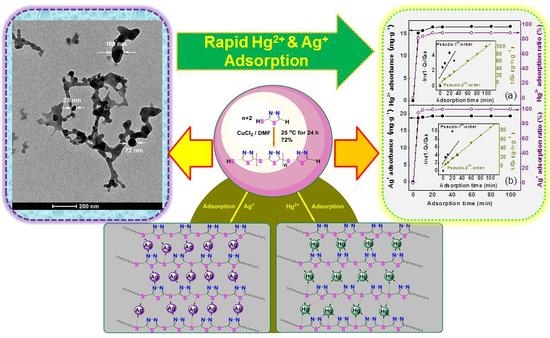
Facile Synthesis, Characterization of Poly-2-mercapto-1,3,4-thiadiazole Nanoparticles for Rapid Removal of Mercury and Silver Ions from Aqueous Solutions
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
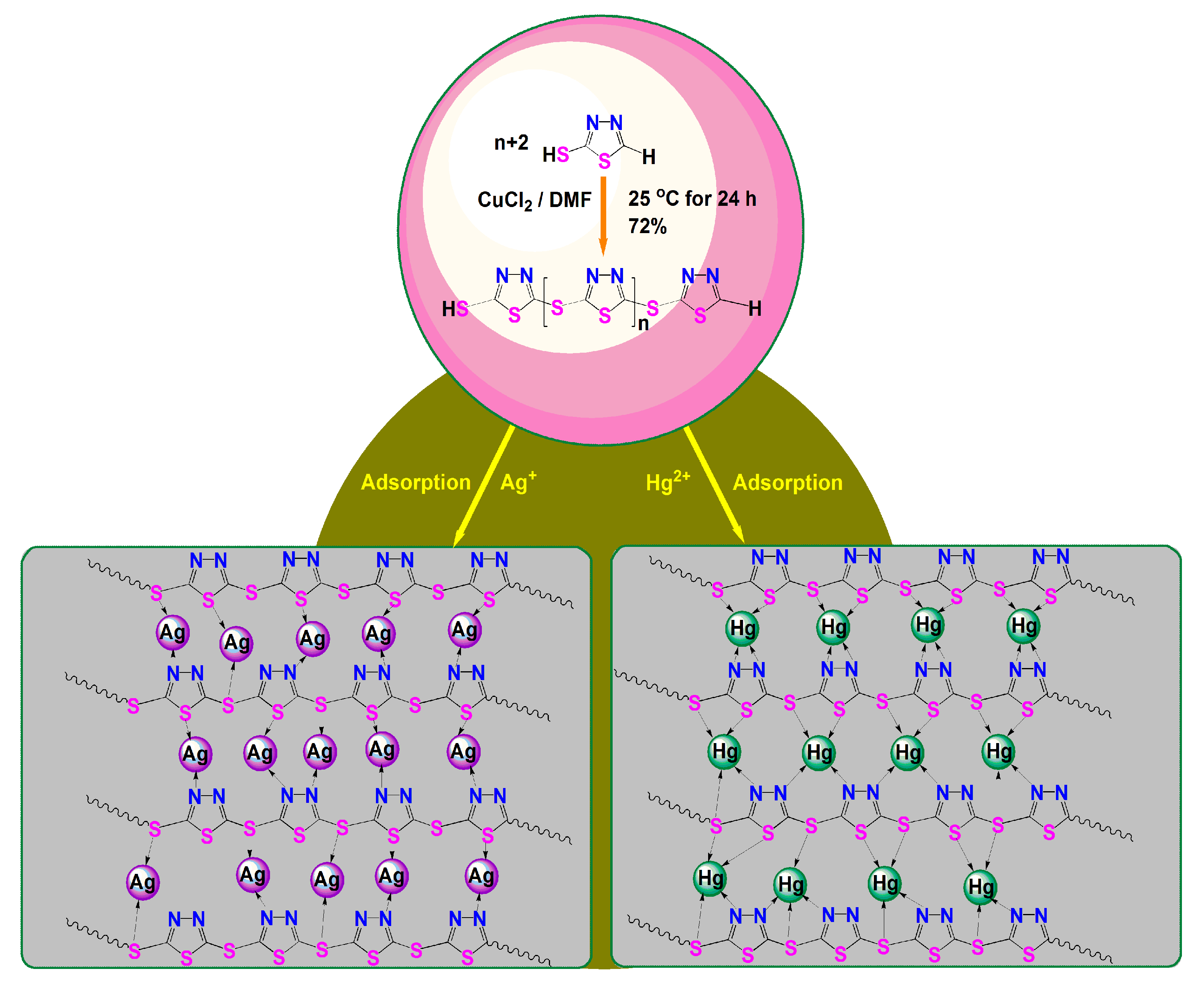
2.2. Synthesis of PTT Nanoparticles
2.3. Adsorption Experiments
2.4. Desorption Experiments
2.5. Characterization and Measurements
3. Results and Discussion
3.1. Synthesis of PTT Nanoparticles
3.1.1. Selection of Oxidant Species
3.1.2. Screening of Polymerization Medium
3.1.3. Optimization of TT Monomer Concentration
3.1.4. Optimization of CuCl2/TT Molar Ratios
3.1.5. Optimization of Polymerization Temperature
3.2. Chemical Structure of PTT Nanoparticles
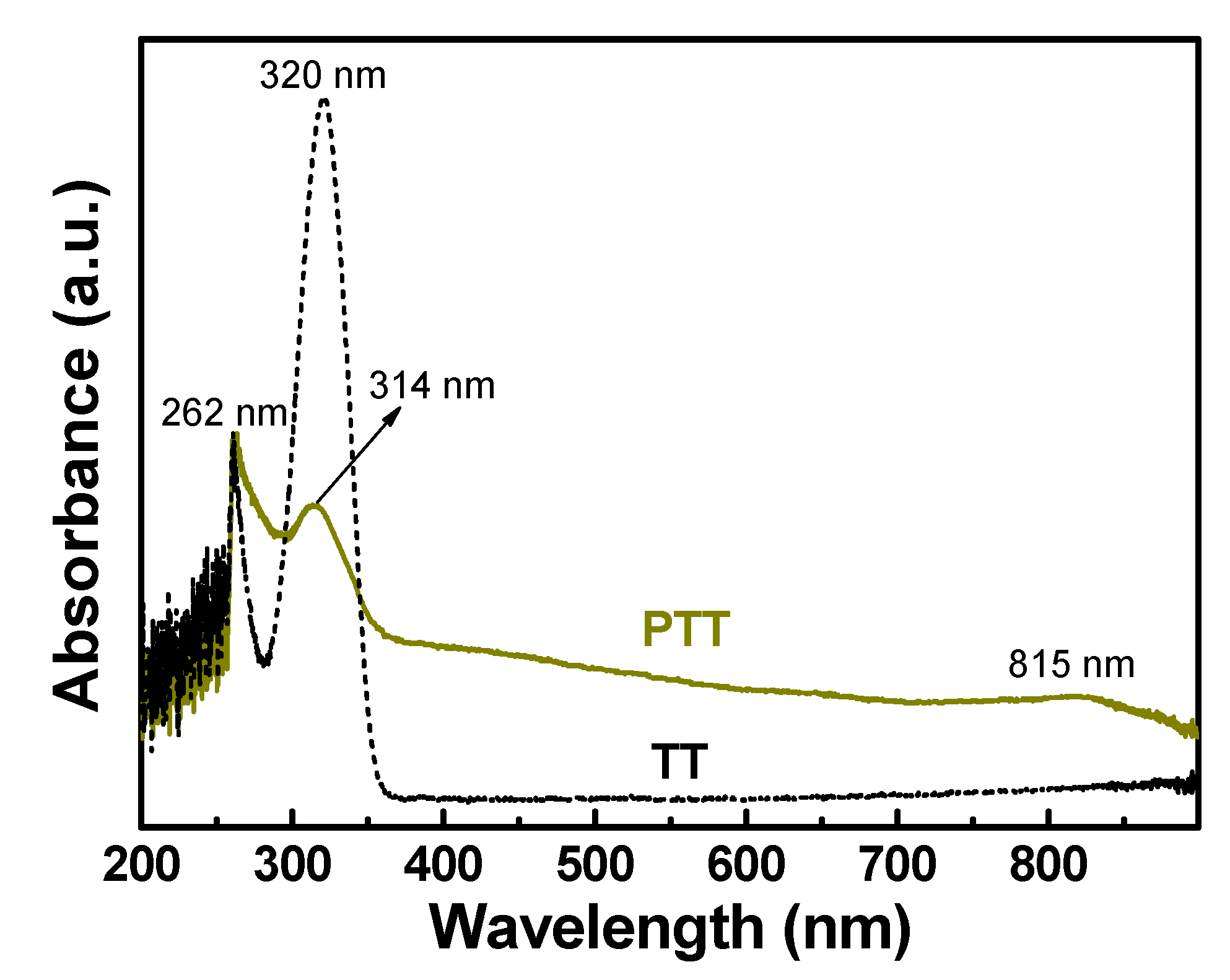
3.2.1. UV-Vis Spectra
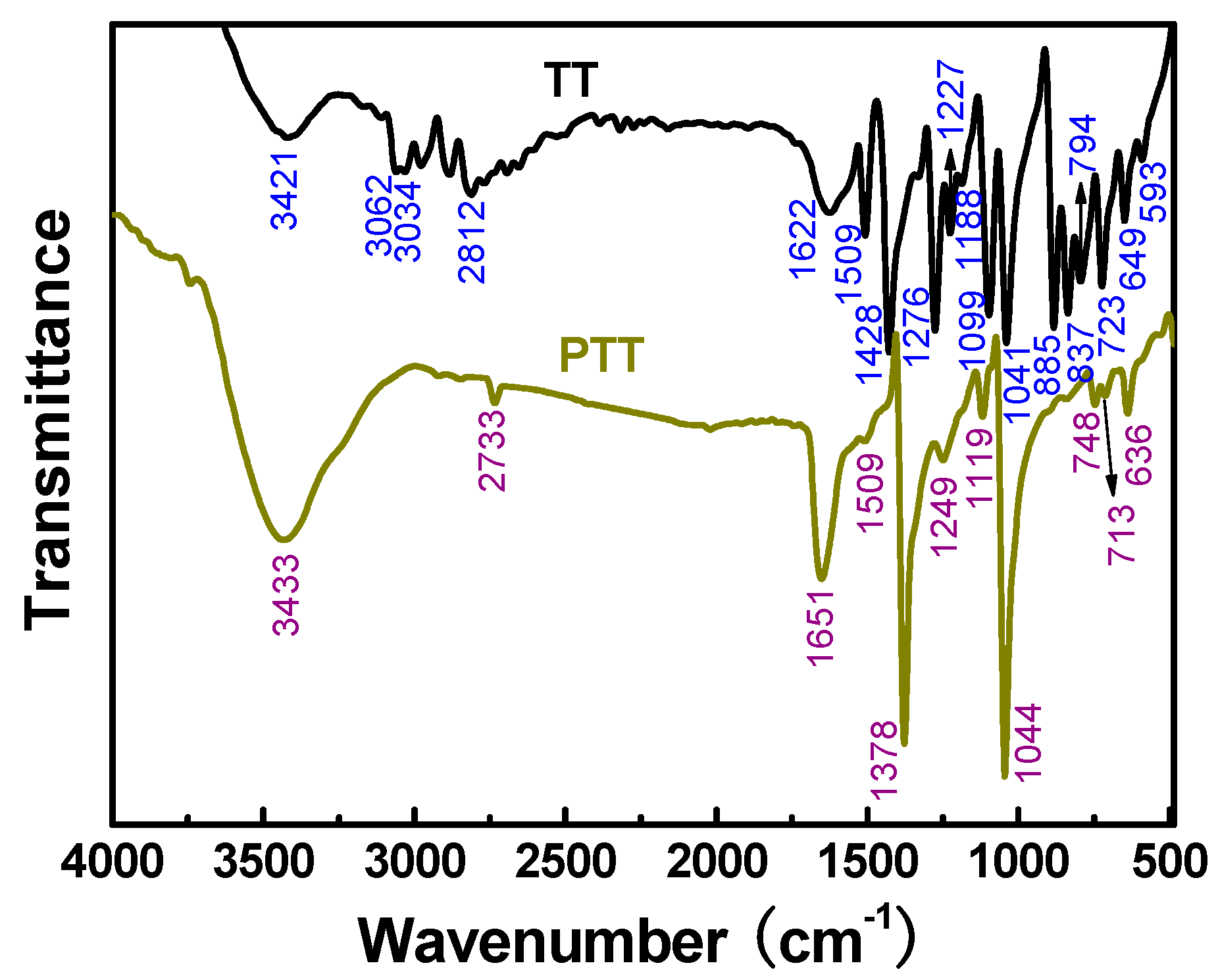
3.2.2. FT-IR Spectra
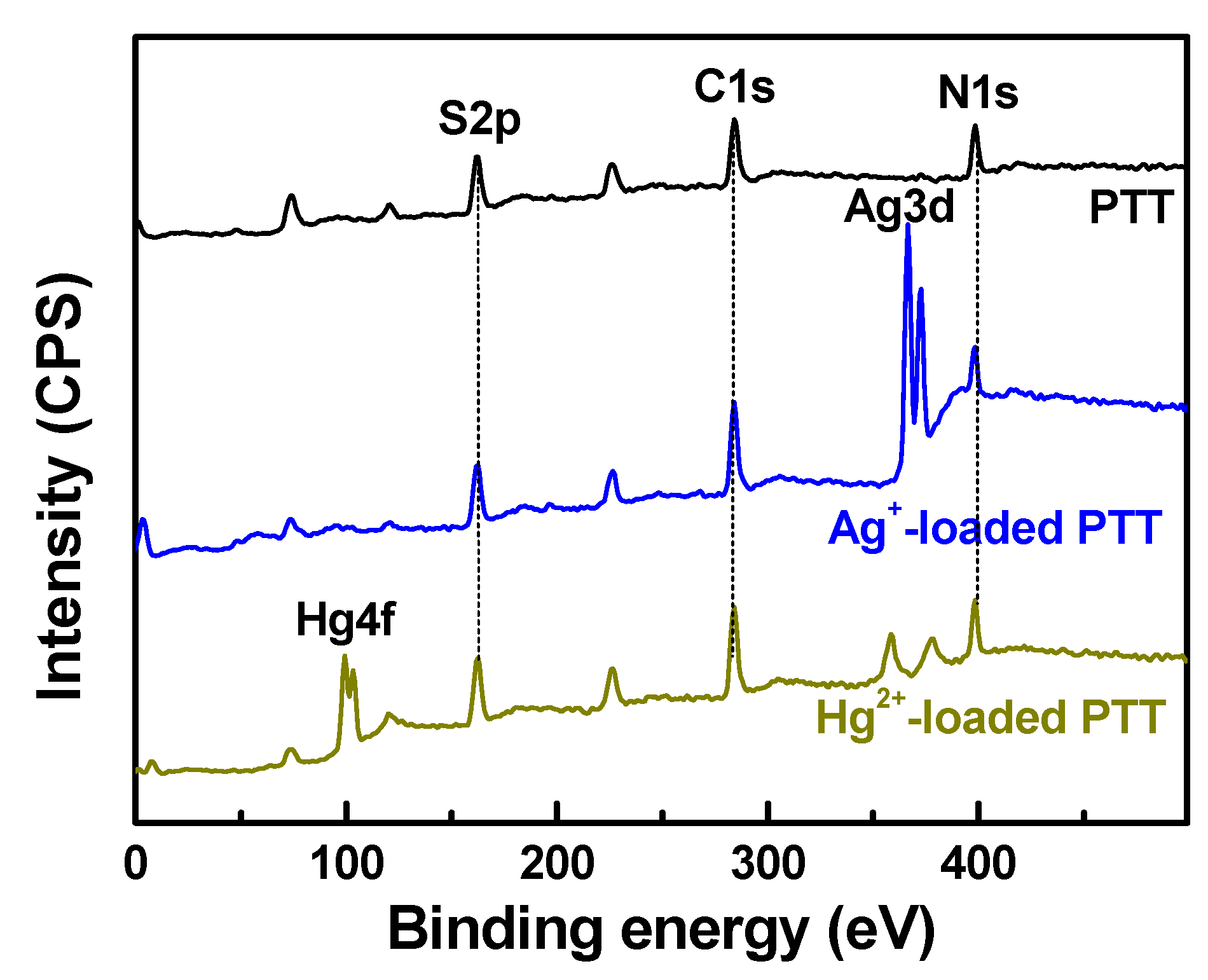
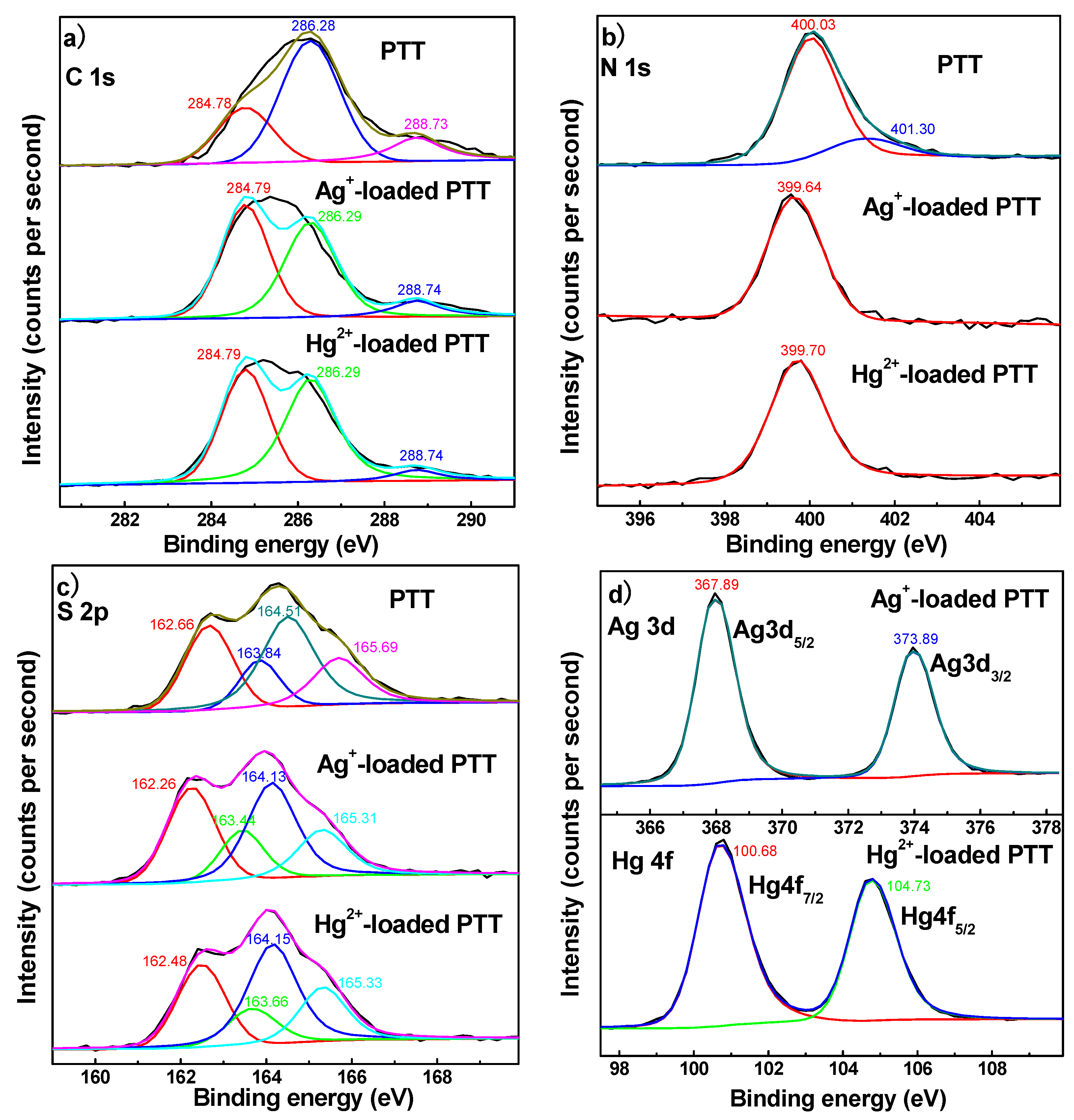
3.2.3. XPS Spectra
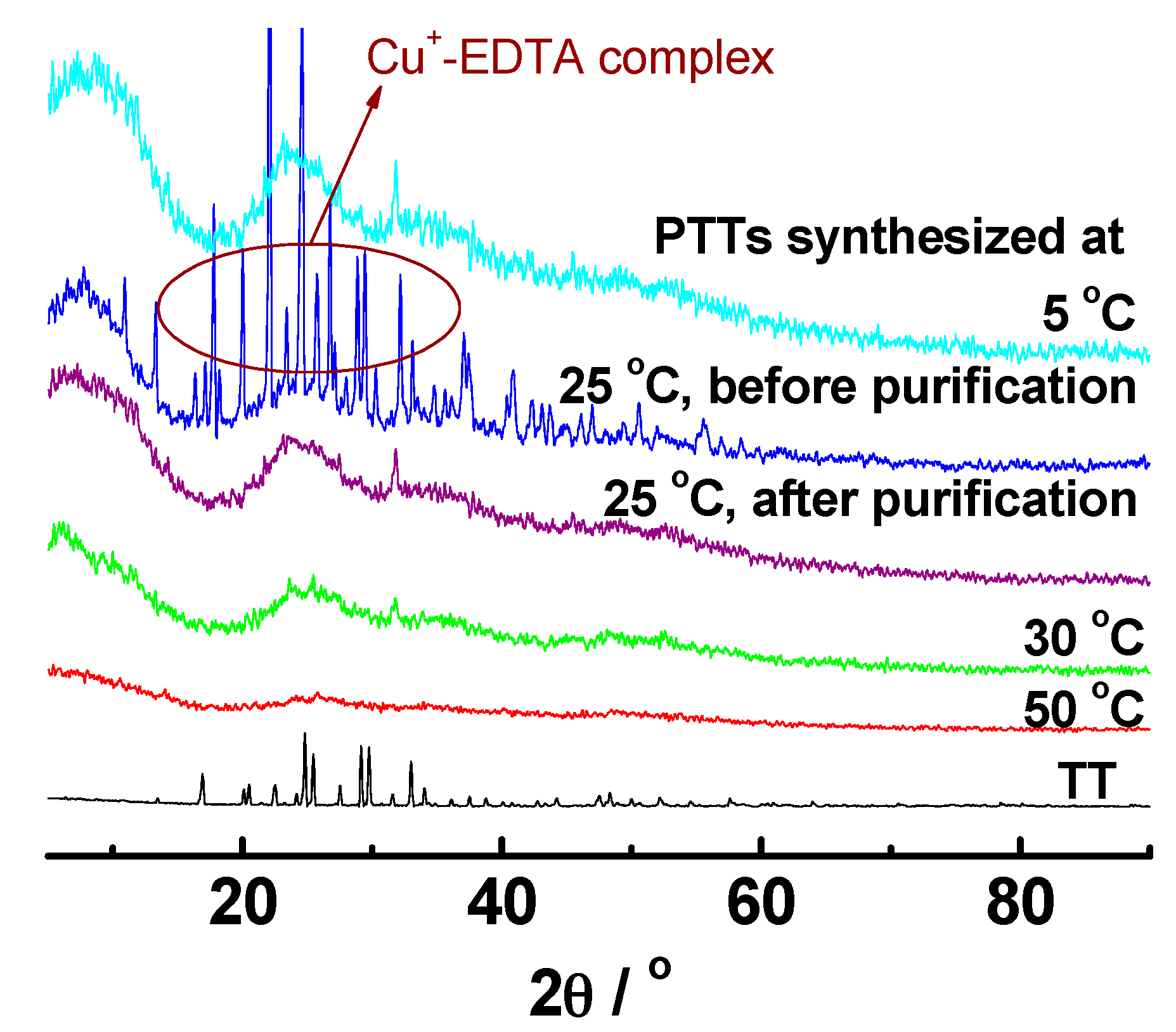
3.2.4. Wide Angle X-Ray Diffraction
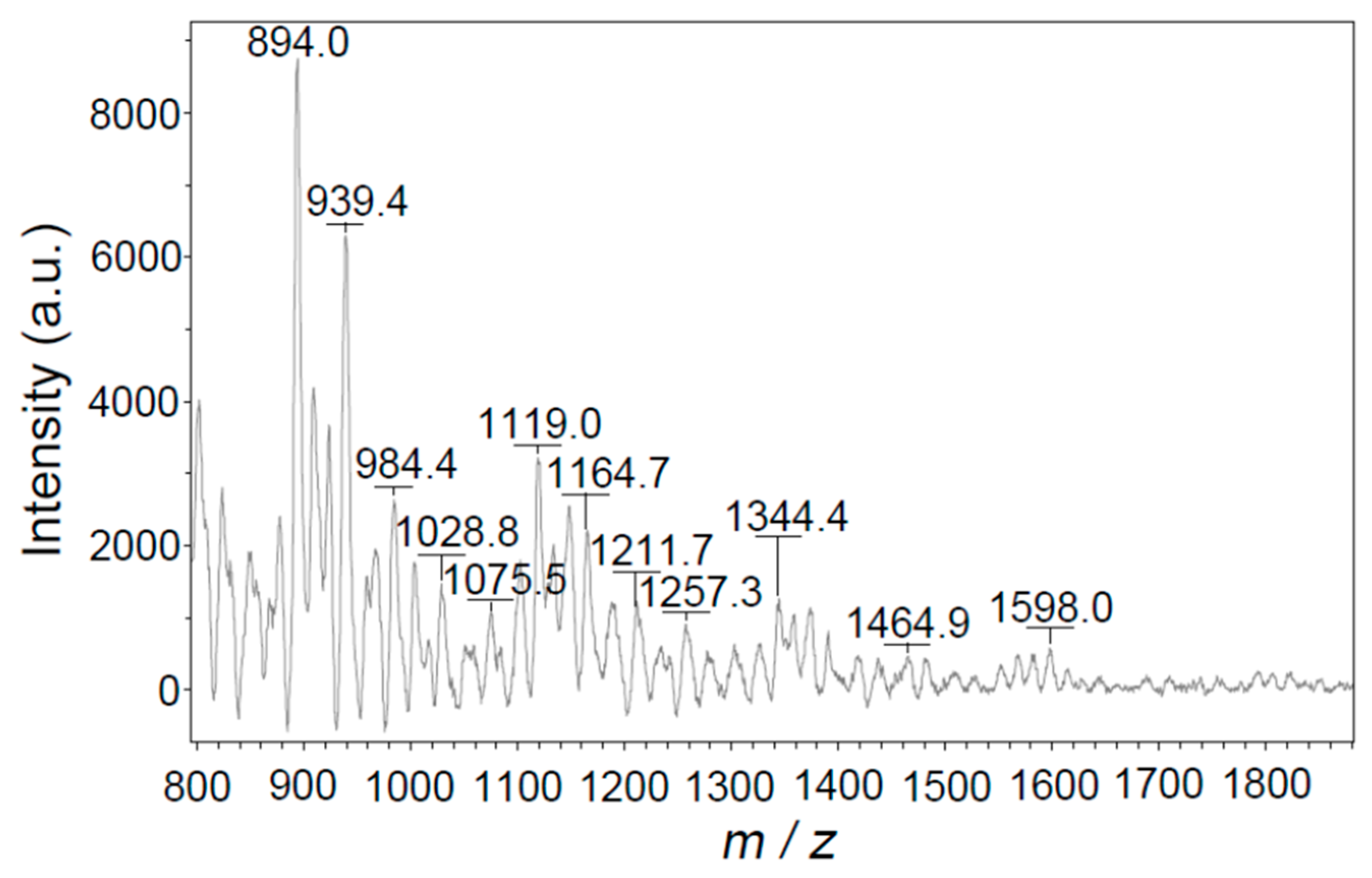
3.2.5. MALDI/TOF Mass Spectra
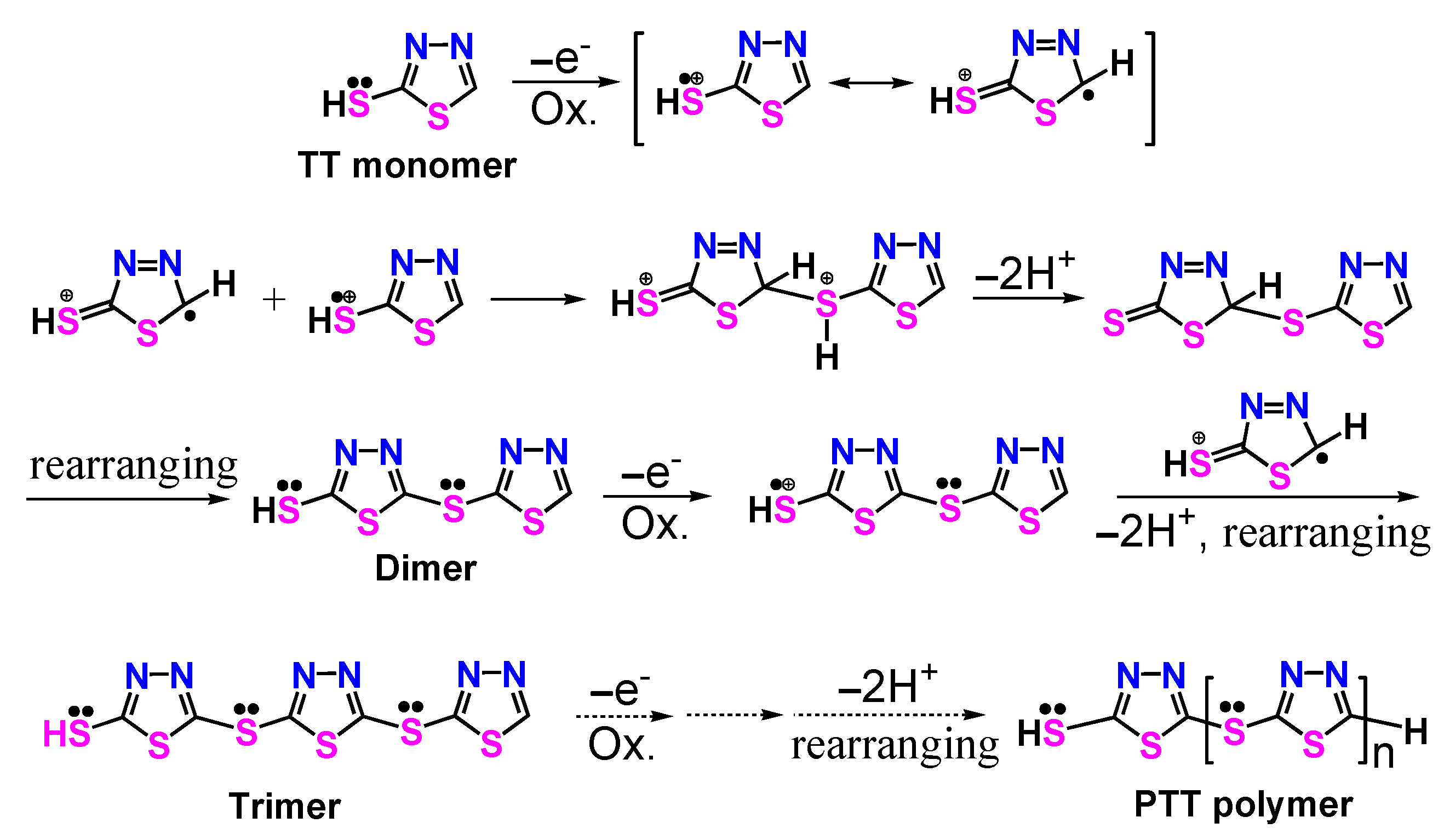
3.3. Polymerization Mechanism of PTT
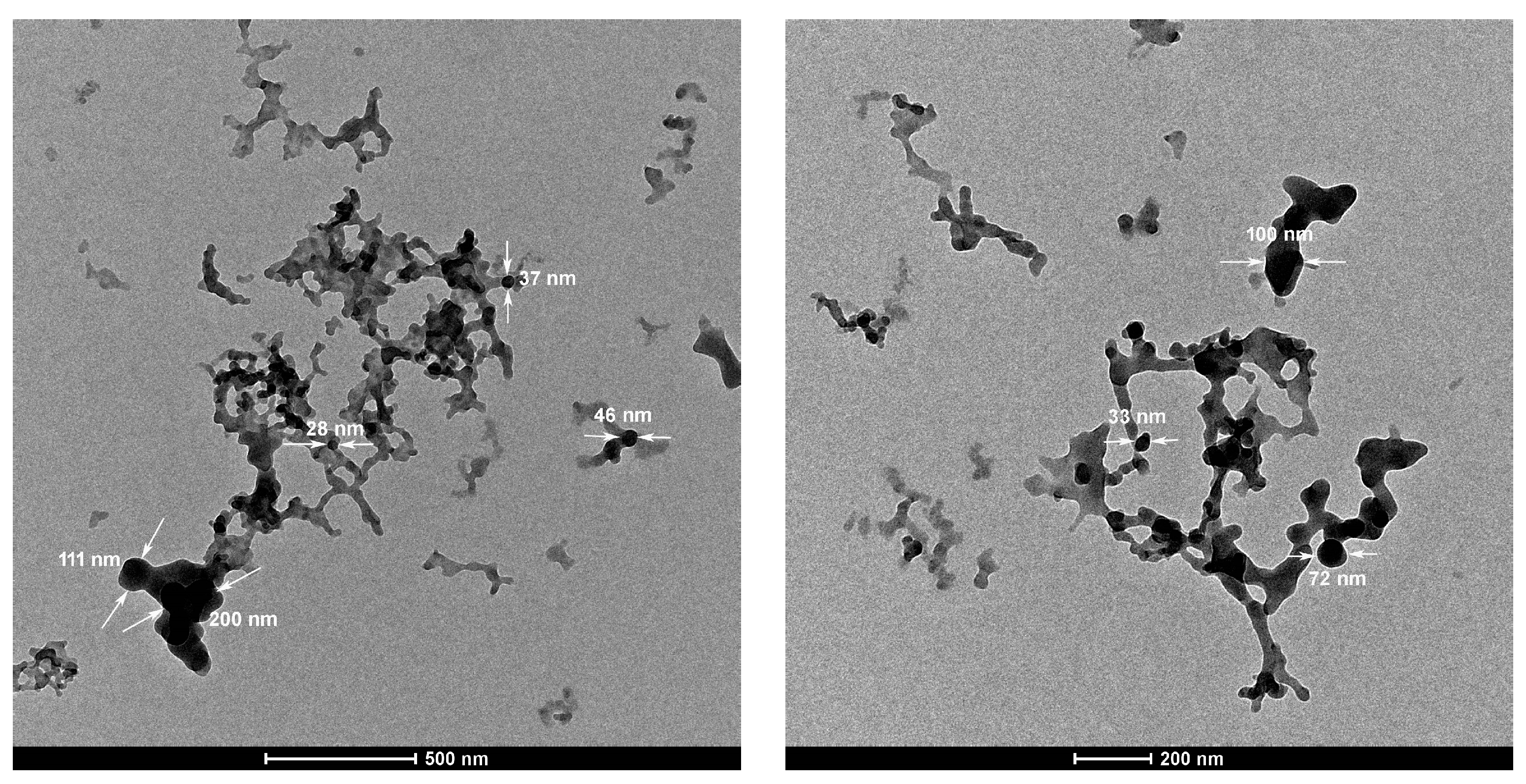
3.4. Morphology of PTT Nanoparticles
3.5. Adsorption of Heavy Metal Ions
3.5.1. Adsorption Capacity
3.5.2. Adsorption Selectivity
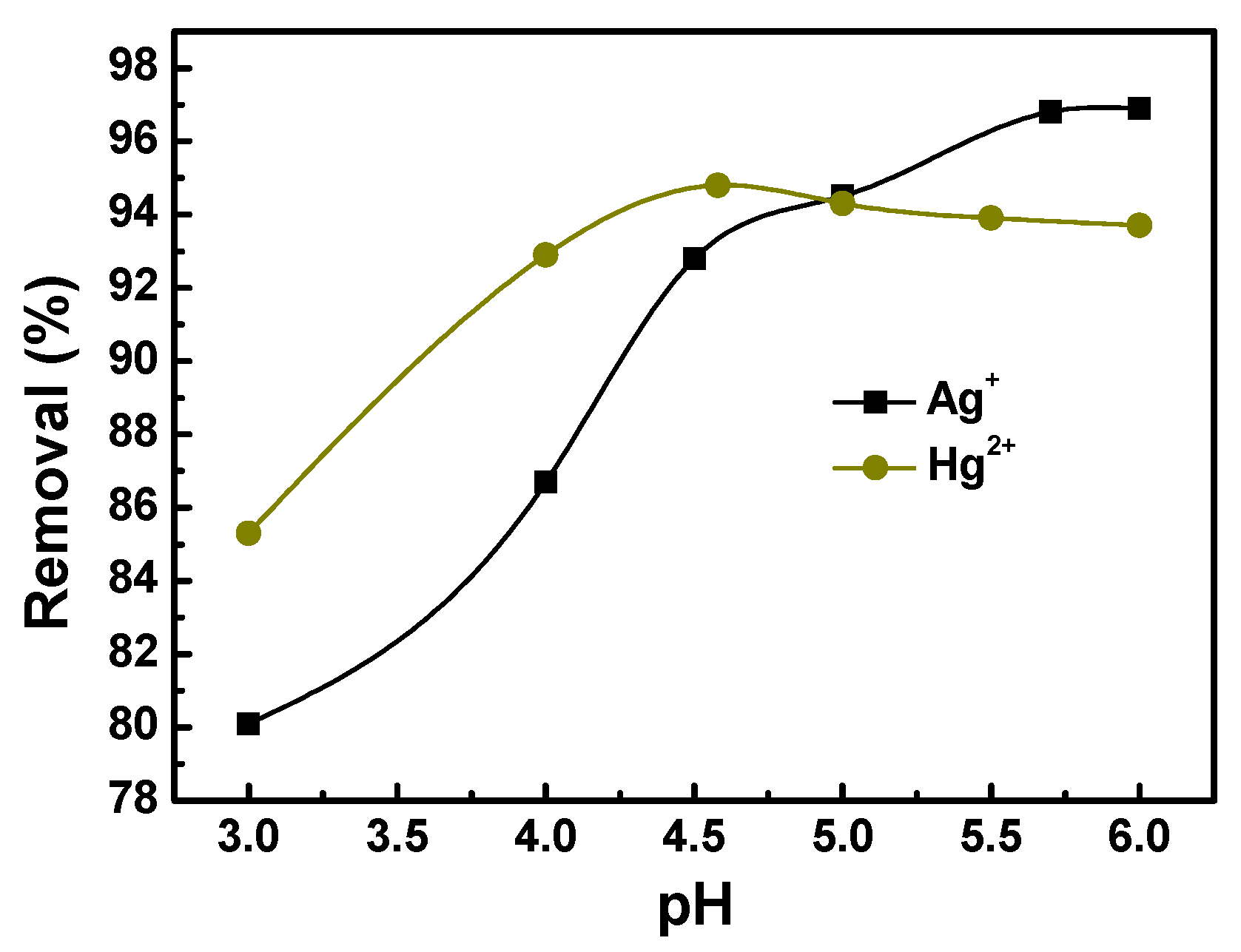
3.5.3. Effect of pH on Metal Adsorption
3.5.4. Adsorption Kinetics
3.5.5. Adsorption Mechanism
3.5.6. Comparison of Ag+ and Hg2+ Adsorbability with Other Adsorbents
3.5.7. Desorption Studies and Regeneration of PTT
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, X.F.; Huang, Y.; Zhang, K.C.; Feng, X.S.; Wang, M.Y. Synthesis and high-performance of carbonaceous polypyrrole nanotubes coated with SnS2 nanosheets anode materials for lithium ion batteries. Chem. Eng. J. 2017, 330, 470–479. [Google Scholar] [CrossRef]
- Baharin, S.N.A.; Sarih, N.M.; Mohamad, S. Novel functionalized polythiophene-coated Fe3O4 nanoparticles for magnetic solid-phase extraction of phthalates. Polymers 2016, 8, 117. [Google Scholar] [CrossRef]
- Parashar, K.; Ballav, N.; Debnath, S.; Pillay, K.; Maity, A. Hydrous ZrO2 decorated polyaniline nanofibres: Synthesis, characterization and application as an efficient adsorbent for water defluoridation. J. Colloid Interf. Sci. 2017, 508, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Xiao, H.; Lackner, K.S.; Chen, X. Capture CO2 from ambient air using nanoconfined ion hydration. Angew. Chem. Int. Ed. Engl. 2016, 55, 4026–4029. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Li, Q.B.; Wang, T.; Lackner, K.S. Kinetic analysis of an anion exchange absorbent for CO2 capture from ambient air. PLoS ONE 2017, 12, e0179828. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, G.; Gunendi, G.; Zeybek, D.K.; Zeybek, B.; Pekyardimci, S. A novel electrochemical DNA biosensor based on poly-(5-amino-2-mercapto-1,3,4-thiadiazole) modified glassy carbon electrode for the determination of nitrofurantoin. Sens. Actuators B Chem. 2014, 197, 211–219. [Google Scholar] [CrossRef]
- Shouji, E.; Oyama, N. Examination of the cleavage and formation of the disulfide bond in poly[dithio-2,5-(1,3,4-thiadiazole)] by redox reaction. J. Electroanal. Chem. 1996, 410, 229–234. [Google Scholar] [CrossRef]
- Jin, L.F.; Wang, G.C.; Li, X.W.; Li, L.B. Poly(2,5-dimercapto-1,3,4-thiadiazole)/sulfonated graphene composite as cathode material for rechargeable lithium batteries. J. Appl. Electrochem. 2011, 41, 377–382. [Google Scholar] [CrossRef]
- Kalimuthu, P.; John, S.A. Modification of electrodes with nanostructured functionalized thiadiazole polymer film and its application to the determination of ascorbic acid. Electrochim. Acta 2009, 55, 183–189. [Google Scholar] [CrossRef]
- He, J.B.; Qi, F.; Wang, Y.; Deng, N. Solid carbon paste-based amperometric sensor with electropolymerized film of 2-amino-5-mercapto-1,3,4-thiadiazole. Sens. Actuators B Chem. 2010, 145, 480–487. [Google Scholar] [CrossRef]
- Varghese, A.; Chitravathi, S.; Munichandraiah, N. Electrocatalytic oxidation and determination of morin at a poly(2,5-dimercapto-1,3,4-thiadiazole) modified carbon fiber paper electrode. J. Electrochem. Soc. 2016, 163, B471–B477. [Google Scholar] [CrossRef]
- Pope, J.M.; Sato, T.; Shoji, E.; Oyama, N.; White, K.C.; Buttry, D.A. Organosulfur/conducting polymer composite cathodes II. Spectroscopic determination of the protonation and oxidation states of 2,5-dimercapto-1,3,4-thiadiazole. J. Electrochem. Soc. 2002, 149, A939–A952. [Google Scholar] [CrossRef]
- Gao, J.; Lowe, M.A.; Conte, S.; Burkhardt, S.E.; Abruna, H.D. Poly(2,5-dimercapto-1,3,4-thiadiazole) as a cathode for rechargeable lithium batteries with dramatically improved performance. Chem.-Eur. J. 2012, 18, 8521–8526. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.S.; Zhang, R.F.; Wang, D.Q. Catena-Poly[[trimethyltin(IV)]-μ-[5-(2-thienyl- methyleneamino)-1,3,4-thiadiazole-2-thiolato-κ2N4:S2]]. Acta Crystallogr. Sect. E 2007, 63, m418–m419. [Google Scholar] [CrossRef]
