Cost-Effective, Highly Selective and Environmentally Friendly Superhydrophobic Absorbent from Cigarette Filters for Oil Spillage Clean up
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
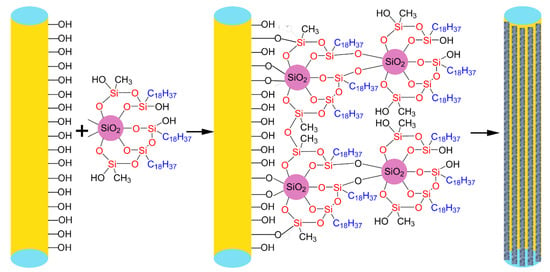
2.2. Fabrication of Hydrophobic SiO2 Particles
2.3. Fabrication of Superhydrophobic Cigarette Filter
2.4. Characterization
2.5. Wettability of Modified CF
2.6. TGA Analyses
2.7. Evaluation of Sorption Capacities of Modified CF for Oils or Organic Solvents
2.8. Recyclability of Modified CF
2.9. Evaluation of Oil Sorption Selectivity of Modified CF
2.10. Qualitative Analysis of Oil-water Separation Efficiency
3. Results and Discussion
3.1. Fabrication of Superhydrophobic Cigarette Filters
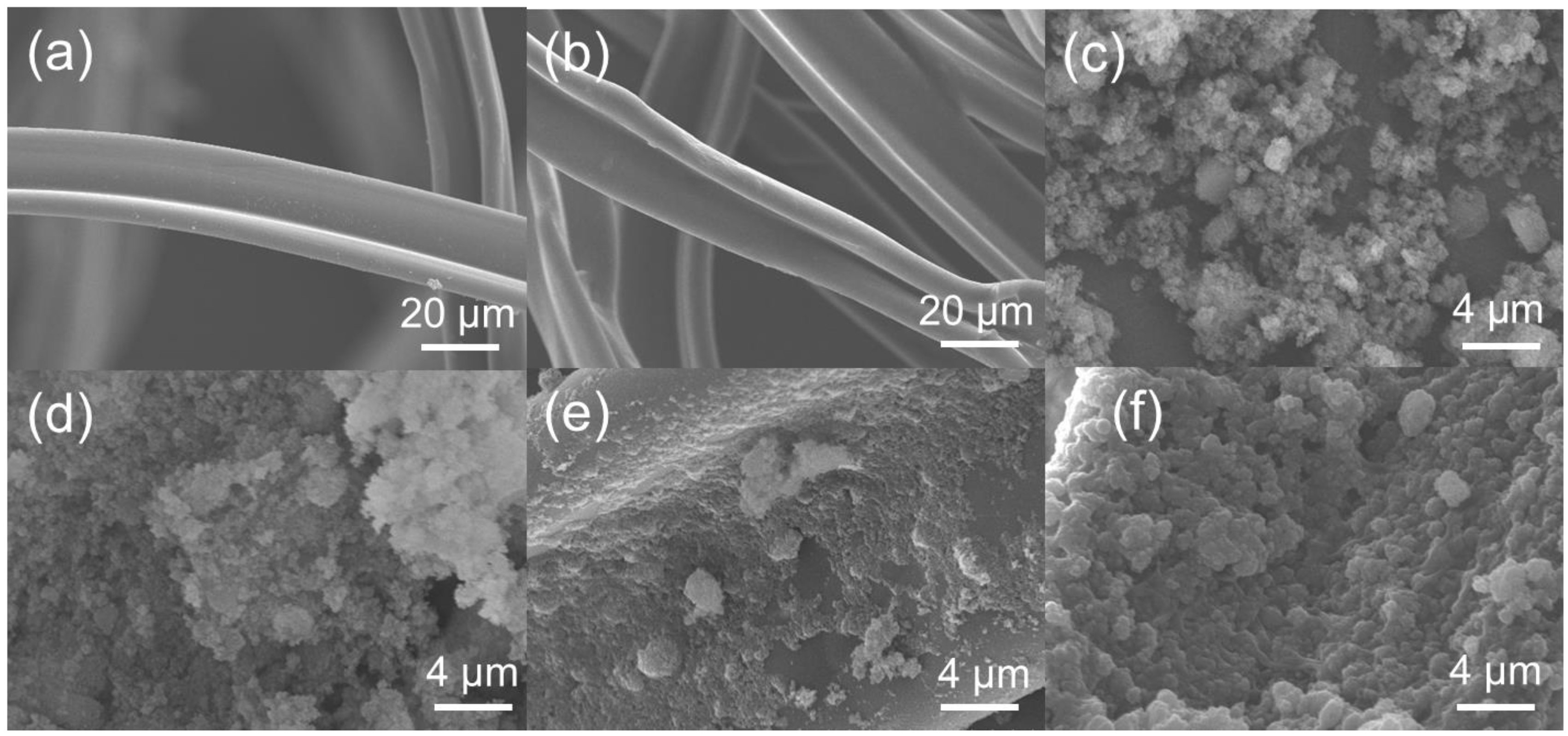
3.2. Surface Morphology and Characterization of Modified Cigarette Filters
3.3. Hydrophobicity of SiO2@OTS@MTMS-modified Cigarette Filters
3.4. Elasticity Property of Modified Cigarette Filters
3.5. Oil Selectivity Absorption
3.6. Reusability
3.7. Oil Adsorption Capacities of Different Hydrophobic Cigarette Filters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mackay, M.E.; Tuteja, A.; Duxbury, P.M.; Hawker, C.J.; Horn, B.V.; Guan, Z.; Chen, G.; Krishnan, R.S. General Strategies for Nanoparticle Dispersion. Science 2006, 311, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.L. Regulating the disposal of cigarette butts as toxic hazardous waste. Tob. Control 2011, 20, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, E.; Gersberg, R.M.; Watanabe, K.; Rudolph, J.; Stransky, C.; Novotny, T.E. Toxicity of cigarette butts, and their chemical components, to marine and freshwater fish. Tob. Control 2011, 20, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Roder Green, A.L.; Putschew, A.; Nehls, T. Littered cigarette butts as a source of nicotine in urban waters. J. Hydrol. 2014, 519, 3466–3474. [Google Scholar] [CrossRef]
- Ou, J.; Wan, B.; Wang, F.; Xue, M.; Wu, H.; Li, W. Superhydrophobic fibers from cigarette filters for oil spill cleanup. RSC Adv. 2016, 6, 44469–44474. [Google Scholar] [CrossRef]
- Mohajerani, A.; Kadir, A.A.; Larobina, L. A practical proposal for solving the world’s cigarette butt problem: Recycling in fired clay bricks. Waste Manag. 2016, 52, 228–244. [Google Scholar] [CrossRef] [PubMed]
- D’HENI Teixeira, M.B.; Duarte, M.A.; Raposo Garcez, L.; Camargo Rubim, J.; Hofmann Gatti, T.; Suarez, P.A. Process development for cigarette butts recycling into cellulose pulp. Waste Manag. 2016, 60, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Novotny, T.E.; Lum, K.; Smith, E.; Wang, V.; Barnes, R. Cigarettes Butts and the Case for an Environmental Policy on Hazardous Cigarette Waste. Int. J. Environ. Res. Public Health 2009, 6, 1691–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beshkar, F.; Khojasteh, H.; Salavati-Niasari, M. Recyclable magnetic superhydrophobic straw soot sponge for highly efficient oil/water separation. J. Colloid Interface Sci. 2017, 497, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, X.; Zhong, L.; Tan, J.; Jing, S.; Cao, X.; Chen, W.; Liu, C.; Sun, R. An ultralight, elastic, cost-effective, and highly recyclable superabsorbent from microfibrillated cellulose fibers for oil spillage cleanup. J. Mater. Chem. A 2015, 3, 8772–8781. [Google Scholar] [CrossRef]
