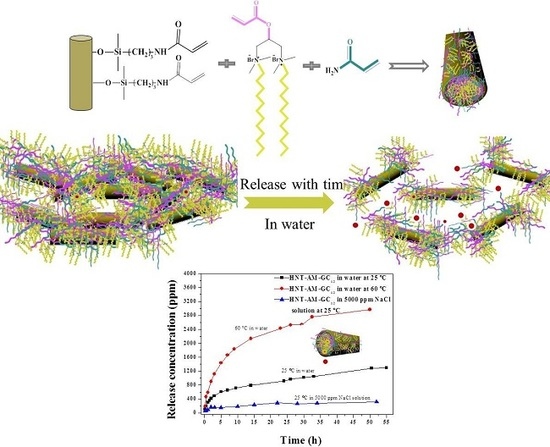
Surface-Engineered Nanocontainers Based on Molecular Self-Assembly and Their Release of Methenamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
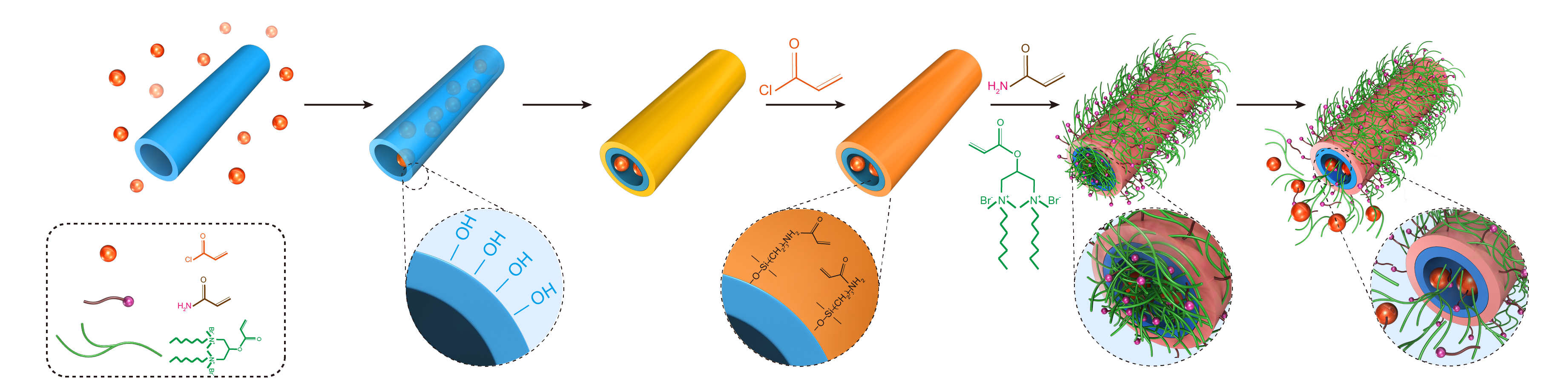
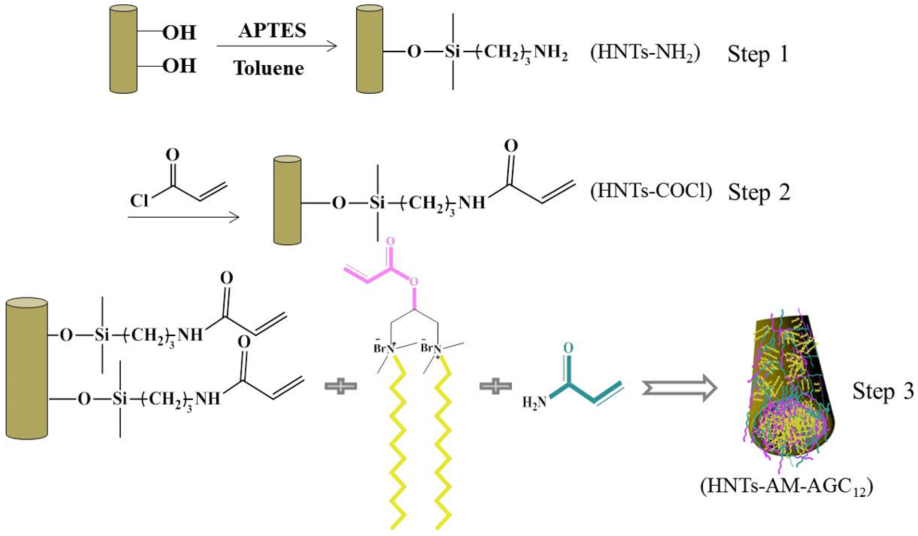
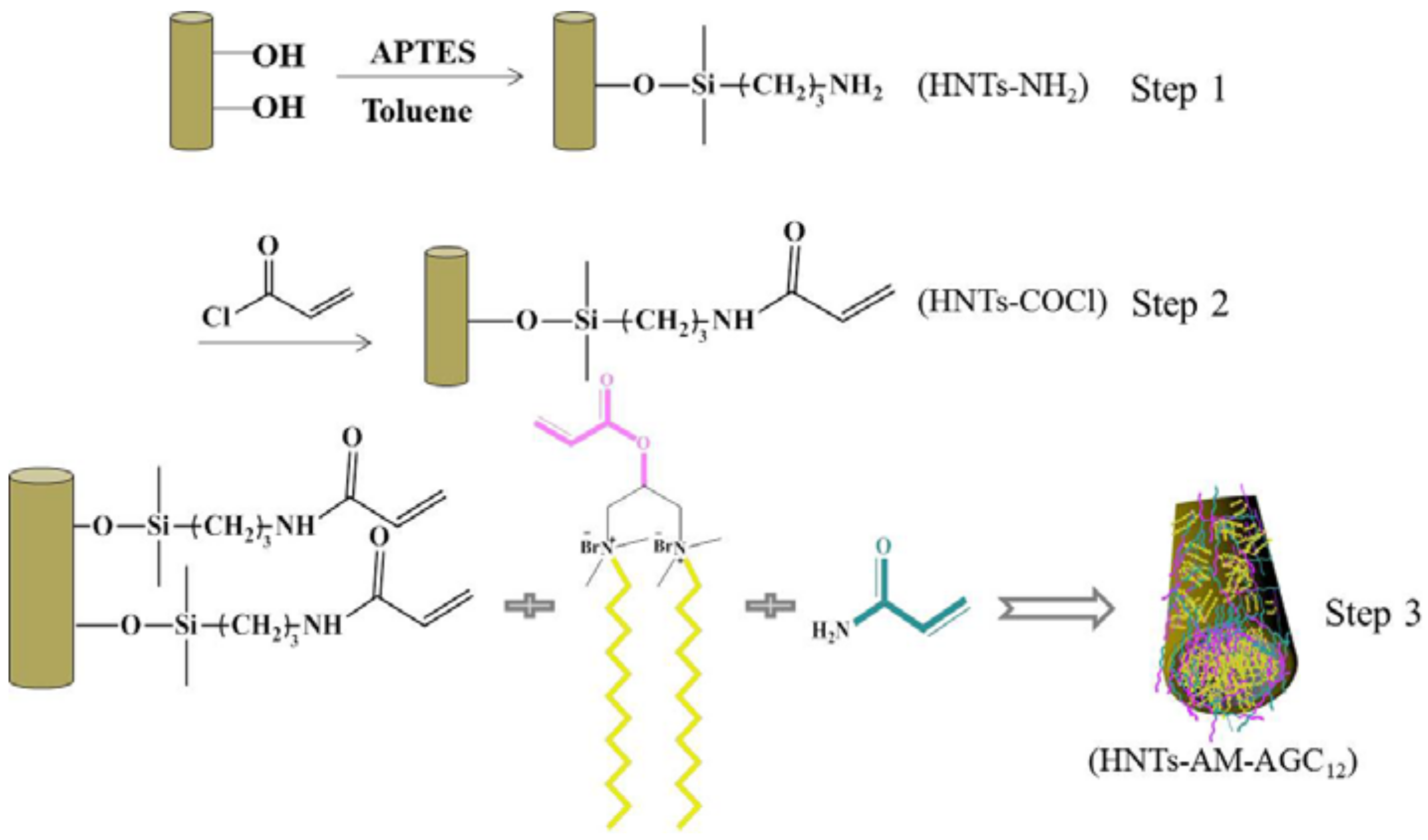
2.2. Sample Preparation
2.3. Characterization
