Preparation and Properties of the 3-pentadecyl-phenol In Situ Modified Foamable Phenolic Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
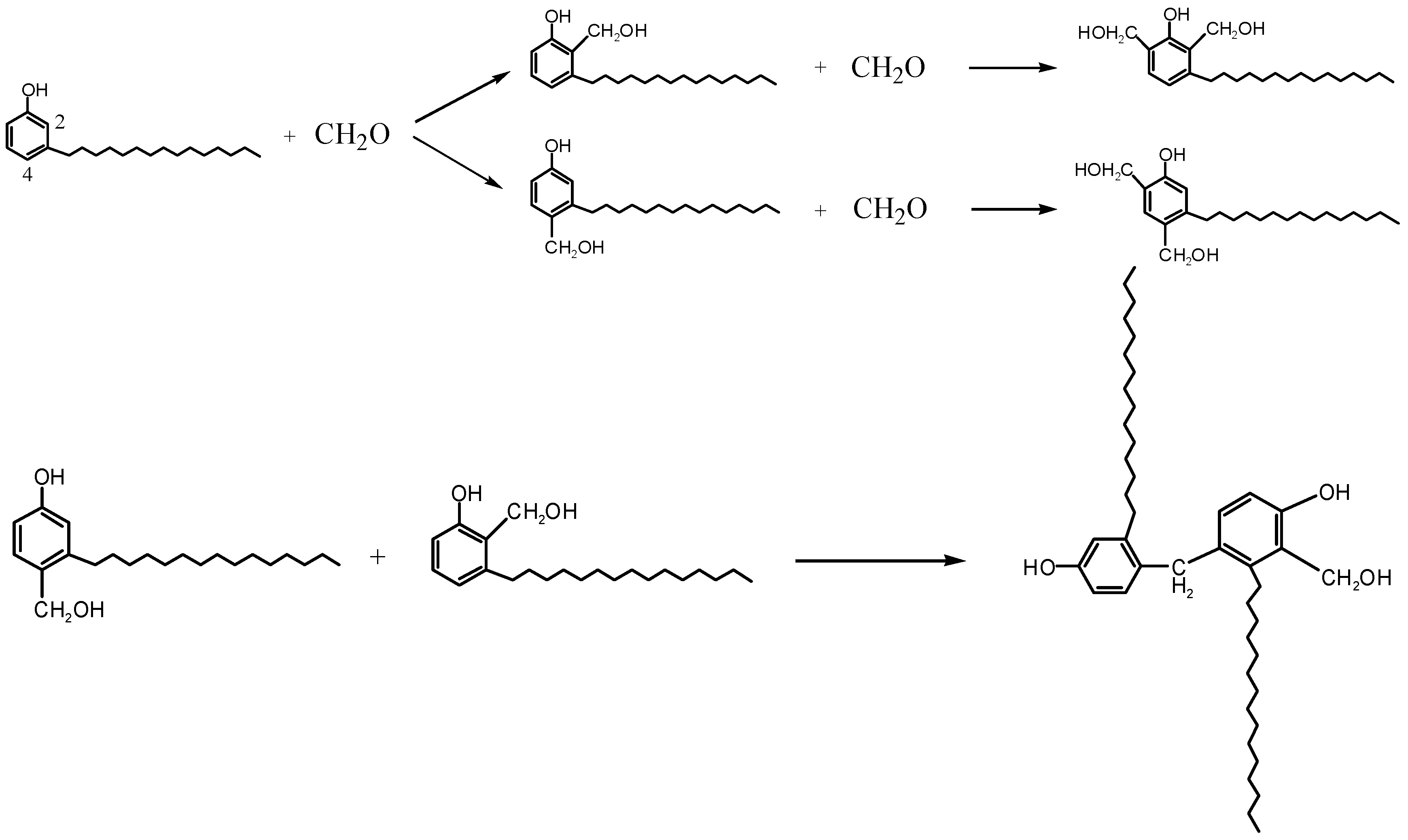
2.2. The Synthesis of Modified Phenolic Resin
2.3. Preparation of Phenolic Foam
2.4. Characterization
3. Results and Discussion
3.1. Resin Structure
3.1.1. FT-IR Analysis
3.1.2. 1H NMR Analysis
3.1.3. 13C NMR Analysis
3.2. Basic Properties of Resin
3.3. Mechanical Properties of the Foam
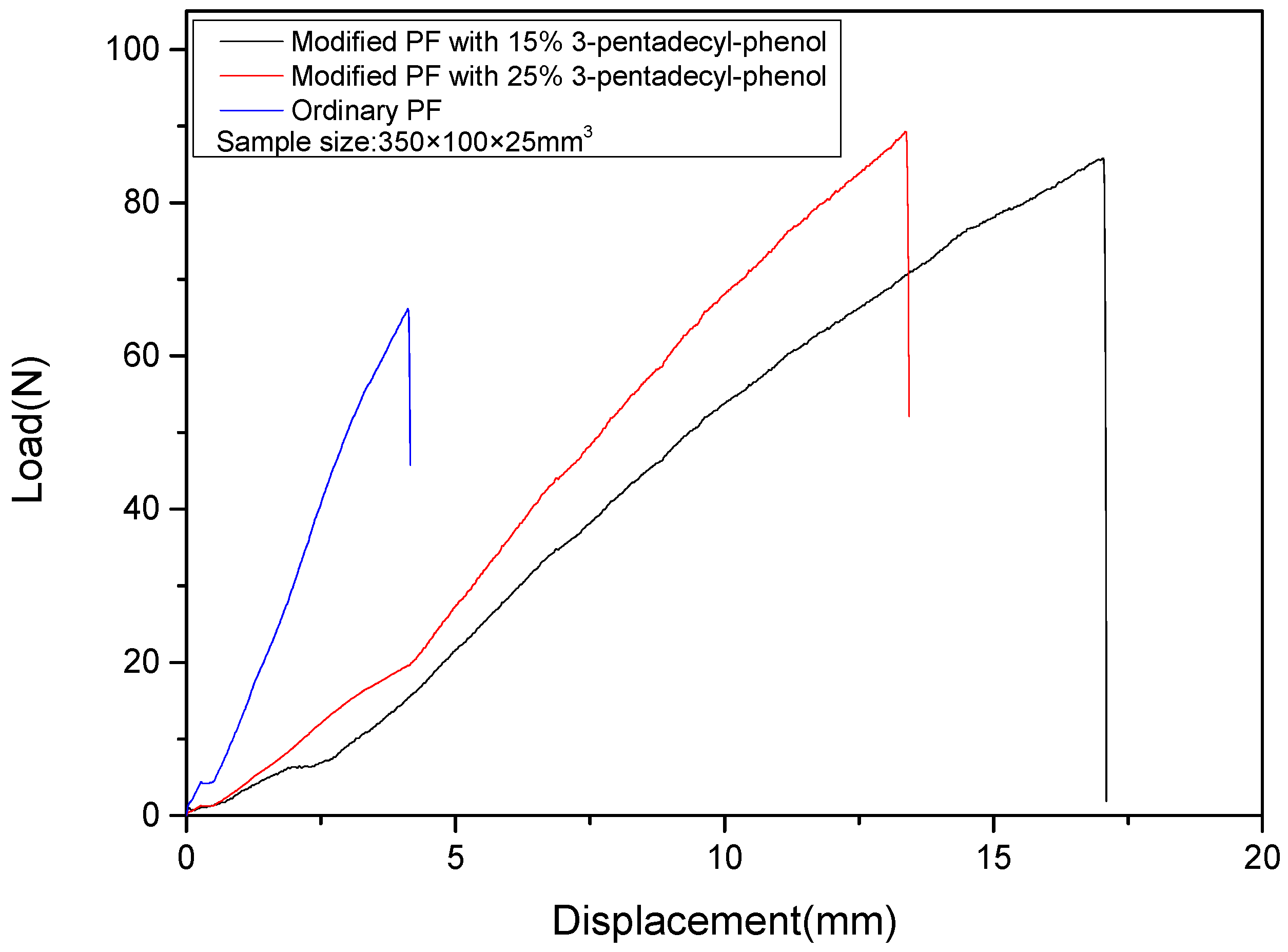
3.3.1. Bending Performance Analysis
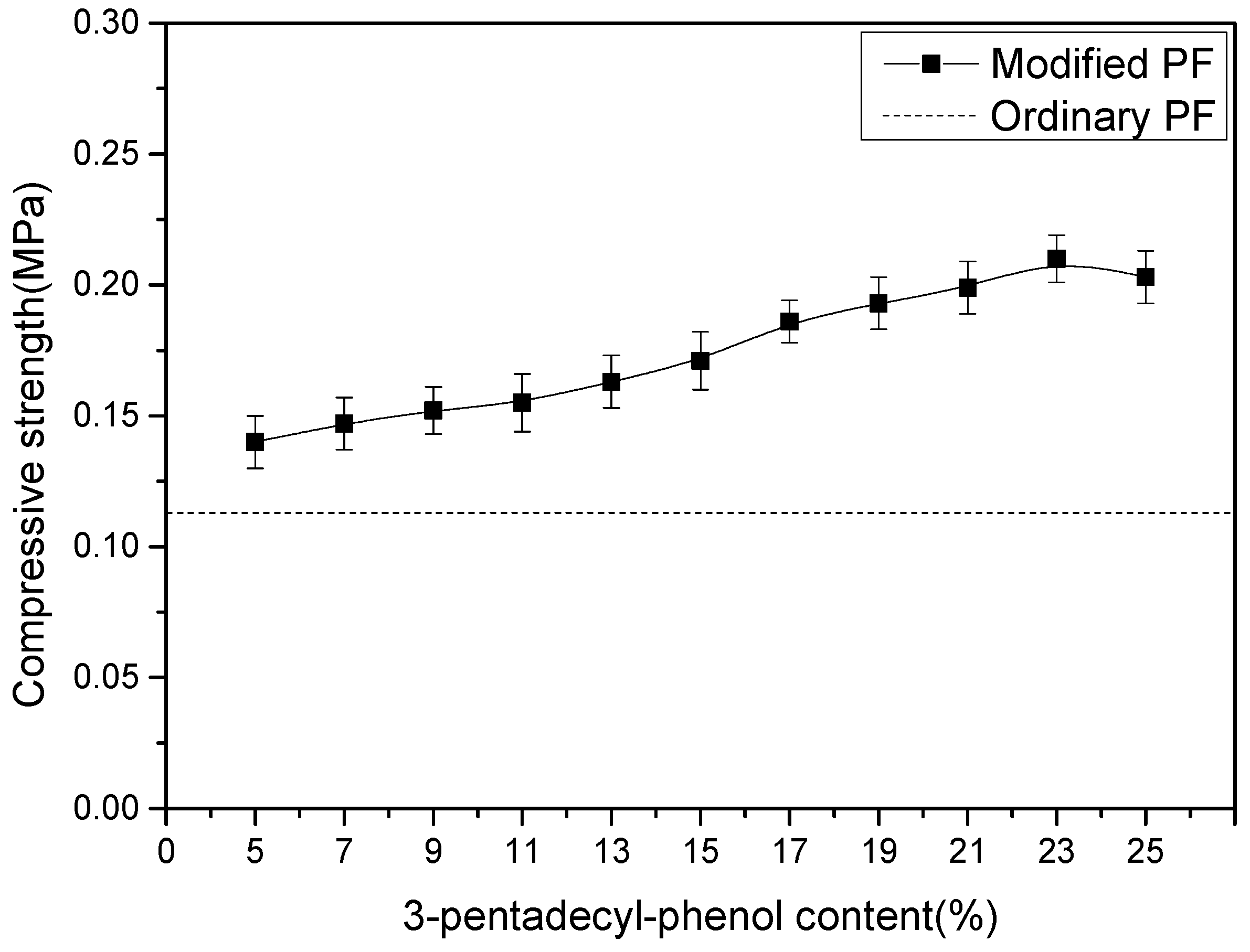
3.3.2. Compressive Strength Analysis
3.4. Foam Cell Structure
3.4.1. Apparent Density Analysis
3.4.2. Analysis of Water Absorption Rate
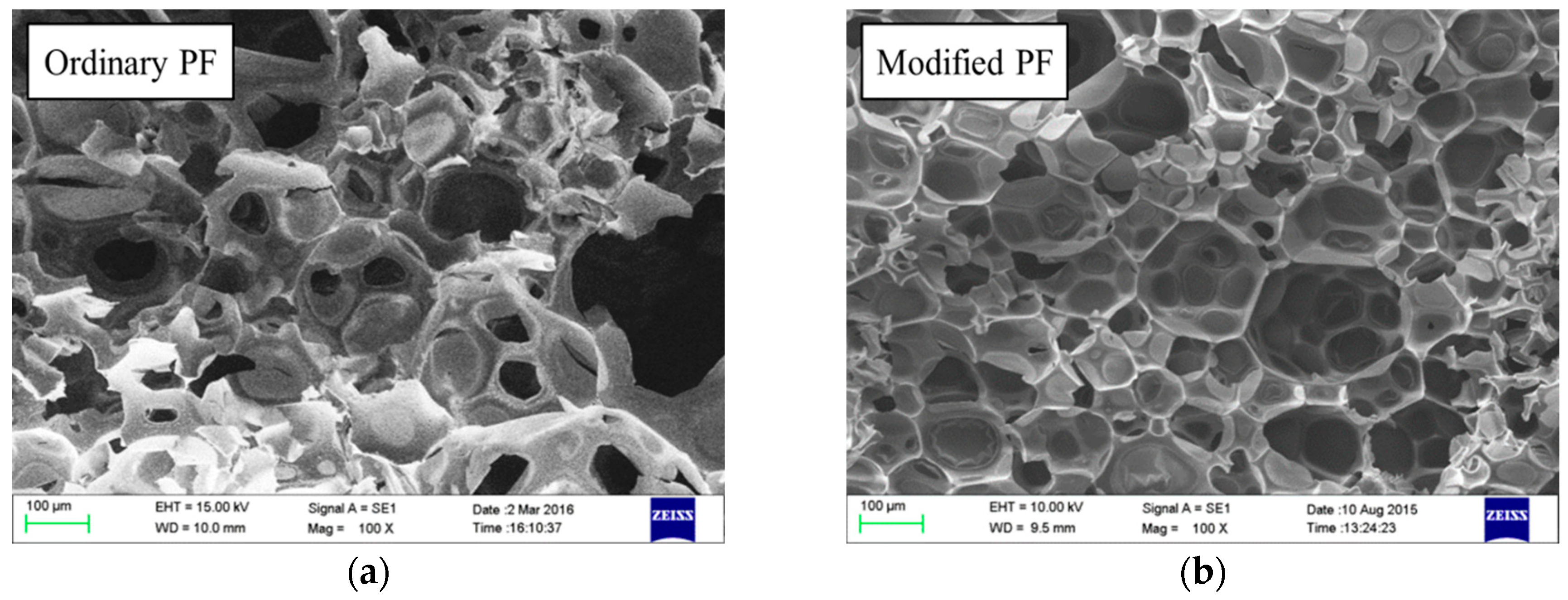
3.4.3. Cell Microstructure
3.5. Foam Heat Resistance
3.5.1. Limited Oxygen Index
3.5.2. Thermogravimetric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, H.; Wang, L.; Gai, G. Performance of modified aramid fiber reinforced phenolic foam. Adv. Mater. Res. 2012, 557, 258–261. [Google Scholar] [CrossRef]
- Choe, J.; Kim, M.; Kim, J.; Lee, D.G. A microwave foaming method for fabricating glass fiber reinforced phenolic foam. Compos. Struct. 2016, 152, 239–246. [Google Scholar] [CrossRef]
- Song, S.A.; Oh, H.J.; Kim, B.G.; Kim, S.S. Novel foaming methods to fabricate activated carbon reinforced microcellular phenolic foams. Compos. Sci. Technol. 2013, 76, 45–51. [Google Scholar] [CrossRef]