- Su, C.; Yang, H.; Song, S.; Lu, B.; Chen, R. A magnetic superhydrophilic/oleophobic sponge for continuous oil-water separation. Chem. Eng. J. 2017, 309, 366–373. [Google Scholar] [CrossRef]
- Narbaitz, R.M.; Rana, D.; Dang, H.T.; Morrissette, J.; Matsuura, T.; Jasim, S.Y.; Tabe, S.; Yang, P. Pharmaceutical and personal care products removal from drinking water by modified cellulose acetate membrane: Field testing. Chem. Eng. J. 2013, 225, 848–856. [Google Scholar] [CrossRef]
- Xia, C.B.; Li, Y.B.; Fei, T.; Gong, W.L. Facile one-pot synthesis of superhydrophobic reduced graphene oxidecoated polyurethane sponge at the presence of ethanol for oil-water separation. Chem. Eng. J. 2018, 345, 648–658. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Li, L.; Wei, X. A versatile biomass derived carbon material for oxygen reduction reaction, supercapacitors and oil/water separation. Nano Energy 2017, 33, 334–342. [Google Scholar] [CrossRef]
- Shetty, D.; Jahovic, I.; Raya, J.; Ravaux, F.; Jouiad, M.; Olsen, J.C.; Trabolsi, A. An ultra-absorbent alkyne-rich porous covalent polycalix 4 arene for water purification. J. Mater. Chem. A 2017, 5, 62–66. [Google Scholar] [CrossRef]
- Rana, D.; Scheier, B.; Narbaitz, R.M.; Matsuura, T.; Tabe, S.; Jasim, S.Y.; Khulbe, K.C. Comparison of cellulose acetate (CA) membrane and novel CA membranes containing surface modifying macromolecules to remove pharmaceutical and personal care product micropollutants from drinking water. J. Membr. Sci. 2012, 409, 346–354. [Google Scholar] [CrossRef]
- Wen, Q.; Di, J.; Jiang, L.; Yu, J.; Xu, R. Zeolite-coated mesh film for efficient oil-water separation. Chem. Sci. 2013, 4, 591–595. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Tan, Y.; Du, J.; Wang, J. Synthesis and characterization of a porous and hydrophobic cellulose-based composite for efficient and fast oil-water separation. Carbohydr. Polym. 2016, 140, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, G.; Gao, C.; Zhang, L.; Chen, M.; Xu, X.; Gao, J.; Pan, C.; Yang, N.; Liu, Y. Biodegradable polylactic acid porous monoliths as effective oil sorbents. Compos. Sci. Technol. 2015, 118, 9–15. [Google Scholar] [CrossRef]
- Tai, M.H.; Gao, P.; Tan, B.Y.L.; Sun, D.D.; Leckie, J.O. Highly efficient and flexible electrospun carbon-silica nanofibrous membrane for ultrafast gravity-driven oil-water separation. ACS Appl. Mater. Interfaces 2014, 6, 9393–9401. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Li, N.; Chen, W.; Chen, G.; Wan, X.; Tian, J. Low-Cost, Sustainable, and Environmentally Sound Cellulose Absorbent with High Efficiency for Collecting Methane Bubbles from Seawater. ACS Sustain. Chem. Eng. 2018, 6, 6370–6377. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Li, B.; Wang, A. Mechanical- and oil-durable superhydrophobic polyester materials for selective oil absorption and oil/water separation. J. Colloid Interface Sci. 2014, 413, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Seeger, S. Polyester Materials with Superwetting Silicone Nanofilaments for Oil/Water Separation and Selective Oil Absorption. Adv. Funct. Mater. 2011, 21, 4699–4704. [Google Scholar] [CrossRef]
