Enhanced Solubilization of Fluoranthene by Hydroxypropyl β-Cyclodextrin Oligomer for Bioremediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
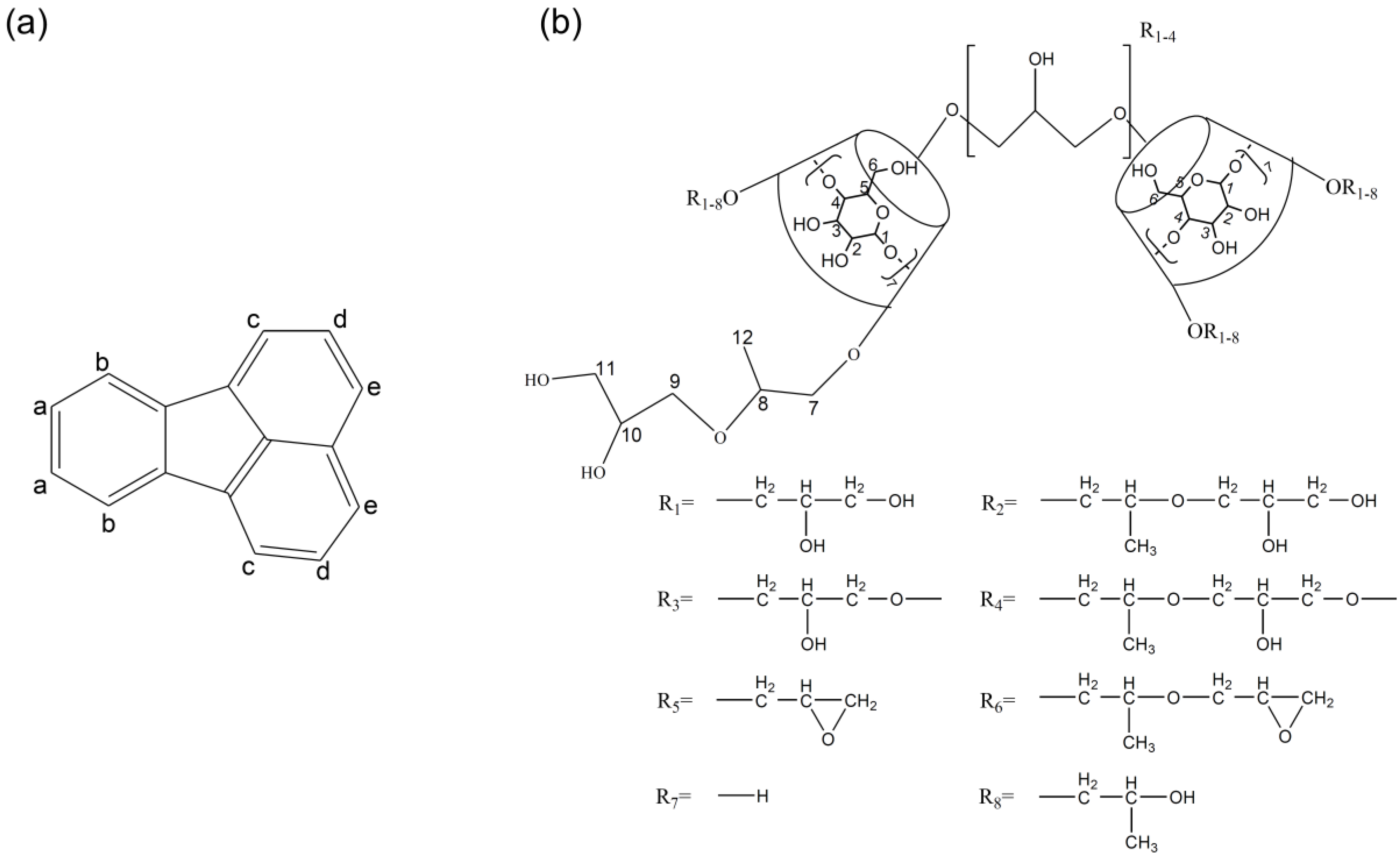
2.2. Synthesis of HP-β-CD-ol
2.3. HP-β-CD-ol Characterization
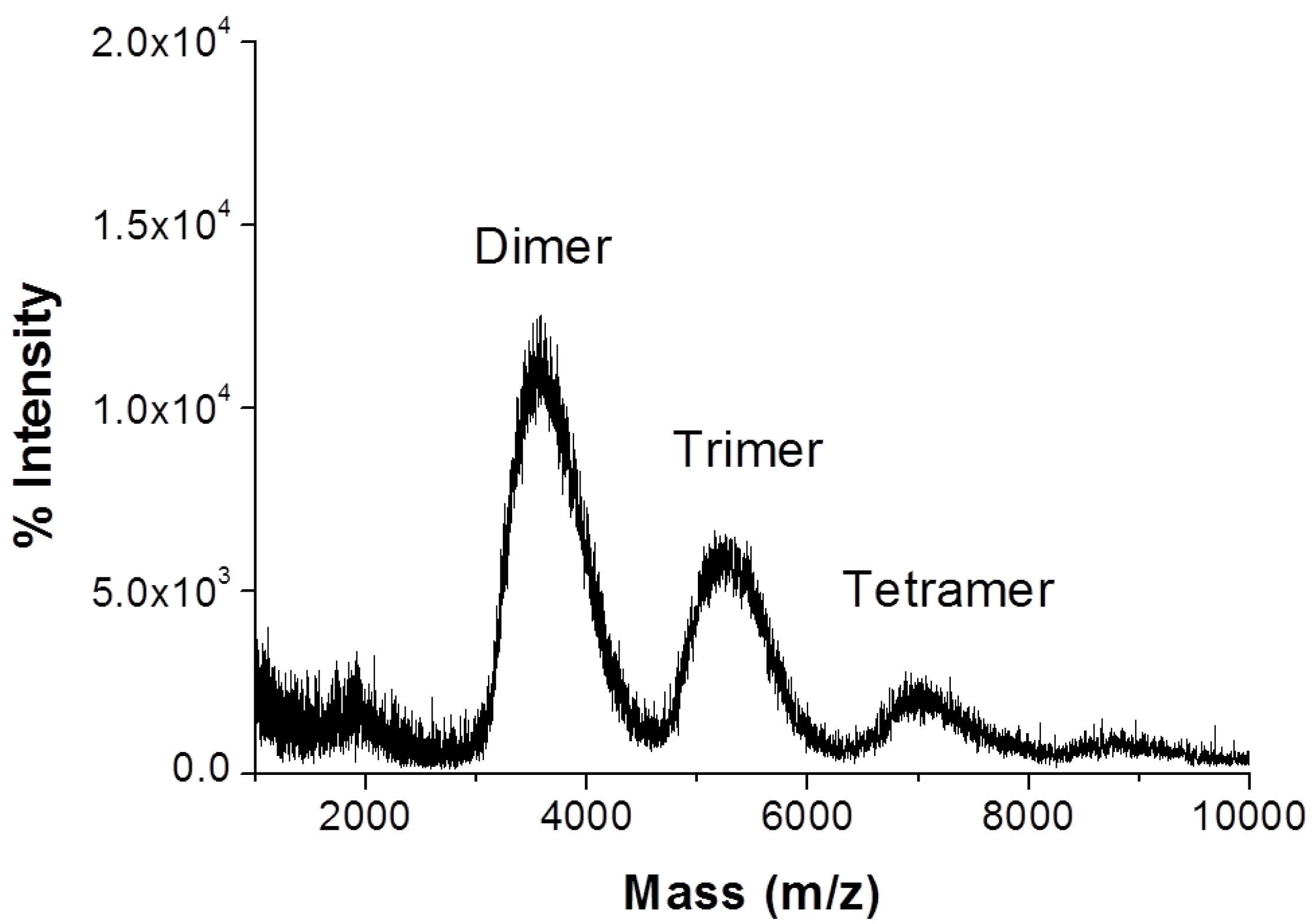
2.3.1. MALDI-TOF MS
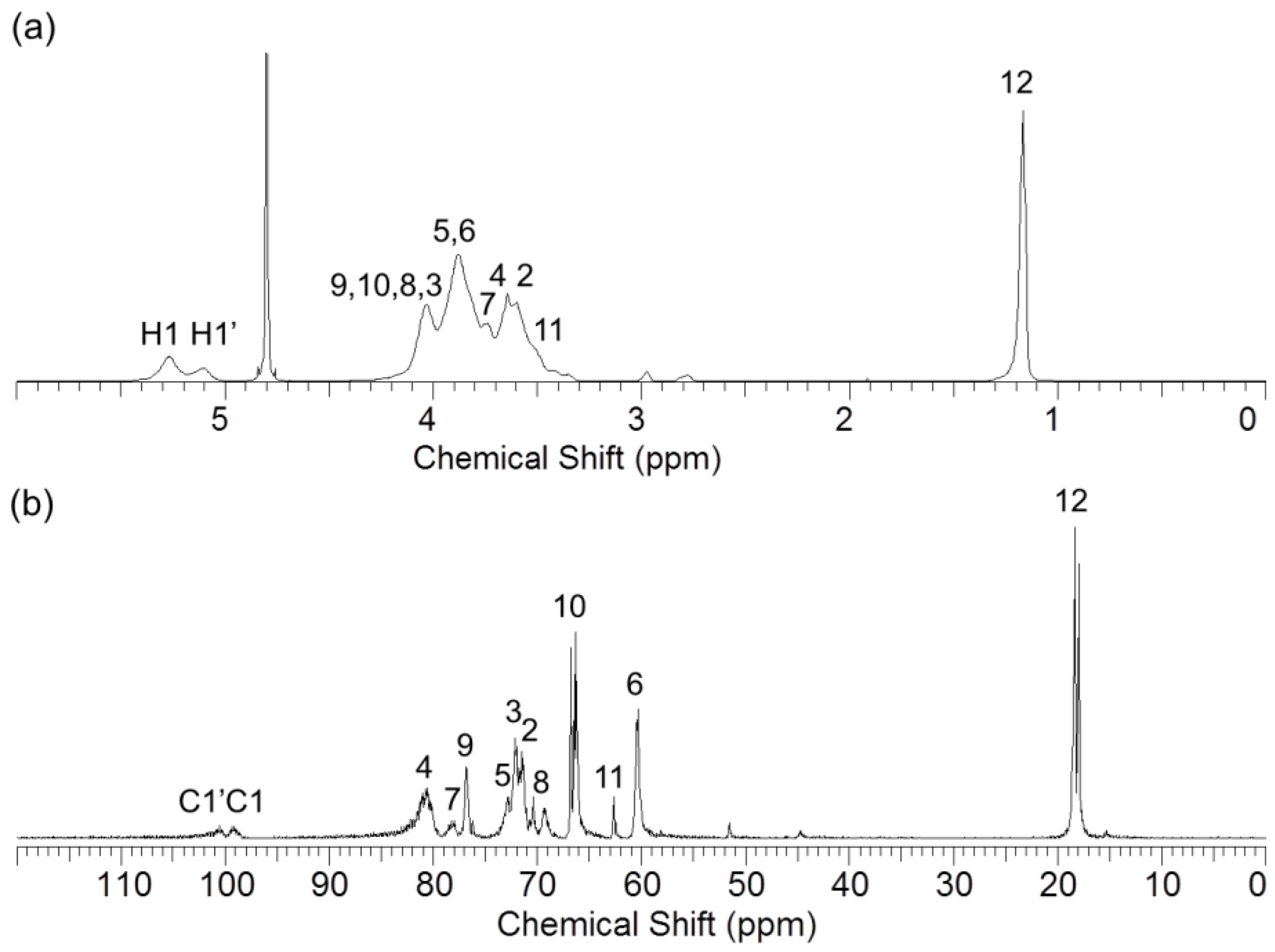
2.3.2. 1H NMR and 13C NMR Spectroscopy
2.4. Solubility Enhancement Test
2.5. Phase Solubility Analysis
2.6. Method of Sample Preparation
2.6.1. Preparation of the Physical Mixture (PM)
2.6.2. Preparation of the Inclusion Complex (IC)
2.7. Inclusion Complex Characterization
2.7.1. Fourier-Transform Infrared (FT-IR)
2.7.2. Differential Scanning Calorimetry (DSC)
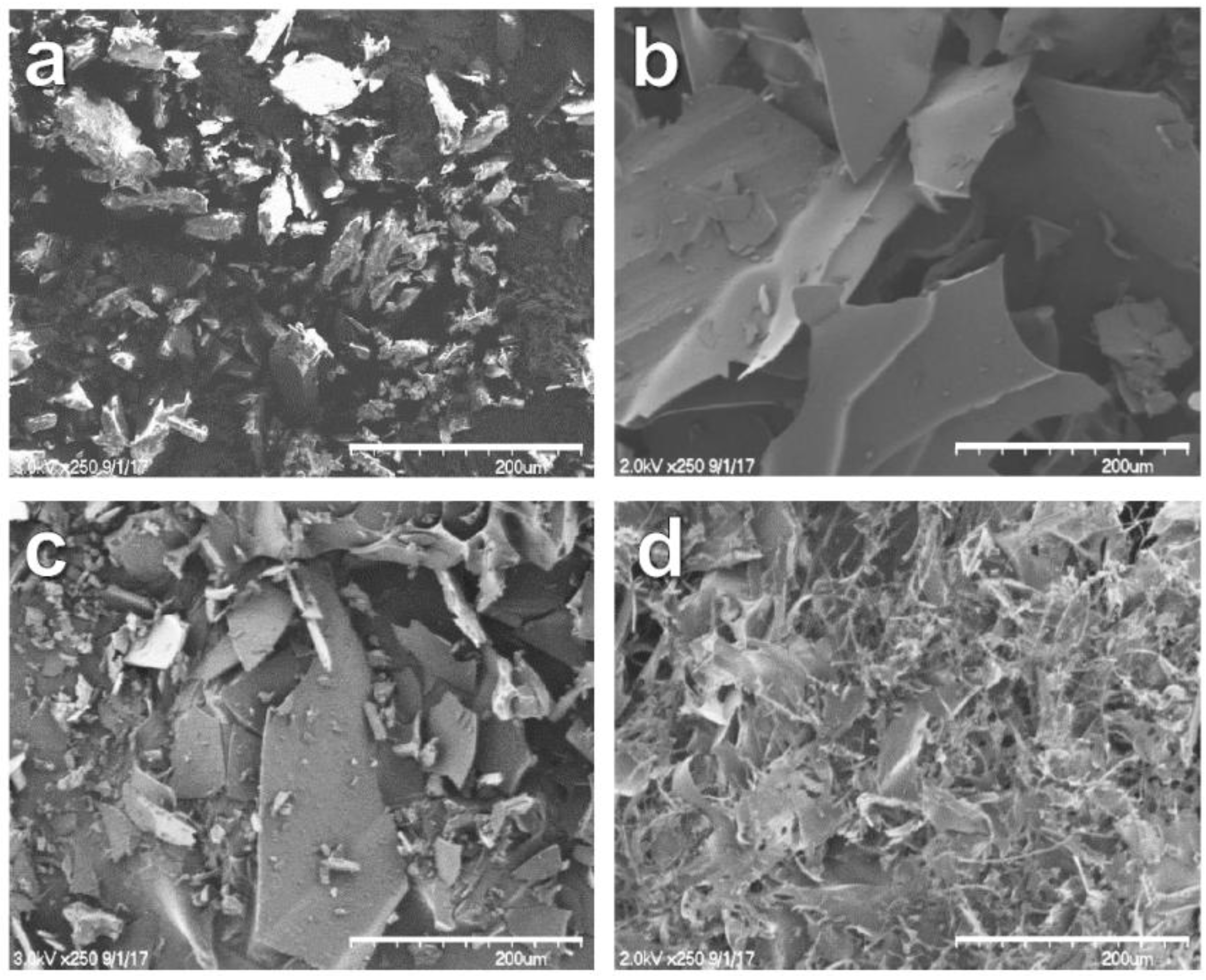
2.7.3. Scanning Electron Microscope (SEM)
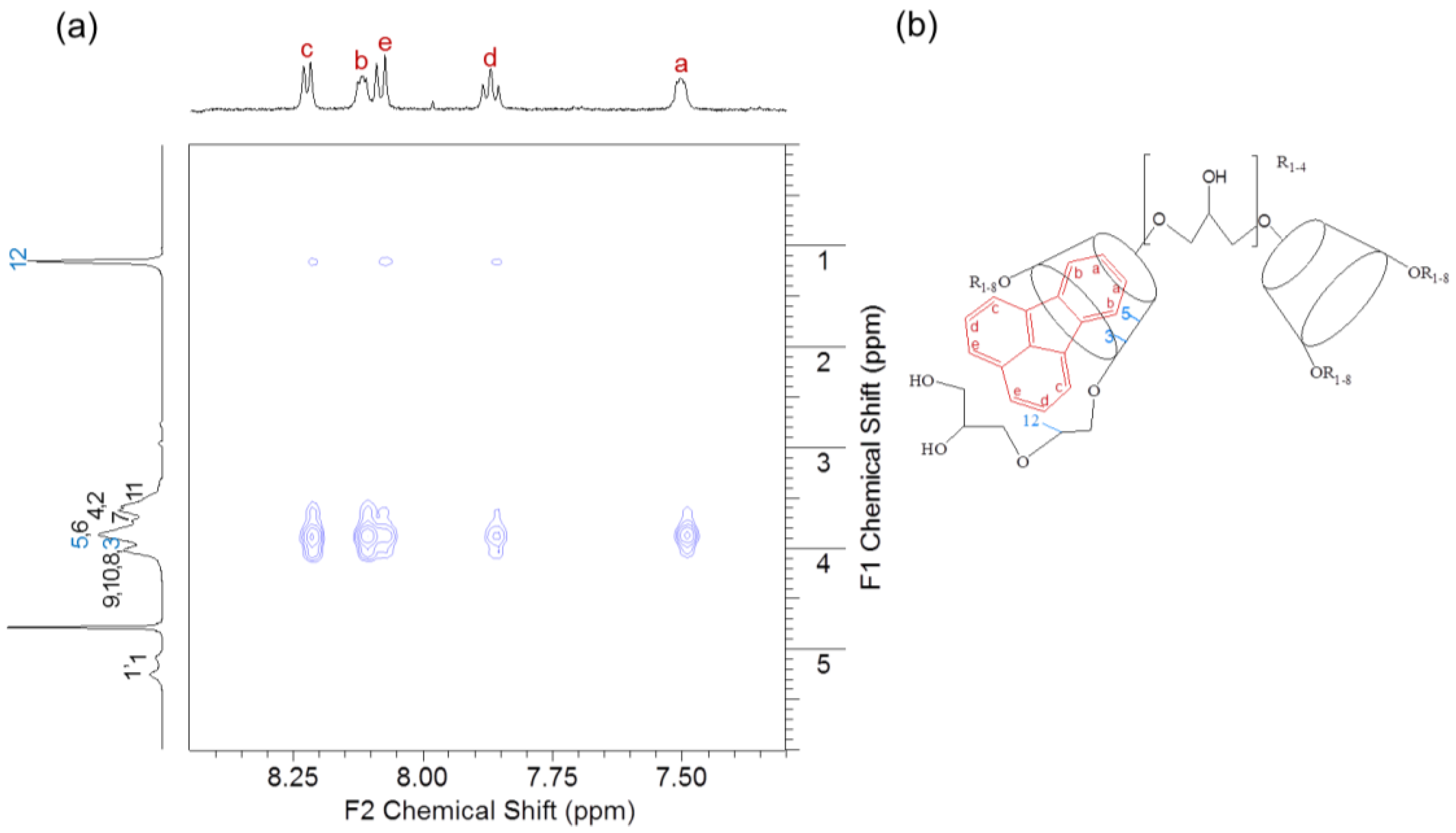
2.7.4. Nuclear Overhauser Effect Spectroscopy (NOESY)
3. Results and Discussion
3.1. Synthesis of Hydroxypropyl β-Cyclodextrin Oligomer (HP-β-CD-ol)
