ciocoiu, O.-N.; Staikos, G.; Vasile, C. Thermoresponsive sodium alginate-g-poly(N-isopropylacrylamide) copolymers iii. Solution properties. J. Appl. Polym. Sci. 2013, 127, 3340–3348. [Google Scholar] [CrossRef]
- Díaz-Silvestre, S.E.; St Thomas, C.; Rivera-Vallejo, C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; de León-Gómez, R.D.; Jiménez-Regalado, E.J. Concentration effect of n-isopropylacrylamide on viscoelastic properties of hydrosoluble thermo-thickening copolymers. Polym. Bull. 2017, 74, 4009–4021. [Google Scholar] [CrossRef]
- Li, X.E.; Xu, Z.; Yin, H.; Feng, Y.; Quan, H. Comparative studies on enhanced oil recovery: Thermoviscosifying polymer versus polyacrylamide. Energy Fuels 2017, 31, 2479–2487. [Google Scholar] [CrossRef]
- Jane, J.-L. Starch: Structure and properties. In Chemical and Functional Properties of Food Saccharides; Tomasik, P., Ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Yoo, S.-H.; Jane, J.-L. Molecular weights and gyration radii of amylopectins determined by high-performance size-exclusion chromatography equipped with multi-angle laser-light scattering and refractive index detectors. Carbohydr. Polym. 2002, 49, 307–314. [Google Scholar] [CrossRef]
- Zobel, H.F. Molecules to granules: A comprehensive starch review. Starch Stärke 1988, 40, 44–50. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Halley, P.J. Starch modification to develop novel starch-biopolymer blends: State of art and perspectives. In Starch Polymers: From Genetic Engineering to Green Applications, 1st ed.; Newnes: Oxford, OK, 2014; pp. 105–143. [Google Scholar]
- Icke, R.N.; Wisegarver, B.B.; Alles, G.A. B-phenylethyldimethylamine. Org. Synth. 1945, 25, 89–92. [Google Scholar]
- Masuelli, M.A.; Sansone, M.G. Hydrodynamic properties of gelatin-studies from intrinsic viscosity measurements. In Products and Applications of Biopolymers; InTech: London, UK, 2012. [Google Scholar]
- Wever, D.A.Z.; Polgar, L.M.; Stuart, M.C.A.; Picchioni, F.; Broekhuis, A.A. Polymer molecular architecture as a tool for controlling the rheological properties of aqueous polyacrylamide solutions for enhanced oil recovery. Ind. Eng. Chem. Res. 2013, 52, 16993–17005. [Google Scholar] [CrossRef]
- Yasuda, K.; Armstrong, R.; Cohen, R. Shear flow properties of concentrated solutions of linear and star branched polystyrenes. Rheol. Acta 1981, 20, 163–178. [Google Scholar] [CrossRef]
- Carreau, P.J. Rheological equations from molecular network theories. Trans. Soc. Rheol. 1972, 16, 99–127. [Google Scholar] [CrossRef]
- Beattie, D.A.; Addai-Mensah, J.; Beaussart, A.; Franks, G.V.; Yeap, K.Y. In situ particle film atr ftir spectroscopy of poly (N-isopropyl acrylamide) (pnipam) adsorption onto talc. Phys. Chem. Chem. Phys. 2014, 16, 25143–25151. [Google Scholar] [CrossRef] [PubMed]