- Prince, J.A.; Bhuvana, S.; Anbharasi, V.; Ayyanar, N.; Boodhoo, K.V.; Singh, G. Ultra-wetting graphene-based PES ultrafiltration membrane—A novel approach for successful oil-water separation. Water Res. 2016, 103, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Arslan, O.; Aytac, Z.; Uyar, T. Superhydrophobic, Hybrid, Electrospun Cellulose Acetate Nanofibrous Mats for Oil/Water Separation by Tailored Surface Modification. ACS Appl. Mater. Interfaces 2016, 8, 19747–19754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prathap, A.; Sureshan, K.M. Organogelator-Cellulose Composite for Practical and Eco-Friendly Marine Oil-Spill Recovery. Angew. Chem. Int. Ed. Engl. 2017, 56, 9405–9409. [Google Scholar] [CrossRef] [PubMed]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, O.; Suopajarvi, T.; Osterberg, M.; Liimatainen, H. Hydrophobic, Superabsorbing Aerogels from Choline Chloride-Based Deep Eutectic Solvent Pretreated and Silylated Cellulose Nanofibrils for Selective Oil Removal. ACS Appl. Mater. Interfaces 2017, 9, 25029–25037. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, M.; Zang, D.; Gao, Z.; Wang, C. Fabrication of superhydrophobic/superoleophilic cotton for application in the field of water/oil separation. Carbohydr. Polym. 2014, 103, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Caschera, D.; Federici, F.; Ingo, G.M.; Gigli, G. Superhydrophobic fabrics for oil–water separation through a diamond like carbon (DLC) coating. J. Mater. Chem. A 2014, 2, 6781–6789. [Google Scholar] [CrossRef]
- Lei, S.; Shi, Z.; Ou, J.; Wang, F.; Xue, M.; Li, W.; Qiao, G.; Guan, X.; Zhang, J. Durable superhydrophobic cotton fabric for oil/water separation. Colloids Surf. A 2017, 533, 249–254. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, Y.; Jiang, G.; Wang, R.; Wang, X.; Hu, R.; Xi, X. Transformation of hydrophilic cotton fabrics into superhydrophobic surfaces for oil/water separation. J. Text. Inst. 2013, 104, 305–311. [Google Scholar] [CrossRef]
- Wen, G.; Guo, Z. Nonflammable superhydrophobic paper with biomimetic layered structure exhibiting boiling-water resistance and repairable properties for emulsion separation. J. Mater. Chem. A 2018, 6, 7042–7052. [Google Scholar] [CrossRef]
- Liu, C.; Chen, B.; Yang, J.; Li, C. One step fabrication of superhydrophobic and superoleophilic cigarette filters for oil water separation. J. Adhes. Sci. Technol. 2015, 29, 2399–2407. [Google Scholar] [CrossRef]
- Avramescu, R.E.; Ghica, M.V.; Dinu-Pirvu, C.; Prisada, R.; Popa, L. Superhydrophobic Natural and Artificial Surfaces-A Structural Approach. Materials 2018, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Gao, H.; He, M.; Zhang, L. Hydrophobic Modification on Surface of Chitin Sponges for Highly Effective Separation of Oil. ACS Appl. Mater. Interfaces 2014, 6, 19933–19942. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Su, X.; Zhu, W.; Hua, W.; Qian, Z.; Liu, L.; Yao, J. Flexible and durable cellulose aerogels for highly effective oil/water separation. RSC Adv. 2016, 6, 63773–63781. [Google Scholar] [CrossRef]