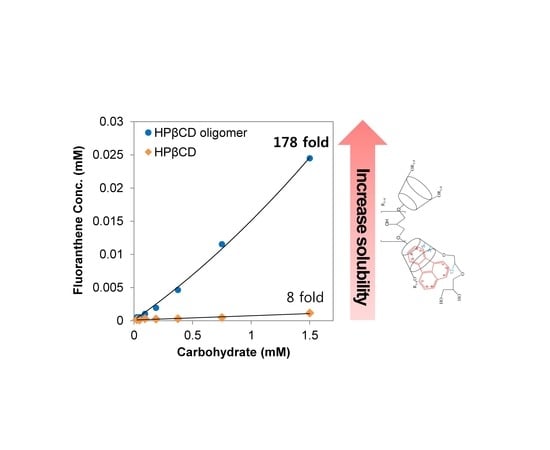
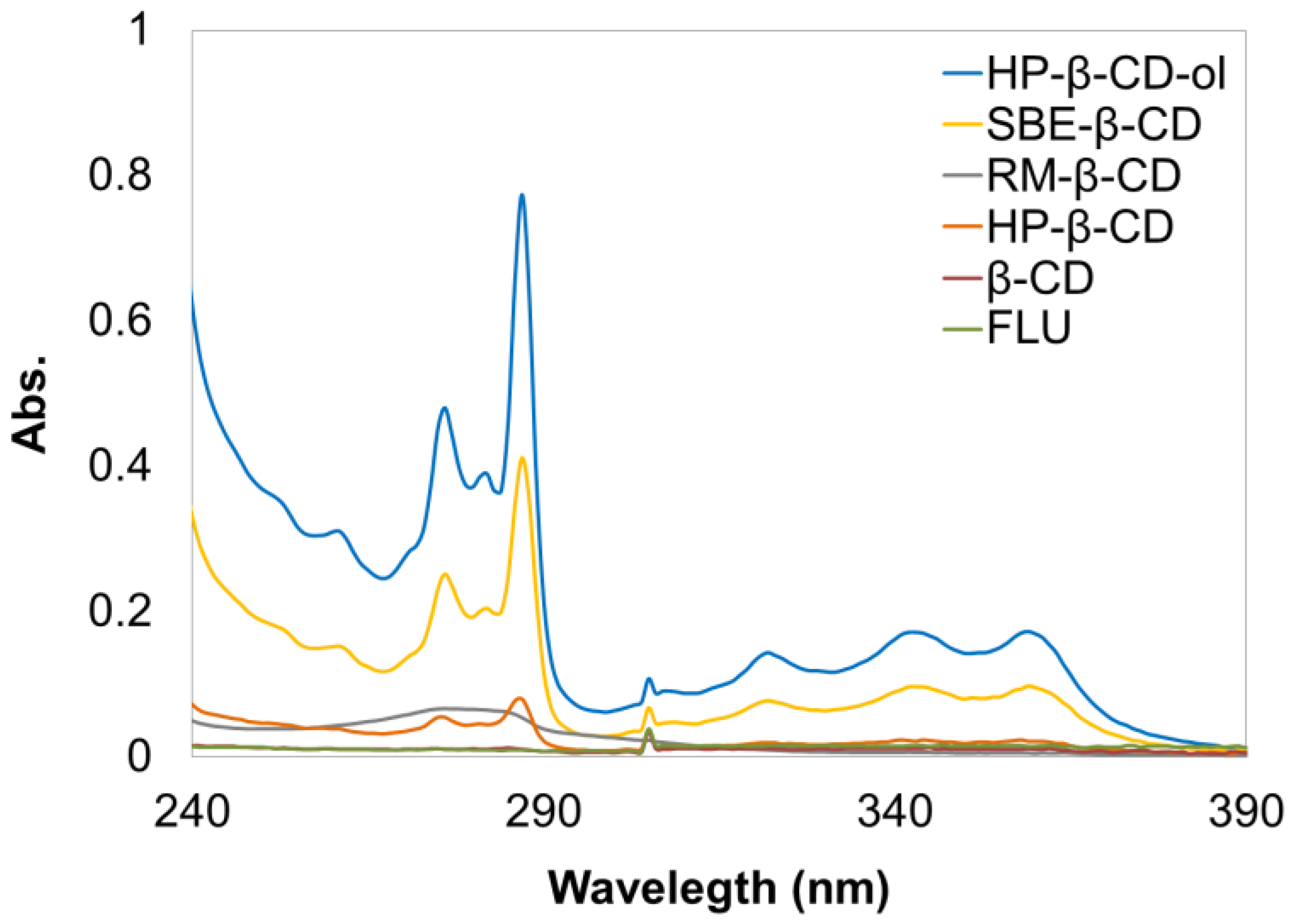
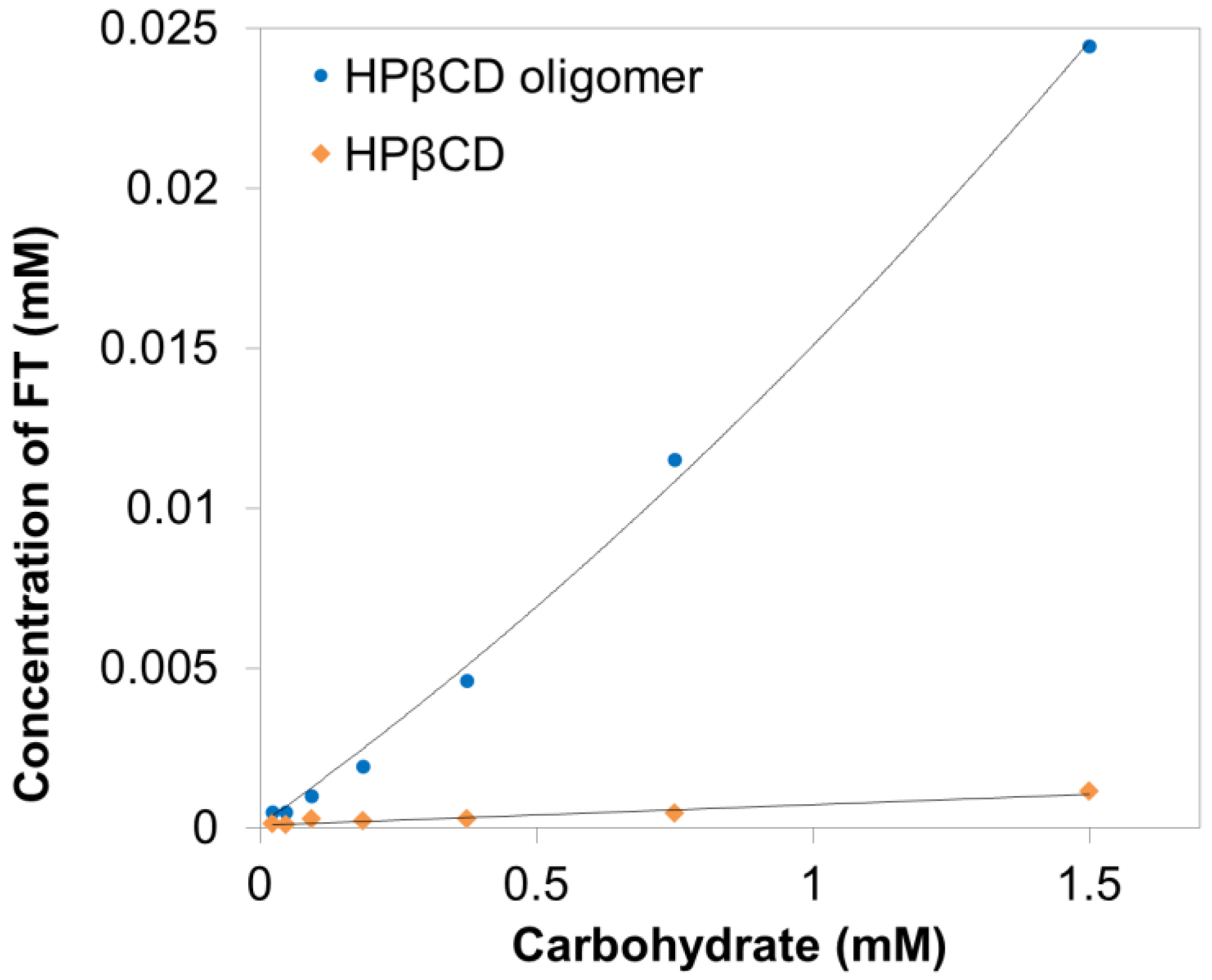
3.2. Solubility Enhancement Test
3.3. Phase Solubility Tests
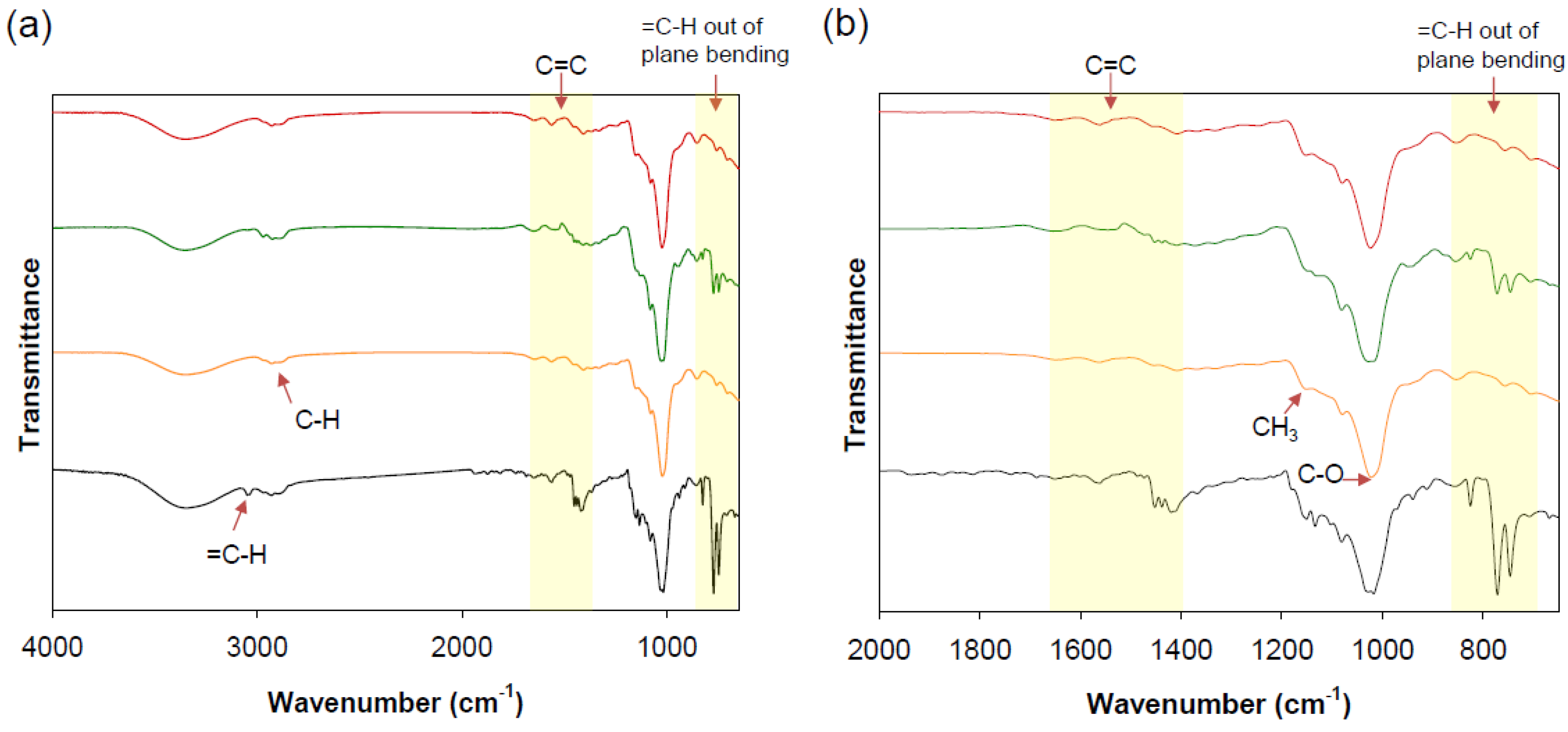
3.4. FT-IR Spectroscopic Analysis
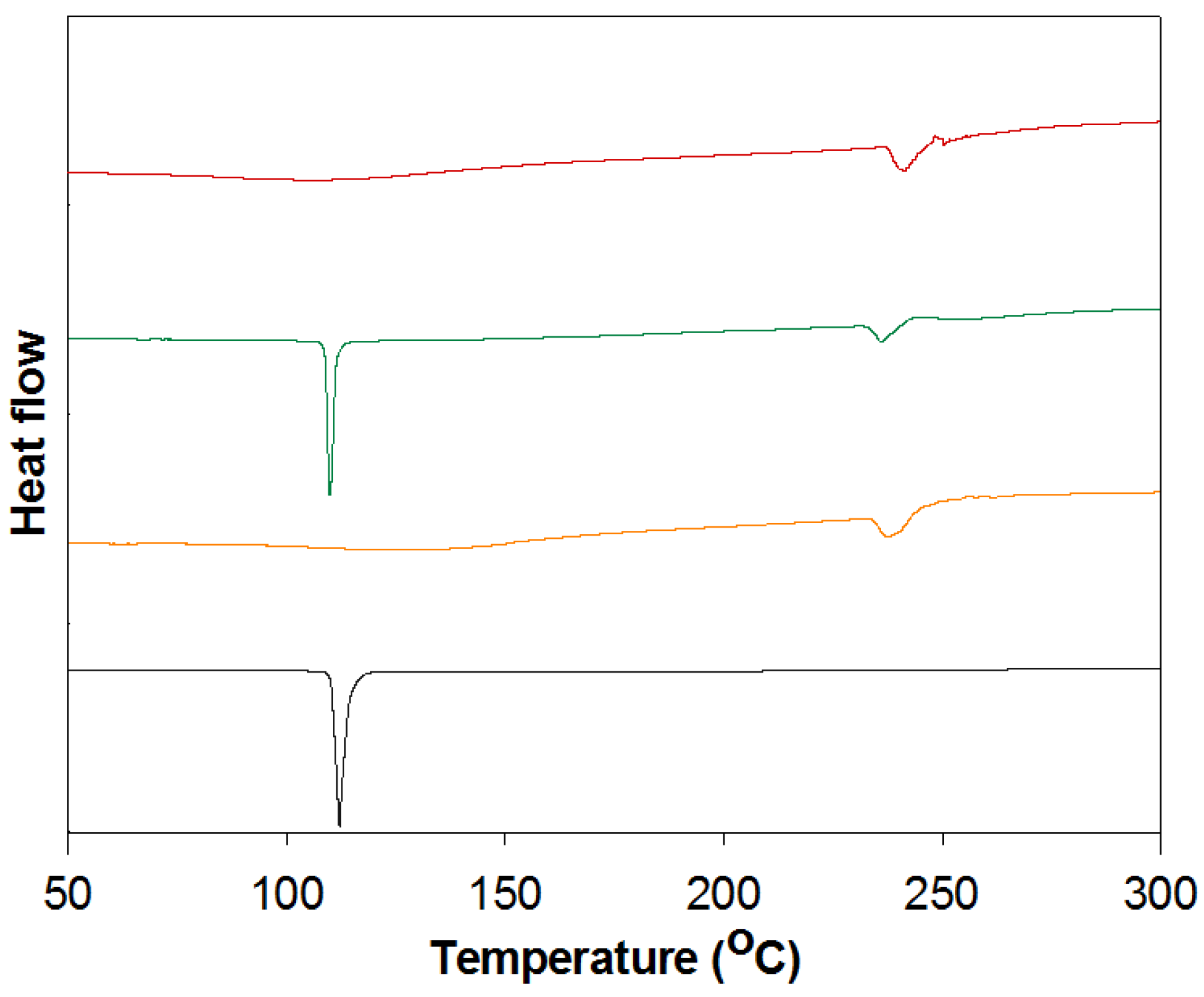
3.5. DSC Analysis
3.6. SEM Analysis
3.7. NOESY Spectroscopy of the FT/HP-β-CD Oligomer Complexes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hilyard, E.J.; Jones-Meehan, J.M.; Spargo, B.J.; Hill, R.T. Enrichment, isolation, and phylogenetic identification of polycyclic aromatic hydrocarbon-degrading bacteria from elizabeth river sediments. Appl. Environ. Microbiol. 2008, 74, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Karcher, W.; Belliardo, J.; Wagstaffe, P. Polycyclic aromatic compounds of environmental and occupational importance. Fresenius Z. Anal. Chem. 1986, 323, 1–10. [Google Scholar] [CrossRef]
- Cerniglia, C.E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 1992, 3, 351–368. [Google Scholar] [CrossRef]