- Qin, J.H.; Wang, J.G.; Hu, P.Z. Catena-Poly[[[bis[2,2′-(propane-1,3-diyl-dithio) bis(1,3,4-thiadiazole)-κN4]-copper(II)]-bis[μ-2,2′-(propane-1,3-diyl-dithio)bis(1,3,4-thiadiazole)-κ2N4:N4′]]bis(perchlorate)]. Acta Crystallogr. Sect. E 2009, 65, m349–m350. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.H.; Wang, J.G.; Hu, P.Z. Crystal structure of ethanolatopenta {2,2′-[1,2-propanedithio-bis(1,3,4-thiadiazole)]} dicopper(II) tetrakisperchlorate, [Cu2(C2H5OH)(C7H8N4S4)5][ClO4]4. Z. Kristallogr. New Cryst. Struct. 2010, 225, 339–342. [Google Scholar]
- Tiwari, M.; Gupta, S.; Prakash, R. One pot synthesis of coordination polymer 2,5-dimercapto-1,3,4-thiadiazole-gold and its application in voltammetric sensing of resorcinol. RSC Adv. 2014, 4, 25675–25682. [Google Scholar] [CrossRef]
- Huang, S.J.; Ma, C.Z.; Liao, Y.Z.; Min, C.G.; Du, P.; Jiang, Y.B. Removal of mercury(II) from aqueous solutions by adsorption on poly(1-amino-5-chloroanthraquinone) nanofibrils: Equilibrium, kinetics, and mechanism studies. J. Nanomater. 2016, 2016, 7245829. [Google Scholar] [CrossRef]
- Kim, B.J.; Bae, K.M.; An, K.H.; Park, S.J. Elemental mercury adsorption behaviors of chemically modified activated carbons. Bull. Korean Chem. Soc. 2011, 32, 1321–1326. [Google Scholar] [CrossRef]
- Yardim, M.F.; Budinova, T.; Ekinci, E.; Petrov, N.; Razvigorova, M.; Minkova, V. Removal of mercury (II) from aqueous solution by activated carbon obtained from furfural. Chemosphere 2003, 52, 835–841. [Google Scholar] [CrossRef]
- Fu, L.K.; Zhang, L.B.; Wang, S.X.; Peng, J.H.; Zhang, G.W. Silica nanoparticles modified with trithiocyanuric acid as a potential adsorbent for removal of Ag+ from aqueous solutions. Water Air Soil Pollut. 2017, 228, 273. [Google Scholar] [CrossRef]
- Pourreza, N.; Rastegarzadeh, S.; Larki, A. Nano-TiO2 modified with 2-mercaptobenzimidazole as an efficient adsorbent for removal of Ag(I) from aqueous solutions. J. Ind. Eng. Chem. 2014, 20, 127–132. [Google Scholar] [CrossRef]
- Celik, Z.; Gulfen, M.; Aydin, A.O. Synthesis of a novel dithiooxamide-formaldehyde resin and its application to the adsorption and separation of silver ions. J. Hazard. Mater. 2010, 174, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Q.; Zhang, W.; Gai, L.G.; Jiang, H.H.; Wang, Y.; Zhao, L.C. Dedoped Fe3O4/PPy nanocomposite with high anti-interfering ability for effective separation of Ag(I) from mixed metal-ion solution. Chem. Eng. J. 2015, 280, 197–205. [Google Scholar] [CrossRef]
- Yang, G.W.; Han, H.Y.; Du, C.Y.; Luo, Z.H.; Wang, Y.J. Facile synthesis of melamine-based porous polymer networks and their application for removal of aqueous mercury ions. Polymer 2010, 51, 6193–6202. [Google Scholar] [CrossRef]