- Bijwe, J.; Majumdar, N.; Satapathy, B.K. Influence of modified phenolic resins on the fade and recovery behavior of friction materials. Wear 2005, 259, 1068–1078. [Google Scholar] [CrossRef]
- Shao, H.; Ji, K.; Liu, Y.; Wang, X.; Deng, W.; Hua, L.; Gao, Y.; Li, Y.; Zhou, T. Research progress of phenolic foam plastics and its application. Eng. Plast. Appl. 2015, 43, 129–132. [Google Scholar]
- Kim, B.G.; Lee, D.G. Development of microwave foaming method for phenolic insulation foams. J. Mater. Process. Technol. 2008, 201, 716–719. [Google Scholar] [CrossRef]
- Li, S.; Ge, D.; Wang, S.; Hu, F. Study of phenolic foam toughening modification. Fiber Reinf. Plast. Compos. 2004, 4, 39–41. [Google Scholar]
- Xiao, W.; Huang, Z.; Ding, J. The mechanical and thermal characteristics of phenolic foam reinforced with kaolin powder and glass fiber fabric. Mater. Sci. Eng. 2017, 274, 012013. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Li, S.; Ma, Y.; Zhang, W.; Xu, Y.Z.; Wang, C.P.; Chu, F.X. Preparation and performance research on phenolic insulation foam used low-temperature foaming technology. Adv. Mater. Res. 2011, 450–454. [Google Scholar] [CrossRef]
- Cui, Y.; Hou, X.; Wang, W.; Chang, J. Synthesis and characterization of bio-oil phenol formaldehyde resin used to fabricate phenolic based materials. Materials 2017, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Nutt, S. Mechanical characterization of short fiber reinforced phenolic foam. Compos. Part A 2003, 34, 899–906. [Google Scholar] [CrossRef]