- Volkering, F.; Breure, A.M.; Van Andel, J.; Rulkens, W.H. Influence of nonionic surfactants on bioavailability and biodegradation of polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 1995, 61, 1699–1705. [Google Scholar] [PubMed]
- Bonten, L.T.; Grotenbuis, T.C.; Rulkens, W.H. Enhancement of pah biodegradation in soil by physicochemical pretreatment. Chemosphere 1999, 38, 3627–3636. [Google Scholar] [CrossRef]
- Ying, G.-G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef] [PubMed]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 1983; Volume 32, pp. 355–364. [Google Scholar]
- Storer, J.S.; DeLeon, I.; Millikan, L.E.; Laseter, J.L.; Griffing, C. Human absorption of crude coal tar products. Arch. Dermatol. 1984, 120, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Faust, R.A. Toxicity Summary for Fluoranthene; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1993. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; World Health Organization: Geneva, Switzerland, 2010; Volume 92, pp. 1–853. [Google Scholar]
- Selck, H.; Palmqvist, A.; Forbes, V.E. Biotransformation of dissolved and sediment-bound fluoranthene in the polychaete, capitella sp. I. Environ. Toxicol. Chem. 2003, 22, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Hentati, D.; Chebbi, A.; Loukil, S.; Kchaou, S.; Godon, J.-J.; Sayadi, S.; Chamkha, M. Biodegradation of fluoranthene by a newly isolated strain of bacillus stratosphericus. Environ. Sci. Pollut. Res. 2016, 23, 15088–15100. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.R.; Vyas, A.; Ahmad, A.; Banerjee, S.; Deshpande, J.; Swamy, K.V.; Jamadar, A.; Dumhe-Klaire, A.C.; Padhye, S.; Sarkar, F.H. Inclusion complex of novel curcumin analogue cdf and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm. Res. 2012, 29, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Guo, G.; Ding, K.; Wang, L.; Liu, T.; Yang, F. Effect of bioaugmentation by bacterial consortium and methyl-β-cyclodextrin on soil functional diversity and removal of polycyclic aromatic hydrocarbons. Polycycl. Aromat. Compd. 