- Fu, L.K.; Zhang, L.B.; Wang, S.X.; Peng, J.H.; Zhang, G.W. Selective adsorption of Ag+ by silica nanoparticles modified with 3-Amino-5-mercapto-1,2,4-triazole from aqueous solutions. J. Mol. Liq. 2017, 241, 292–300. [Google Scholar] [CrossRef]
- Li, X.G.; Feng, H.; Huang, M.R. Strong adsorbability of mercury ions on aniline/sulfoanisidine copolymer nanosorbents. Chem. A Eur. J. 2009, 15, 4573–4581. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Z.; Deng, B.L.; Yang, J.; Gang, D.C. Modifying activated carbon with hybrid ligands for enhancing aqueous mercury removal. Carbon 2009, 47, 2014–2025. [Google Scholar] [CrossRef]
- Song, X.T.; Niu, Y.Z.; Qiu, Z.M.; Zhang, Z.X.; Zhou, Y.Z.; Zhao, J.J.; Chen, H. Adsorption of Hg(II) and Ag(I) from fuel ethanol by silica gel supported sulfur-containing PAMAM dendrimers: Kinetics, equilibrium and thermodynamics. Fuel 2017, 206, 80–88. [Google Scholar] [CrossRef]
- Jia, Y.F.; Demopoulos, G.P. Adsorption of silver onto activated carbon from acidic media: Nitrate and sulfate media. Ind. Eng. Chem. Res. 2003, 42, 72–79. [Google Scholar] [CrossRef]
- Billinge, S.J.L.; McKimmy, E.J.; Shatnawi, M.; Kim, H.J.; Petkov, V.; Wermeille, D.; Pinnavaia, T.J. Mercury binding sites in thiol-functionalized mesostructured silica. J. Am. Chem. Soc. 2005, 127, 8492–8498. [Google Scholar] [CrossRef] [PubMed]
- Denizli, A.; Senel, S.; Alsancak, G.; Tuzmen, N.; Say, R. Mercury removal from synthetic solutions using poly(2-hydroxyethylmethacrylate) gel beads modified with poly(ethyleneimine). React. Funct. Polym. 2003, 55, 121–130. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Modification and characterization of PET fibers for fast removal of Hg(II), Cu(II) and Co(II) metal ions from aqueous solutions. J. Hazard. Mater. 2013, 250, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.M.; Li, C.X.; Qu, R.J.; Zhang, Y.; Zhang, B.D.; Kuang, Y.Z. Syntheses of diethylenetriamine-bridged polysilsesquioxanes and their structure-adsorption properties for Hg(II) and Ag(I). Chem. Eng. J. 2014, 240, 369–378. [Google Scholar] [CrossRef]
- Monier, M.; Nawar, N.; Abdel-Latif, D.A. Preparation and characterization of chelating fibers based on natural wool for removal of Hg(II), Cu(II) and Co(II) metal ions from aqueous solutions. J. Hazard. Mater. 2010, 184, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Helleur, R. Selective adsorption of Ag+ by ion-imprinted O-carboxymethyl chitosan beads grafted with thiourea-glutaraldehyde. Chem. Eng. J. 2015, 264, 56–65. [Google Scholar] [CrossRef]
- Huang, S.J.; Ma, C.Z.; Liao, Y.Z.; Min, C.G.; Du, P.; Zhu, Y.Q.; Jiang, Y.B. Superb adsorption capacity and mechanism of poly(1-amino-5-chloroanthraquinone) nanofibrils for lead and trivalent chromium ions. React. Funct. Polym. 2016, 106, 76–85. [Google Scholar] [CrossRef]