2.4. Nanotube Loading Procedure
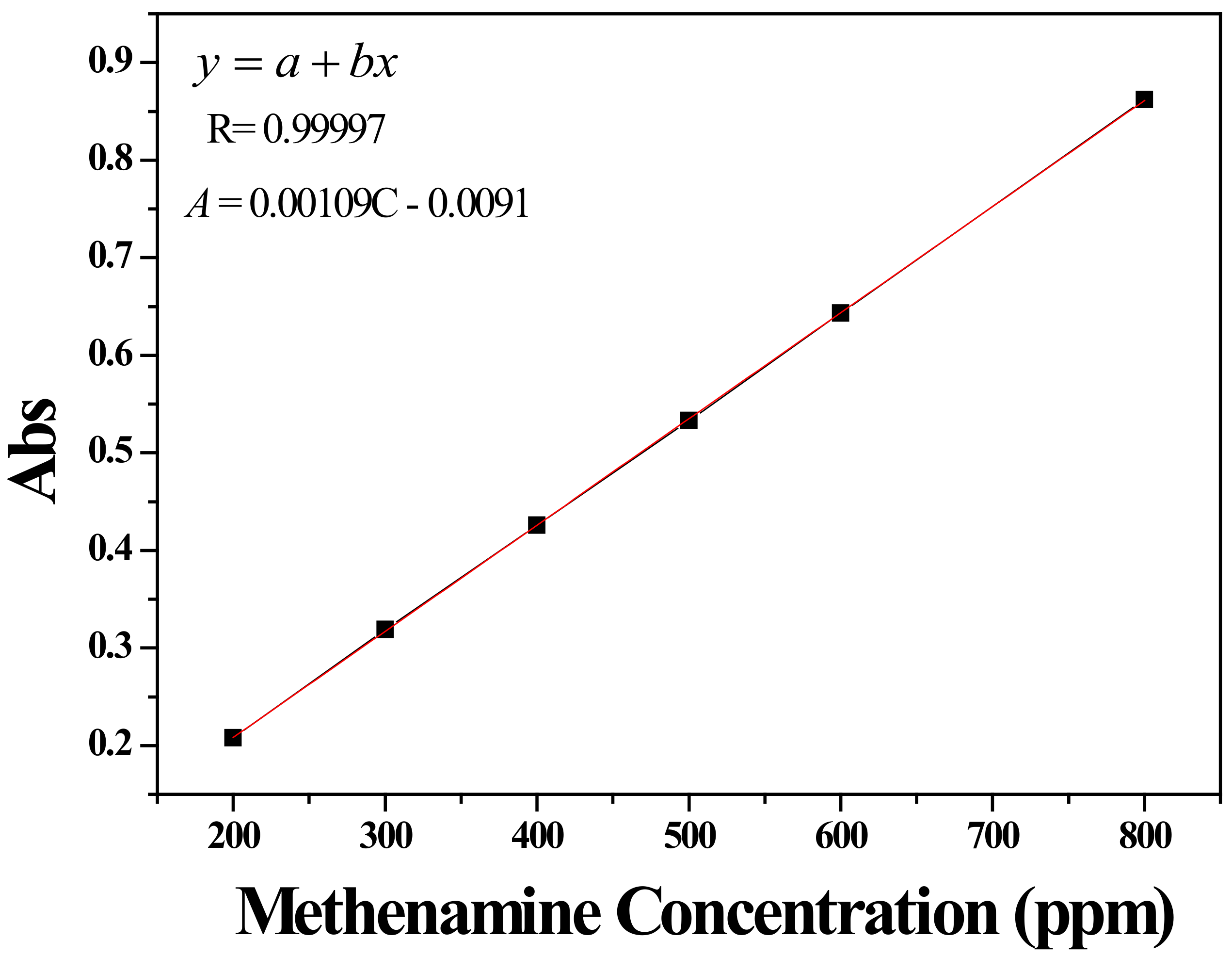
2.5. Functional Chemicals Release Performance of Surface Engineered HNTs
2.6. Change of Particle Size with Methenamine Release Dynamics