- Auad, M.L.; Zhao, L.; Shen, H.; Nutt, S.R.; Sorathia, U. Flammability properties and mechanical performance of epoxy modified phenolic foams. J. Appl. Polym. Sci. 2010, 104, 1399–1407. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, Z.; Chen, Y.; Wei, D.; Wu, Y. Thermomechanical analyses of phenolic foam reinforced with glass fiber mat. Mater. Des. 2013, 51, 131–135. [Google Scholar] [CrossRef]
- Yang, Y.; He, J. Mechanical characterization of phenolic foams modified by short glass fibers and polyurethane prepolymer. Polym. Compos. 2015, 36, 1584–1589. [Google Scholar] [CrossRef]
- Dong, H.; Peng, J.; Zhang, L.; Zou, W. Preparation and performances of phenolic resin/aluminium phosphate composite. Eng. Plast. Appl. 2015, 43, 29–33. [Google Scholar]
- Wu, C.; Gao, S.; Zhang, L. Phenolic foam modified with polyurethane prepolymer. Chem. Ind. Eng. Prog. 2016, 35, 1144–1148. [Google Scholar]
- Hu, X.M.; Wang, D.M.; Cheng, W.M.; Zhou, G. Effect of polyethylene glycol on the mechanical property, microstructure, thermal stability, and flame resistance of phenol–urea–formaldehyde foams. J. Mater. Sci. 2014, 49, 1556–1565. [Google Scholar] [CrossRef]
- De Carvalho, G.; Pimenta, J.A.; dos Santos, W.N.; Frollini, E. Phenolic and lignophenolic closed cells foams: thermal conductivity and other properties. Polym.-Plast. Technol. Eng. 2003, 42, 605–626. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Preparation and characterization of lignin demethylated at atmospheric pressure and its application in fast curing biobased phenolic resins. RSC Adv. 2016, 6, 67435–67443. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Fast curing bio-based phenolic resins via lignin demethylated under mild reaction condition. Polymers 2017, 9, 428. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, X.; Liao, C.; Geng, X.; Wang, C.; Chu, F. Preparation and characterization of DOPO-ITA modified ethyl cellulose and its application in phenolic foams. Polymers 2018, 10, 1049. [Google Scholar] [CrossRef]
- Turunen, M.; Alvila, L.; Pakkanen, T.T.; Rainio, J. Modification of phenol-formaldehyde resol resins by lignin, starch, and urea. J. Appl. Polym. Sci. 2010, 88, 582–588. [Google Scholar] [CrossRef]
- Mirski, R.; Dziurka, D.; Łęcka, J. Properties of phenol-formaldehyde resin modified with organic acid esters. J. Appl. Polym. Sci. 2010, 107, 3358–3366. [Google Scholar] [CrossRef]
- Hou, C.Y.; Ma, G.Z.; Yuan, L.P. Preparation and application of alkylphenol-acetone-formaldehyde resins. Appl. Chem. Ind. 2010, 39, 11–400. [Google Scholar]
- Zoss, A.O.; Hanford, W.E.; Schildknecht, C.E. Preparation and properties of alkylphenol-acetylene resins. Ind. Eng. Chem. Res. 1949, 41, 73–77. [Google Scholar] [CrossRef]
- Miller, D.; Feustel, M.; Vollmer, A.; Vybiral, R.; Hoffmann, D. Synergistic Mixtures of Alkylphenol-Formaldehyde Resins with Oxalkylated Amines as Asphaltene Dispersants. U.S. Patent EP 0 975 420 B1, 2 February 2002. [Google Scholar]
- Kifer, E.W.; Colton, J.P.; Stickel, J.T. Closed Cell Phenolic Foam Containing Alkyl Glucosides. U.S. Patent US4956394A, 11 September 1990. [Google Scholar]