- Xue, Y.; Sheng, Z.H.; Zhao, H.; Wu, Z.J.; Li, X.J.; He, Y.J.; Yuan, Z.B. Electrochemical synthesis and characterization of a novel thiazole-based copolymer and its application in biosensor. Electrochim. Acta 2012, 59, 256–263. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Zou, H.; Qian, W.; Liao, Y.Z. Simple synthesis of conducting poly(2-aminothiazole) with high molecular weight. Colloid Polym. Sci. 2015, 293, 2027–2034. [Google Scholar] [CrossRef]
- Wei, Y.; Focke, W.W.; Wnek, G.E.; Ray, A.; MacDiarmid, A.G. Synthesis and electrochemistry of alkyl ring-substituted polyanilines. J. Phys. Chem. 1989, 93, 495–499. [Google Scholar] [CrossRef]
- Kalimuthu, P.; John, S.A. Simultaneous determination of epinephrine, uric acid and xanthine in the presence of ascorbic acid using an ultrathin polymer film of 5-amino-1,3,4-thiadiazole-2-thiol modified electrode. Anal. Chim. Acta 2009, 647, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Sun, X.Y. Spectrometric Identification of Organic Molecular Structures, 1st ed.; Science Press: Beijing, China, 2003; pp. 373–388. ISBN 7-03-010866-3. [Google Scholar]
- Vieira, R.S.; Oliveira, M.L.M.; Guibal, E.; Rodriguez-Castellon, E.; Beppu, M.M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf. A 2011, 374, 108–114. [Google Scholar] [CrossRef]
- Kalimuthu, P.; John, S.A. Nanostructured electropolymerized film of 5-amino-2-mercapto-1,3,4-thiadiazole on glassy carbon electrode for the selective determination of L-cysteine. Electrochem. Commun. 2009, 11, 367–370. [Google Scholar] [CrossRef]
- Huang, S.J.; Min, C.G.; Liao, Y.Z.; Du, P.; Sun, H.; Zhu, Y.Q.; Ren, A.M. Intrinsically conducting polyaminoanthraquinone nanofibrils: Interfacial synthesis, formation mechanism and lead adsorbents. RSC Adv. 2014, 4, 47657–47669. [Google Scholar] [CrossRef]
- Huang, M.R.; Huang, S.J.; Li, X.G. Facile Synthesis of polysulfoaminoanthraquinone nanosorbents for rapid removal and ultrasensitive fluorescent detection of heavy metal ions. J. Phys. Chem. C 2011, 115, 5301–5315. [Google Scholar] [CrossRef]
- Ando, S.; Ueda, M. Density functional theory calculations of the local spin densities of 3-substituted thiophenes and the oligomerization mechanism of 3-methylsulfanyl thiophene. Synth. Met. 2002, 129, 207–213. [Google Scholar] [CrossRef]