3. Results and Discussion
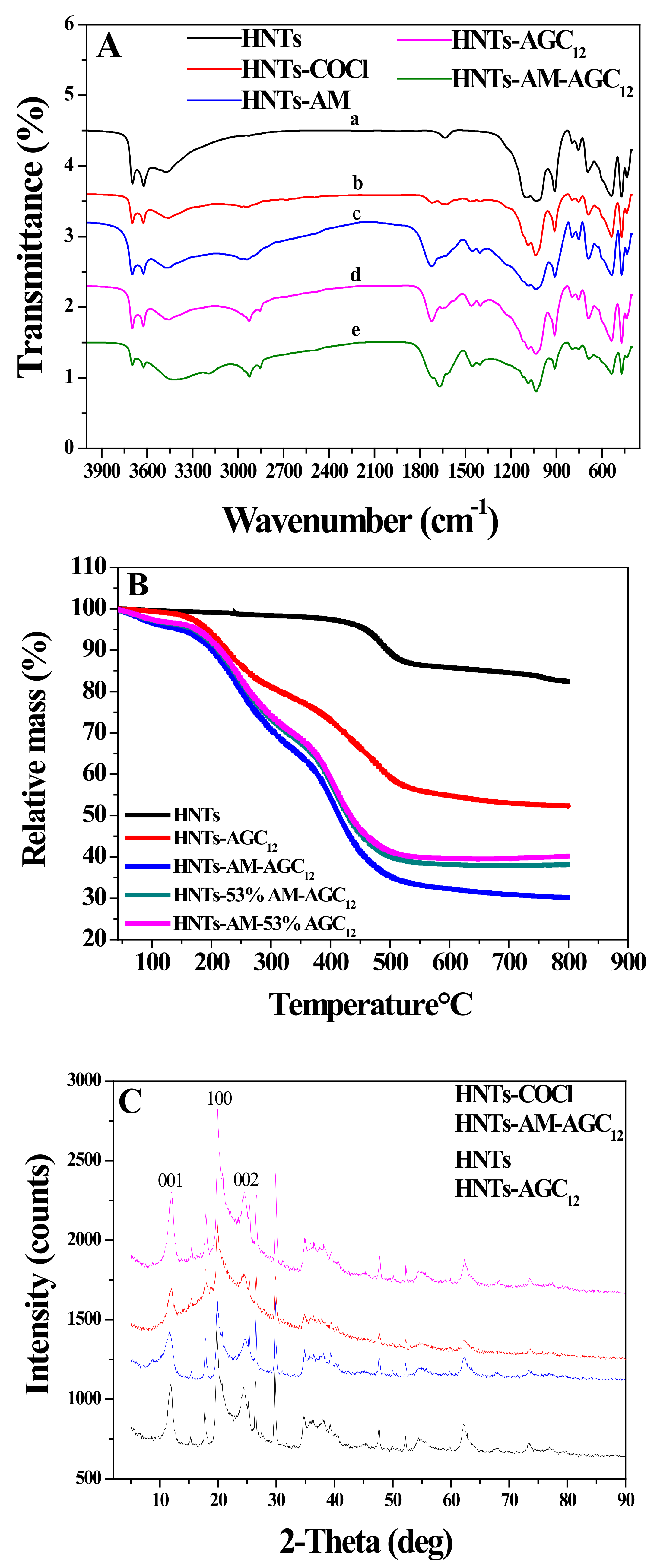
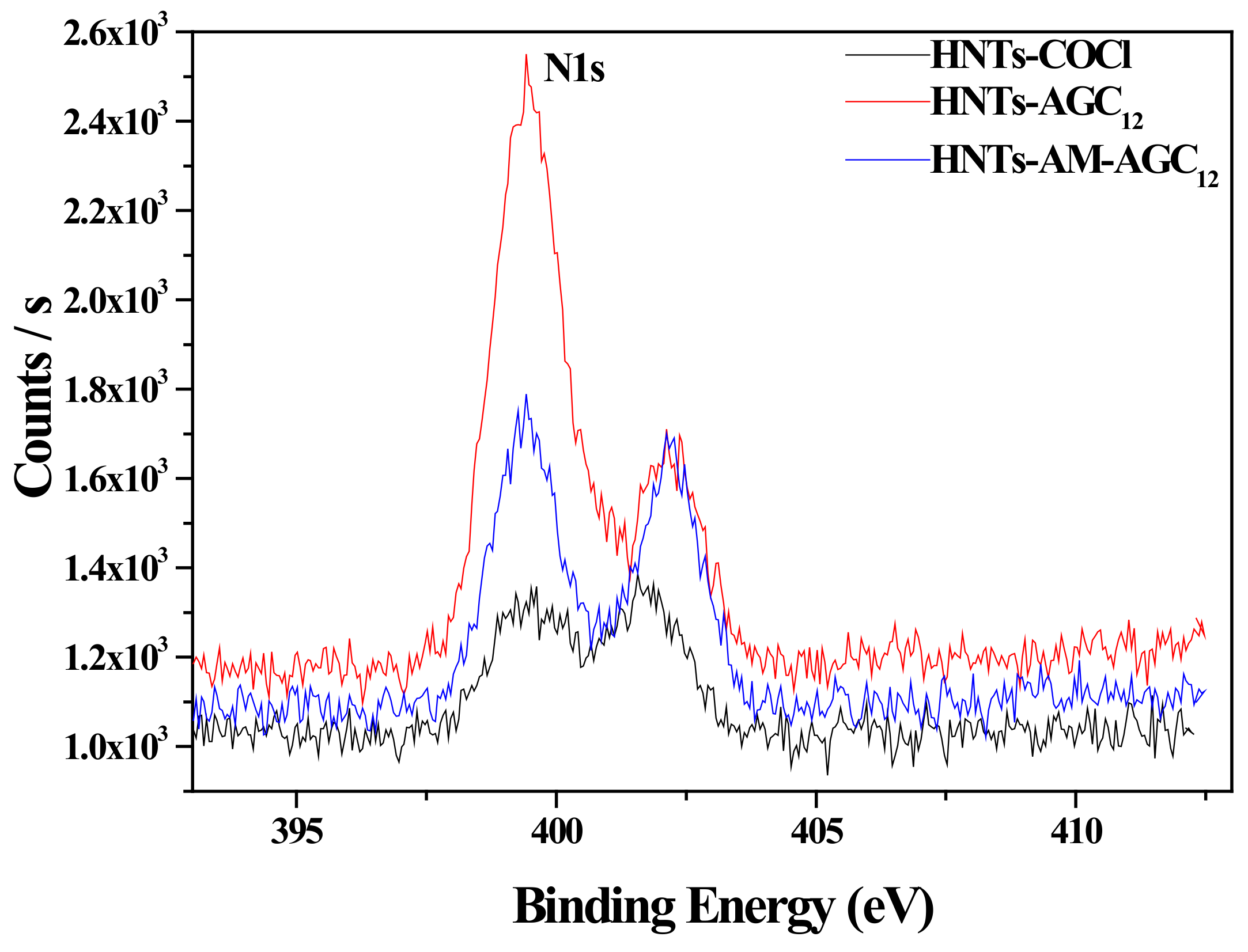
3.1. Synthesis and Characterization of Modified HNTs