- Paul, U.; Fragouli, D.; Bayer, I.; Athanassiou, A. Functionalized Cellulose Networks for Efficient Oil Removal from Oil–Water Emulsions. Polymers 2016, 8, 52. [Google Scholar] [CrossRef]
- Naseem, S.; Wu, C.-M.; Xu, T.-Z.; Lai, C.-C.; Rwei, S.-P. Oil-Water Separation of Electrospun Cellulose Triacetate Nanofiber Membranes Modified by Electrophoretically Deposited TiO2/Graphene Oxide. Polymers 2018, 10, 746. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef]
- Zhang, Z.; Sèbe, G.; Rentsch, D.; Zimmermann, T.; Tingaut, P. Ultralightweight and Flexible Silylated Nanocellulose Sponges for the Selective Removal of Oil from Water. Chem. Mater. 2014, 26, 2659–2668. [Google Scholar] [CrossRef]
- Zheng, Y.; He, Y.; Qing, Y.; Zhuo, Z.; Mo, Q. Formation of SiO2/polytetrafluoroethylene hybrid superhydrophobic coating. Appl. Surf. Sci. 2012, 258, 9859–9863. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne Superhydrophobic and Superoleophobic Coatings for the Protection of Marble and Sandstone. Materials 2018, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, L.; Li, B.; Zhang, J.; Wang, A. Magnetic, durable, and superhydrophobic polyurethane@Fe3O4@SiO2@fluoropolymer sponges for selective oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 4936–4946. [Google Scholar] [CrossRef] [PubMed]
- Obaid, M.; Tolba, G.M.K.; Motlak, M.; Fadali, O.A.; Khalil, K.A.; Almajid, A.A.; Kim, B.; Barakat, N.A.M. Effective polysulfone-amorphous SiO2 NPs electrospun nanofiber membrane for high flux oil/water separation. Chem. Eng. J. 2015, 279, 631–638. [Google Scholar] [CrossRef]
- Micevska, T.; Warne, M.S.; Pablo, F.; Patra, R. Variation in, and causes of, toxicity of cigarette butts to a cladoceran and microtox. Arch. Environ. Contam. Toxicol. 2006, 50, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, N.; Qu, C.; Wu, X.; Zhang, J.; Zhang, X. Cigarette Butts and Their Application in Corrosion Inhibition for N80 Steel at 90 °C in a Hydrochloric Acid Solution. Ind. Eng. Chem. Res. 2010, 49, 3986–3991. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Zhang, N.; Qu, C.; Zhang, X. Synergized Action of CuCl on Recycled Cigarette Butts as Corrosion Inhibitor for N80 Steel at 90 °C in 15% HCl. Ind. Eng. Chem. Res. 2011, 50, 7264–7272. [Google Scholar] [CrossRef]
- Lee, M.; Kim, G.-P.; Don Song, H.; Park, S.; Yi, J. Preparation of energy storage material derived from a used cigarette filter for a supercapacitor electrode. Nanotechnology 2014, 25, 345601–345609. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ding, Y.; Yuan, Y.; He, X.; Wu, S.; Hu, S.; Zou, M.; Zhao, W.; Yang, L.; Cao, A.; et al. Graphene aerogel composites derived from recycled cigarette filters for electromagnetic wave absorption. J. Mater. Chem. C 2015, 3, 11893–11901. [Google Scholar] [CrossRef]