- Doskocz, J.; Doskocz, M.; Roszak, S.; Soloducho, J.; Leszcynski, J. Theoretical studies of symmetric five-membered heterocycle derivatives of carbazole and fluorene: Precursors of conducting polymers. J. Phys. Chem. A 2006, 110, 13989–13994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Li, X.J.; Liang, H.J.; Ning, J.L.; Zhou, Z.D.; Li, G.Y. Equilibrium, kinetics and mechanism of Au3+, Pd2+ and Ag+ ions adsorption from aqueous solutions by graphene oxide functionalized persimmon tannin. Mater. Sci. Eng. C 2017, 79, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Huang, C.F.; Hsu, W.J.; Chang, F.C. Removal of Hg2+ from aqueous solution using alginate gel containing chitosan. J. Appl. Polym. Sci. 2007, 104, 2896–2905. [Google Scholar] [CrossRef]
- Zhou, L.M.; Liu, Z.R.; Liu, J.H.; Huang, Q.W. Adsorption of Hg(II) from aqueous solution by ethylenediamine-modified magnetic crosslinking chitosan microspheres. Desalination 2010, 258, 41–47. [Google Scholar] [CrossRef]
- Atia, A.A.; Donia, A.M.; Shahin, A.E. Studies on the uptake behavior of a magnetic Co3O4-containing resin for Ni(II), Cu(II) and Hg(II) from their aqueous solutions. Sep. Purif. Technol. 2005, 46, 208–213. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, R.B. Mechanisms and kinetics of humic acid adsorption onto chitosancoated granules. J. Colloid Interface Sci. 2003, 264, 30–38. [Google Scholar] [CrossRef]
- Huang, M.R.; Lu, H.J.; Li, X.G. Synthesis and strong heavy-metal ion sorption of copolymer microparticles from phenylenediamine and its sulfonate. J. Mater. Chem. 2012, 22, 17685–17699. [Google Scholar] [CrossRef]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Wang, D.F.; Xie, H.Z.; Won, S.W.; Cui, L.Z.; Wu, G.P. Adsorption of Ag (I) from aqueous solution by waste yeast: Kinetic, equilibrium and mechanism studies. Bioprocess Biosyst. Eng. 2015, 38, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ghadikolaei, N.J.; Bahramifar, N.; Ashrafi, F. Synthesis of NH2-MCM-41 nano porous adsorbent and using it for Zn and Ag metals removal from aqueous solutions by adsorption method and studying effect of some physicochemical parameters on it. Res. J. Appl. Sci. Eng. Technol. 2013, 6, 26–32. [Google Scholar] [CrossRef]
- Genc, O.; Arpa, C.; Bayramoglu, G.; Arica, M.Y.; Bektas, S. Selective recovery of mercury by Procion Brown MX 5BR immobilized poly(hydroxyethylmethacrylate/chitosan) composite membranes. Hydrometallurgy 2002, 67, 53–62. [Google Scholar] [CrossRef]