- Chiklis, C.K.; Grasshoff, J.M. Swelling of thin films. I. Acrylamide–n-isopropylacrylamide copolymers in water. J. Polym. Sci. Part A-2 Polym. Phys. 1970, 8, 1617–1626. [Google Scholar] [CrossRef]
- Arvidson, S.A.; Rinehart, B.T.; Gadala-Maria, F. Concentration regimes of solutions of levan polysaccharide from bacillus sp. Carbohydr. Polym. 2006, 65, 144–149. [Google Scholar] [CrossRef]
- Lee, J.; Tripathi, A. Intrinsic viscosity of polymers and biopolymers measured by microchip. Anal. Chem. 2005, 77, 7137–7147. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, D.; Zhong, H. Study on flow behavoirs of viscoelastic polymer solution in micropore with dead end. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: Richardson, TX, USA, 2006. [Google Scholar]
- Zhu, D.; Zhang, J.; Han, Y.; Wang, H.; Feng, Y. Laboratory study on the potential eor use of hpam/ves hybrid in high-temperature and high-salinity oil reservoirs. J. Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Picout, D.R.; Ross-Murphy, S.B. Thermoreversible and Irreversible Physical Gels from Biopolymers; Marcel Dekker, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Saito, S. Salt effect on polymer solutions. J. Polym. Sci. Part A Polym. Chem. 1969, 7, 1789–1802. [Google Scholar] [CrossRef]
- Gou, S.; He, Y.; Ma, Y.; Luo, S.; Zhang, Q.; Jing, D.; Guo, Q. A water-soluble antimicrobial acrylamide copolymer containing sulfitobetaine for enhanced oil recovery. RSC Adv. 2015, 5, 51549–51558. [Google Scholar] [CrossRef]
- Du, H.; Wickramasinghe, R.; Qian, X. Effects of salt on the lower critical solution temperature of poly(N-isopropylacrylamide). J. Phys. Chem. B 2010, 114, 16594–16604. [Google Scholar] [CrossRef] [PubMed]





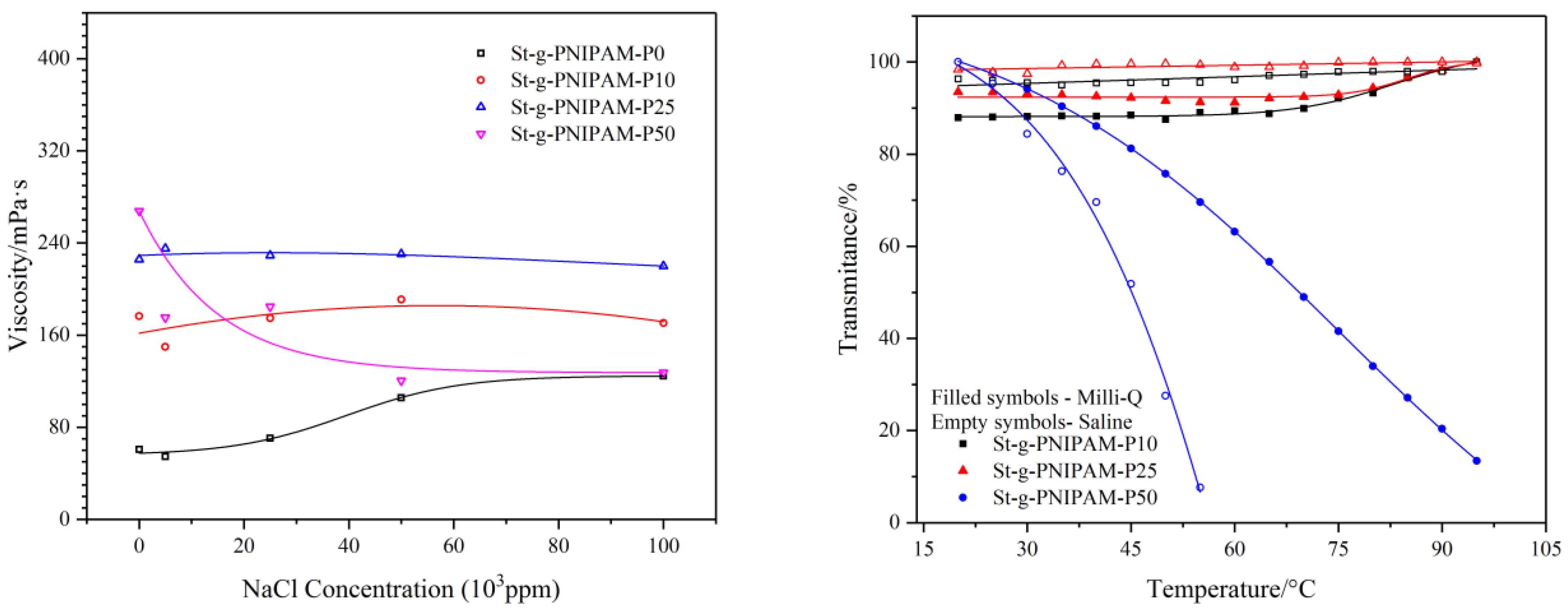
| Sample | Monomer Ratio a (AM:NIPAM) | Time/min | Conversion/% b | Ratio c (NIPAM) | DP d | PDI e | |
|---|---|---|---|---|---|---|---|
| AM | NIPAM | ||||||
| St-g-PNIPAM-P0 | 100:0 | 12 | 91.56 | - | 0 | 5554 | 1.36 |
| St-g-PNIPAM-P10 | 90:10 | 12 | 80.66 | 80.66 | 10 | 4840 | 1.64 |
| St-g-PNIPAM-P25 | 75:25 | 15 | 92.57 | 82.95 | 23 | 5410 | - f |
| St-g-PNIPAM-P50 | 50:50 | 12 | 87.08 | 77.22 | 47 | 4929 | - f |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Boulif, N.; Picchioni, F. Thermo-Responsive Starch-g-(PAM-co-PNIPAM): Controlled Synthesis and Effect of Molecular Components on Solution Rheology. Polymers 2018, 10, 92. https://doi.org/10.3390/polym10010092
Fan Y, Boulif N, Picchioni F. Thermo-Responsive Starch-g-(PAM-co-PNIPAM): Controlled Synthesis and Effect of Molecular Components on Solution Rheology. Polymers. 2018; 10(1):92. https://doi.org/10.3390/polym10010092
Chicago/Turabian StyleFan, Yifei, Nadia Boulif, and Francesco Picchioni. 2018. "Thermo-Responsive Starch-g-(PAM-co-PNIPAM): Controlled Synthesis and Effect of Molecular Components on Solution Rheology" Polymers 10, no. 1: 92. https://doi.org/10.3390/polym10010092