2017, 1–10. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Pitha, J.; Milecki, J.; Fales, H.; Pannell, L.; Uekama, K. Hydroxypropyl-β-cyclodextrin: Preparation and characterization; effects on solubility of drugs. Int. J. Pharm. 1986, 29, 73–82. [Google Scholar] [CrossRef]
- Gould, S.; Scott, R.C. 2-hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Deratani, A.; Volet, G.; Sebille, B. Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur. Polym. J. 1997, 33, 49–57. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K. Phase Solubility Studies. In Advances in analytical chemistry and instrumentation; Wiley-Interscience: New York, NY, USA, 1965; pp. 117–212. [Google Scholar]
- Higuchi, T.; Connors, A. Phase-solubility techniques. Adv. Chem. Instrume. 1965, 4, 212–217. [Google Scholar]
- Mackay, D.; Bobra, A.; Shiu, W.; Yalkowsky, S. Relationships between aqueous solubility and octanol-water partition coefficients. Chemosphere 1980, 9, 701–711. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins and Their Inclusion Complexes; Akadaemiai Kiadao: Budapest, Hungary, 1982. [Google Scholar]
- Kamphorst, A.; de Sá, I.M.; Faria, A.; Sinisterra, R. Association complexes between ovalbumin and cyclodextrins have no effect on the immunological properties of ovalbumin. Eur. J. Pharm. Biopharm. 2004, 57, 199–205. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, D.R.; Tsuneda, S.S.; Cereda, C.M.; Carvalho, F.D.G.; Preté, P.S.; Fernandes, S.A.; Yokaichiya, F.; Franco, M.K.; Mazzaro, I.; Fraceto, L.F. Development and pharmacological evaluation of ropivacaine-2-hydroxypropyl-β-cyclodextrin inclusion complex. Eur. J. Pharm. Sci. 2008, 33, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Baboota, S.; Dhaliwal, M.; Kohli, K. Physicochemical characterization, in vitro dissolution behavior, and pharmacodynamic