- Say, R.; Birlik, E.; Erdemgil, Z.; Denizli, A.; Ersoz, A. Removal of mercury species with dithiocarbamate-anchored polymer/organosmectite composites. J. Hazard. Mater. 2008, 150, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Nouri, R.; Tahmasebi, E.; Morsali, A. Capability of magnetic functional metal-organic nanocapsules for removal of mercury(II) ions. Mater. Chem. Phys. 2017, 198, 310–316. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg(II), Cd(II) and Zn(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 209, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Bandegharaei, A.; Hosseini, M.S.; Jalalabadi, Y.; Sarwghadi, M.; Nedaie, M.; Taherian, A.; Ghaznavi, A.; Eftekhari, A. Removal of Hg(II) from aqueous solutions using a novel impregnated resin containing 1-(2-thiazolylazo)-2-naphthol (TAN). Chem. Eng. J. 2011, 168, 1163–1173. [Google Scholar] [CrossRef]

| Metal Ion Solutions | Adsorption Capacity (mg·g−1) | Adsorption Ratio (%) | Theoretical Selectivity Coefficient | |
|---|---|---|---|---|
| Ag+ | Hg2+ | |||
| AgNO3 | 193.1 | 96.8 | 1.00 | 0.97 |
| Hg(NO3)2 | 186.9 | 94.8 | 1.03 | 1.00 |
| Zn(NO3)2 | 24.8 | 12.4 | 7.79 | 7.54 |
| Cd(NO3)2 | 24.5 | 12.3 | 7.88 | 7.63 |
| Ni(NO3)2 | 12.0 | 6.0 | 16.09 | 15.58 |
| Cr(NO3)3 | 10.1 | 5.1 | 19.12 | 18.50 |
| Pb(NO3)2 | 70.6 | 35.9 | 2.74 | 2.65 |
| Cu(NO3)2 | 15.6 | 7.9 | 12.38 | 11.98 |
| Fe(NO3)3 | 27.6 | 13.8 | 7.00 | 6.77 |
| Co(NO3)2 | 9.9 | 5.2 | 19.51 | 18.88 |
| Mixed Solution | Metal Ions | Adsorption Capacity (mg·g−1) | Adsorption Ratio (%) |
|---|---|---|---|
| AgNO3 | Ag+ | 7.6 | 75.5 |
| Hg(NO3)2 | Hg2+ | 8.7 | 86.6 |
| Zn(NO3)2 | Zn2+ | 5.6 | 55.5 |
| Cd(NO3)2 | Cd2+ | 5.8 | 58.0 |
| Ni(NO3)2 | Ni2+ | 3.5 | 35.0 |
| Cr(NO3)3 | Cr3+ | 5.4 | 53.5 |
| Pb(NO3)2 | Pb2+ | 5.9 | 59.0 |
| Cu(NO3)2 | Cu2+ | 6.4 | 64.0 |
| Fe(NO3)3 | Fe3+ | 6.6 | 66.0 |
| Co(NO3)2 | Co2+ | 6.1 | 60.5 |
| Mathematical Model | Equation | R2 | Standard Deviation | k1(h−1) k2(g·mg−1·h−1) h0(mg·g−1·h−1) | Qe Found by | |
|---|---|---|---|---|---|---|
| Experiment | Equation | |||||
| Ag+ adsorption | ||||||
| Pseudo-1st-order | −ln(1 − Qt/Qe) = 9.9918t + 2.1009 | 0.3191 | 3.3821 | k1 = 9.9918 k2 = 14.4009 h0 = 5.3642 × 103 | 19.30 | – |
| Pseudo-2nd-order | t/Qt = 0.05168t + 2.0793 × 10−4 | 0.99998 | 1.8462 × 10−4 | 19.30 | 19.35 | |
| Hg2+ adsorption | ||||||
| Pseudo-1st-order | −ln(1 − Qt/Qe) = 5.9904t + 1.3080 | 0.5576 | 1.2366 | k1 = 5.9904 k2 = 5.4834 h0 = 1.5001 × 103 | 16.54 | – |
| Pseudo-2nd-order | t/Qt = 0.06002t + 6.6663 × 10−4 | 0.9992 | 3.6159 × 10−4 | 16.54 | 16.66 | |
| Adsorbents | C0 (mg·L−1) | pH | Qe (mg·g−1) | q (%) | TQe (h) |
|---|---|---|---|---|---|
| Ag+ adsorption | |||||
| PTT (this work) | 200 | 5.7 | 193.1 | 96.8 | 0.33 |
| Activated carbon [30] | 120 | 4.5 | 38.8 | 97 | 5 |
| Thiourea/glutaraldehyde grafted O-carboxymethyl chitosan [36] | 160.5 | 5.0 | 156.32 | 97.4 | 48 |
| Cellulose nanocrystals [55] | 107.8 | 6.39 | 34.4 | 64 | 12 |