- Ma, L.; Zhang, Y. Synthesis of boron alkylphenol modified phenolic resin and its heat resistance. J. Beijing Union Univ. 1997, 11, 40–44. [Google Scholar]
- Xiao, X.; Tang, X.; Wang, D. Study on a new environment-friendly composition technology of nonyl phenol and formaldehyde resin. New Chem. Mater. 2013, 41, 55–56. [Google Scholar]
- Geng, X.D.; Zhang, Z.R. Preparation of rosin modified long chain alkyl phenolic resin for high-end thermoset ink. Thermosetting Resin 2017, 5, 34–37. [Google Scholar]
- Li, P.; Wu, J.; Hou, C. Effect of alkyl phnolic condensation compound on the performance of modified resin. Thermosetting Resin 2017, 2, 1–4. [Google Scholar]
- Ge, T.; Yang, S. Study on toughening modification of phenolic foam with compound modified phenolic resin. Plast. Sci. Technol. 2015, 43, 67–71. [Google Scholar]
- Linul, E.; Movahedi, N.; Marsavina, L. On the lateral compressive behavior of empty and ex-situ aluminum foam-filled tubes at high temperature. Materials 2018, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- King, P.W.; Mitchell, R.H.; Westwood, A.R. Structural analysis of phenolic resole resins. J. Appl. Polym. Sci. 2010, 18, 1117–1130. [Google Scholar] [CrossRef]
- Lei, S.; Guo, Q.; Zhang, D.; Shi, J.; Liu, L.; Wei, X. Preparation and properties of the phenolic foams with controllable nanometer pore structure. J. Appl. Polym. Sci. 2010, 117, 3545–3550. [Google Scholar] [CrossRef]
- Qiao, D.P.; Wei-Min, L.I.; Wang, S.P.; Wen, X.D.; Ding, L.; Han, X.L. Study on phenolic foam plastic toughening modification with flexible epoxy resin. Henan Chem. Ind. 2006, 23, 22–23. [Google Scholar]