- Kim, G.-P.; Lee, M.; Song, H.D.; Bae, S.; Yi, J. Highly efficient supporting material derived from used cigarette filter for oxygen reduction reaction. Catal. Commun. 2016, 78, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, M.; Yang, Y.; Ran, F. Hybrid Electrode Material of Vanadium Nitride and Carbon Fiber with Cigarette Butt/Metal Ions Wastes as the Precursor for Supercapacitors. Electrochim. Acta 2016, 222, 1914–1921. [Google Scholar] [CrossRef]
- Sun, H.; La, P.; Yang, R.; Zhu, Z.; Liang, W.; Yang, B.; Li, A.; Deng, W. Innovative nanoporous carbons with ultrahigh uptakes for capture and reversible storage of CO2 and volatile iodine. J. Hazard. Mater. 2017, 321, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Ge, B.; Men, X.; Zhou, X.; Xue, Q. A versatile approach to produce superhydrophobic materials used for oil-water separation. J. Colloid Interface Sci. 2014, 432, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Uyama, H. Facile synthesis of flexible macroporous polypropylene sponges for separation of oil and water. Sci. Rep. 2016, 6, 21265–21271. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, W. Superhydrophobic/Superoleophilic and Reinforced Ethyl Cellulose Sponges for Oil/Water Separation: Synergistic Strategies of Cross-linking, Carbon Nanotube Composite, and Nanosilica Modification. ACS Appl. Mater. Interfaces 2017, 9, 29167–29176. [Google Scholar] [CrossRef] [PubMed]








| Time | Materials | Oil Recovery and Reuse Method | Reuse Efficiency | Cost | Environment Friendly | Ref |
|---|---|---|---|---|---|---|
| 2014 | polyester fabric | squeezing and solvent extraction | XX | + | [46] | |
| 2014 | nanofibrillated cellulose | solvent extraction | X | + | Yes | [34] |
| 2014 | cotton | vacuum filtration | X | + | Yes | [27] |
| 2015 | polyurethane | extrusion by pump | √ | + | [32] | |
| 2015 | cellulose fiber | squeezing | √√ | −− | [9] | |
| 2015 | bacterial cellulose | compression, rinsing and freeze-drying | XX | ++ | [25] | |
| 2015 | cigarette filters | extrusion and washed with ethanol | √ | − | Yes | [28] |
| 2016 | polypropylene | squeezing | √√ | − | [47] | |
| 2017 | ethyl cellulose | heating | √ | − | [48] | |
| 2018 | cigarette filters | squeezing | √√ | −− | Yes | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Q.; Bai, Q.; Li, C.; Lei, H.; Liu, C.; Shen, Y.; Uyama, H. Cost-Effective, Highly Selective and Environmentally Friendly Superhydrophobic Absorbent from Cigarette Filters for Oil Spillage Clean up. Polymers 2018, 10, 1101. https://doi.org/10.3390/polym10101101
Xiong Q, Bai Q, Li C, Lei H, Liu C, Shen Y, Uyama H. Cost-Effective, Highly Selective and Environmentally Friendly Superhydrophobic Absorbent from Cigarette Filters for Oil Spillage Clean up. Polymers. 2018; 10(10):1101. https://doi.org/10.3390/polym10101101
Chicago/Turabian StyleXiong, Qiancheng, Qiuhong Bai, Cong Li, Huan Lei, Chaoyun Liu, Yehua Shen, and Hiroshi Uyama. 2018. "Cost-Effective, Highly Selective and Environmentally Friendly Superhydrophobic Absorbent from Cigarette Filters for Oil Spillage Clean up" Polymers 10, no. 10: 1101. https://doi.org/10.3390/polym10101101