studies of rofecoxib-cyclodextrin inclusion compounds. Preparation and properties of rofecoxib hydroxypropyl β-cyclodextrin inclusion complex: A technical note. AAPS PharmSciTech 2005, 6, E83–E90. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef] [PubMed]
- Keepers, J.W.; James, T.L. A theoretical study of distance determinations from NMR. Two-dimensional nuclear overhauser effect spectra. J. Magn. Reson. (1969) 1984, 57, 404–426. [Google Scholar] [CrossRef]
- Kandoth, N.; Choudhury, S.D.; Mukherjee, T.; Pal, H. Host-guest interaction of 1, 4-dihydroxy-9, 10-anthraquinone (quinizarin) with cyclodextrins. Photochem. Photobiol. Sci. 2009, 8, 82–90. [Google Scholar] [CrossRef] [PubMed]
| Apparent stability constants, Km:n (M−1) | Solubilization efficiency (SE) | ||
|---|---|---|---|
| K(1:1) | K(1:2) | ||
| HP-β-CD | 6004 | 8.3 | |
| HP-β-CD-ol | 159,504 | 2195 | 178.3 |
| FT | HP-β-CD oligomer | Physical mixture (PM) | Inclusion complex (IC) | |
|---|---|---|---|---|
| O–H | 3354 | 3356 | 3356 | |
| C–H | 2931 | 2930 | 2926 | 2930 |
| CH2 | 1408 | 1409 | 1408 | |
| CH3 | 1367 | 1372 | 1366 | |
| C–O–C | 1080 | 1081 | 1080 | |
| C–O | 1022 | 1027 | 1024 | |
| ≡C–H | 3348 | |||
| =C–H | 3050 | 3050 | ||
| C=C | 1650 1562 1474 1452 1438 1420 | 1650 1561 1452 1438 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.H.; Choi, J.M.; Cho, E.; Jung, S. Enhanced Solubilization of Fluoranthene by Hydroxypropyl β-Cyclodextrin Oligomer for Bioremediation. Polymers 2018, 10, 111. https://doi.org/10.3390/polym10020111
Park KH, Choi JM, Cho E, Jung S. Enhanced Solubilization of Fluoranthene by Hydroxypropyl β-Cyclodextrin Oligomer for Bioremediation. Polymers. 2018; 10(2):111. https://doi.org/10.3390/polym10020111
Chicago/Turabian StylePark, Kyeong Hui, Jae Min Choi, Eunae Cho, and Seunho Jung. 2018. "Enhanced Solubilization of Fluoranthene by Hydroxypropyl β-Cyclodextrin Oligomer for Bioremediation" Polymers 10, no. 2: 111. https://doi.org/10.3390/polym10020111