- Wang, J.; Wang, N.; Liu, X.; Ding, J.; Xia, X.; Chen, X.; Zhao, W. Compressive deformation behavior of closed-cell micro-pore magnesium composite foam. Materials 2018, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Guo, Q.; Shi, J.; Liu, L. Preparation of phenolic-based carbon foam with controllable pore structure and high compressive strength. Carbon 2010, 48, 2644–2646. [Google Scholar] [CrossRef]
- Yuan, H.; Xing, W.; Yang, H.; Song, L.; Hu, Y.; Yeoh, G.H. Mechanical and thermal properties of phenolic/glass fiber foam modified with phosphorus-containing polyurethane prepolymer. Polym. Int. 2013, 62, 273–279. [Google Scholar] [CrossRef]
- Qian, J.; Jin, Z.; Wang, J. Structure and basic properties of woodceramics made from phenolic resin–basswood powder composite. Mater. Sci. Eng. 2004, 368, 71–79. [Google Scholar] [CrossRef]
- Faulstich de Paiva, J.M.; Frollini, E. Unmodified and modified surface sisal fibers as reinforcement of phenolic and lignophenolic matrices composites: Thermal analyses of fibers and composites. Macromol. Mater. Eng. 2006, 291, 405–417. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, W.; Nie, W.; Wang, D. Flame retardant, thermal, and mechanical properties of glass fiber/nanoclay reinforced phenol–urea–formaldehyde foam. Polym. Compos. 2016, 37, 2323–2332. [Google Scholar] [CrossRef]
- Qian, B. New advances of phenolic foam plastics. World Plast. 2011, 3, 36–41. [Google Scholar]