| Diethylenetriamine-bridged polysilsesquioxanes [34] | 539.3 | – | 162.9 | 30.2 | 12 |
| NH2-MCM-41 nano porous adsorbent [57] | 300 | 7 | 101.45 | 94.32 | 3 |
| Waste yeast [56] | 100 | 3 | 27.9 | 93 | 1 |
| Silica nanoparticles modified with 3-amino-5-mercapto-1,2,4-triazole [26] | 400 | 5 | 124.52 | 62.3 | 0.33 |
| Silica nanoparticles modified with trithiocyanuric acid [21] | 500 | 5.0 | 80 | 32 | 0.5 |
| Hg2+ adsorption | |||||
| PTT (this work) | 200 | 4.6 | 186.9 | 94.8 | 0.33 |
| Poly(hydroxyethylmethacrylate/chitosan) membranes [58] | 400 | 5.0 | 68.8 | – | 0.75 |
| Poly(2-hydroxyethylmethacrylate) gel beads modified with poly(ethyleneimine) [32] | 300 | 5.0 | 335 | – | 1.0 |
| Dithiocarbamate-anchored polymer/organosmectite composites [59] | 400 | 6.0 | 157.3 | – | 1.0 |
| Fe3O4@Zn(btb) nanospheres [60] | 200 | 8 | 129.87 | 64.93 | 0.5 |
| Cross-linked magnetic chitosan-phenylthiourea [61] | 200 | 5 | 135 | 67.50 | 3 |
| Poly(1-amino-5-chloroanthraquinone) [18] | 100.3 | 6 | 83.25 | 83 | 2 |
| Wool-grafted-poly(cyano-acetic acid α-amino-acrylichydrazide) (wool-g-PCAH) chelating fibers [35] | 100 | 5 | 154.32 | 93.3 | 1.5 |
| Modified poly(ethylene terephthalate) chelating fibers [33] | 150 | 5 | 120.02 | 80.01 | 0.83 |
| Cycle Number | Desorption Percentage (%) | Adsorption Capacity (%) | ||||
|---|---|---|---|---|---|---|
| EDTA Solution | HNO3 Solution | Ag+ | Hg2+ | |||
| Ag+ | Hg2+ | Ag+ | Hg2+ | |||
| 1 | 98.4 | 99.2 | 96.5 | 97.4 | 100 | 100 |
| 2 | 95.9 | 97.8 | 91.7 | 92.2 | 97.2 | 97.6 |
| 3 | 94.1 | 95.3 | 90.3 | 91.8 | 96.3 | 96.8 |
| 4 | 92.6 | 93.1 | 85.9 | 86.5 | 94.4 | 95.7 |
| 5 | 87.2 | 89.5 | 80.1 | 82.6 | 91.5 | 92.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Ma, C.; Li, C.; Min, C.; Du, P.; Xia, Y.; Yang, C.; Huang, Q. Facile Synthesis, Characterization of Poly-2-mercapto-1,3,4-thiadiazole Nanoparticles for Rapid Removal of Mercury and Silver Ions from Aqueous Solutions. Polymers 2018, 10, 150. https://doi.org/10.3390/polym10020150
Huang S, Ma C, Li C, Min C, Du P, Xia Y, Yang C, Huang Q. Facile Synthesis, Characterization of Poly-2-mercapto-1,3,4-thiadiazole Nanoparticles for Rapid Removal of Mercury and Silver Ions from Aqueous Solutions. Polymers. 2018; 10(2):150. https://doi.org/10.3390/polym10020150
Chicago/Turabian StyleHuang, Shaojun, Chengzhang Ma, Chao Li, Chungang Min, Ping Du, Yi Xia, Chaofen Yang, and Qiuling Huang. 2018. "Facile Synthesis, Characterization of Poly-2-mercapto-1,3,4-thiadiazole Nanoparticles for Rapid Removal of Mercury and Silver Ions from Aqueous Solutions" Polymers 10, no. 2: 150. https://doi.org/10.3390/polym10020150