- Domínguez, J.C.; del Saz-Orozco, B.; Oliet, M.; Alonso, M.V.; Rodriguez, F. Thermal properties and thermal degradation kinetics of phenolic and wood flour-reinforced phenolic foams. J. Compos. Mater. 2016, 51, 125–138. [Google Scholar] [CrossRef]










| Phenolic resin (phr) | Foaming agent (phr) | Surfactant (phr) | Curing agent (phr) |
|---|---|---|---|
| 100 | 8 | 8 | 12 |
| The content of 3-pentadecyl-phenol of total phenol (%) | Gel time (s) | Free formaldehyde content (%) | Solid content (%) |
|---|---|---|---|
| 0 | 218 | 0.22 | 87.6 |
| 5 | 221 | 0.20 | 87.9 |
| 7 | 208 | 0.18 | 88.0 |
| 9 | 197 | 0.17 | 88.4 |
| 11 | 184 | 0.17 | 88.6 |
| 13 | 181 | 0.15 | 89.1 |
| 15 | 182 | 0.16 | 88.8 |
| 17 | 180 | 0.17 | 88.1 |
| 19 | 179 | 0.21 | 87.7 |
| 21 | 181 | 0.24 | 87.0 |
| 23 | 177 | 0.27 | 86.4 |
| 25 | 175 | 0.28 | 86.3 |
| Parameter | PF | Modified PF |
|---|---|---|
| Tmax (°C) | 503.7 | 498.5 |
| Residual mass (%) | 9.09 | 8.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, T.; Tang, K.; Yu, Y.; Tan, X. Preparation and Properties of the 3-pentadecyl-phenol In Situ Modified Foamable Phenolic Resin. Polymers 2018, 10, 1124. https://doi.org/10.3390/polym10101124
Ge T, Tang K, Yu Y, Tan X. Preparation and Properties of the 3-pentadecyl-phenol In Situ Modified Foamable Phenolic Resin. Polymers. 2018; 10(10):1124. https://doi.org/10.3390/polym10101124
Chicago/Turabian StyleGe, Tiejun, Kaihong Tang, Yang Yu, and Xiapeng Tan. 2018. "Preparation and Properties of the 3-pentadecyl-phenol In Situ Modified Foamable Phenolic Resin" Polymers 10, no. 10: 1124. https://doi.org/10.3390/polym10101124