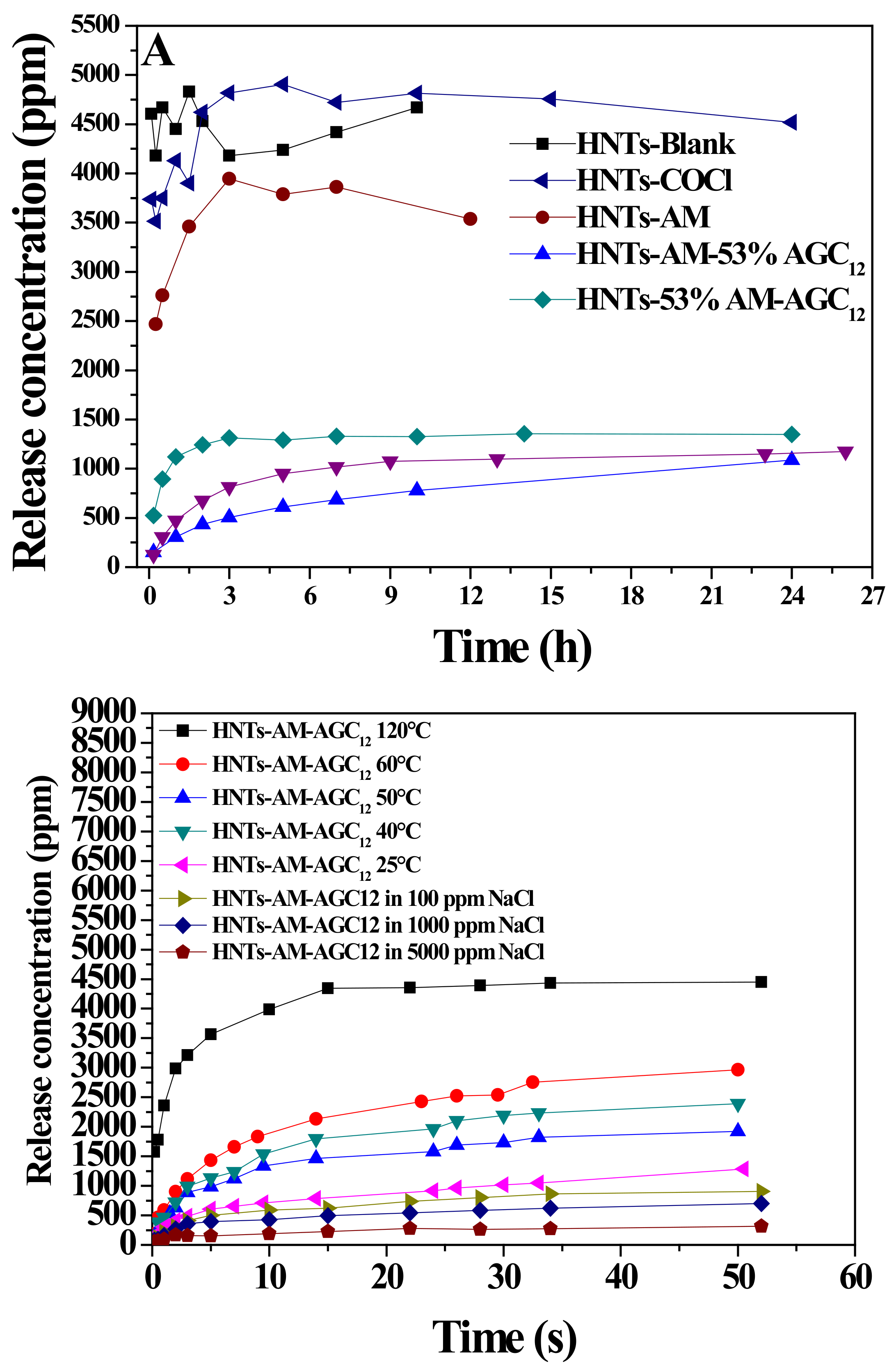
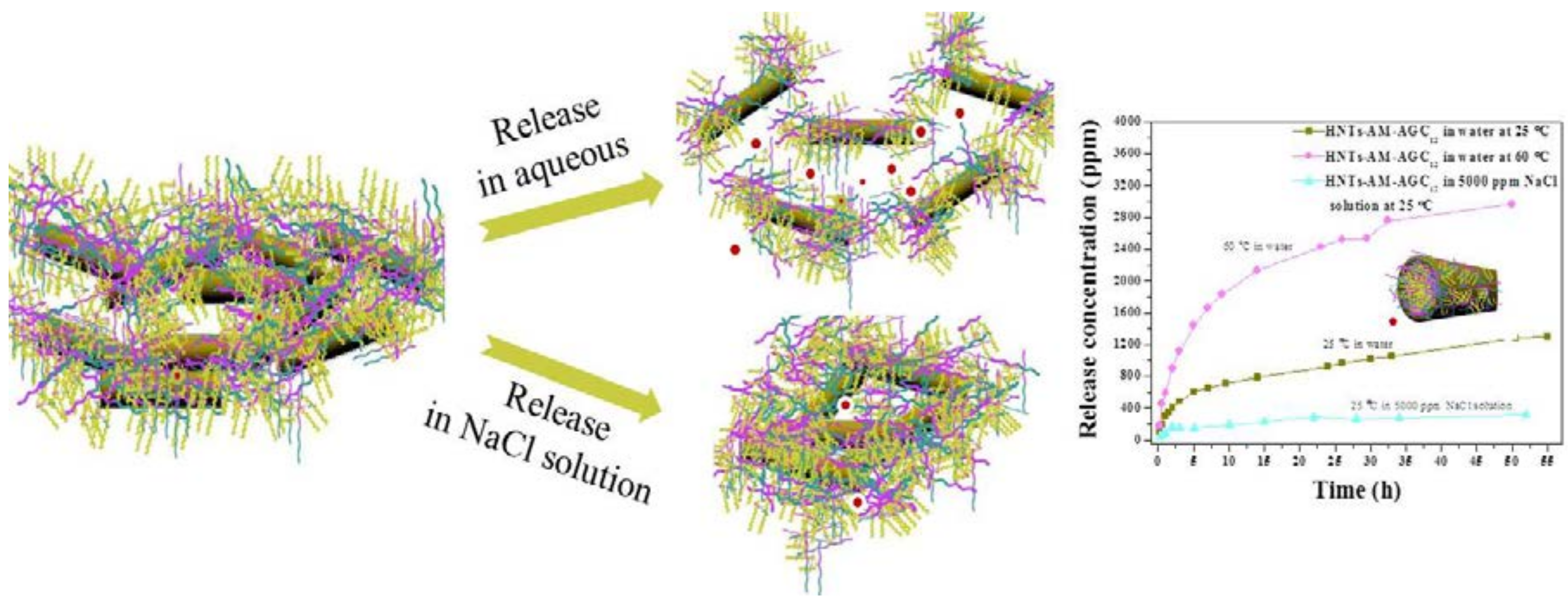
3.2. Taking Surface-Engineered HNTs as Carriers for Methenamine Release
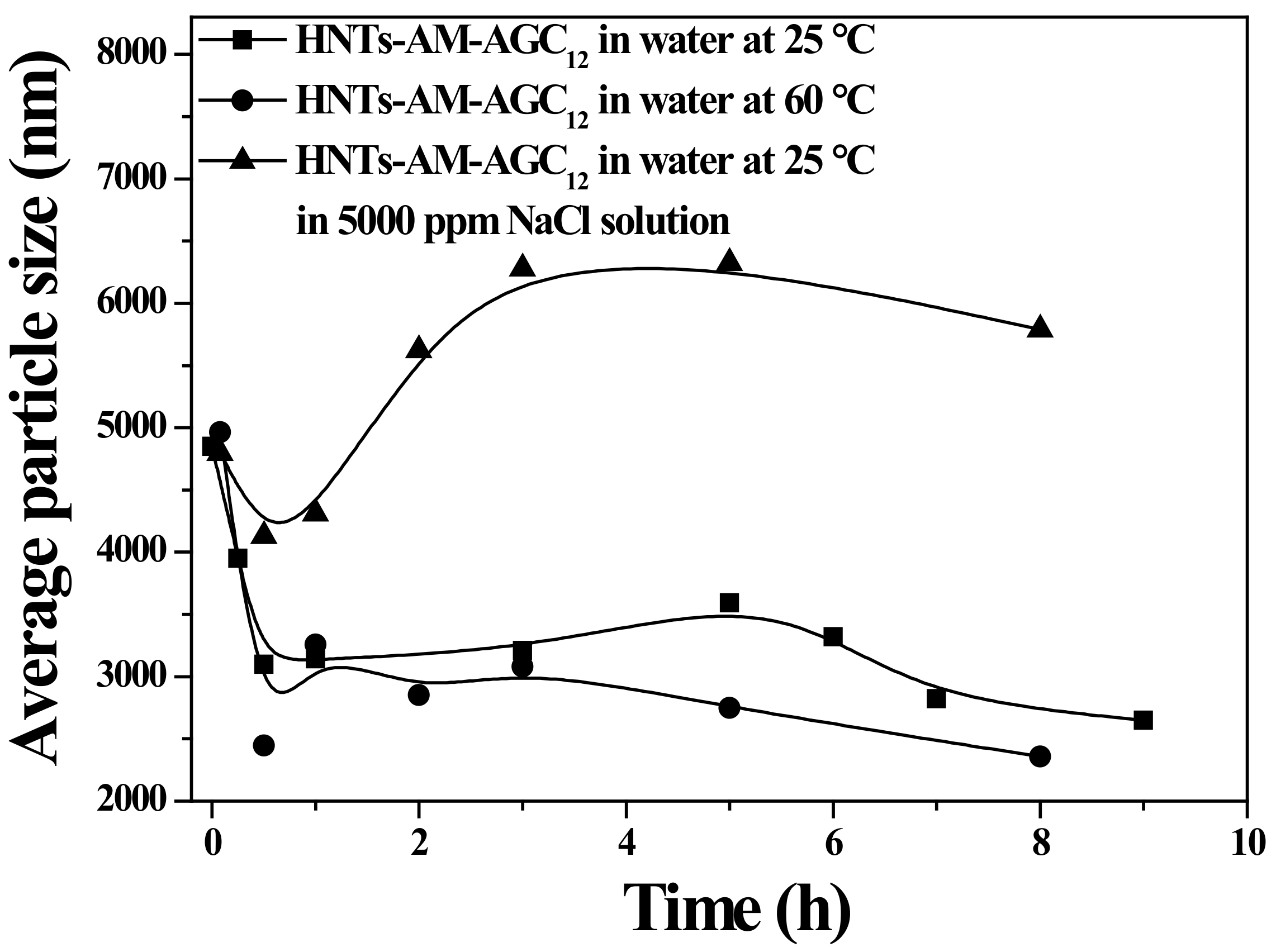
3.3. Particle Size Variations of the Nanocarriers in the Releasing Process under Various Conditions
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdullayev, E.; Price, R.; Shchukin, D.; Lvov, Y. Halloysite Tubes as Nanocontainers for Anticorrosion Coating with Benzotriazole. ACS Appl. Mater. Interfaces 2009, 1, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Guo, B.; Jia, D. Newly emerging applications of halloysite nanotubes: A review. Polym. Int. 2010, 59, 574–582. [Google Scholar] [CrossRef]
- Antill, S.J. Halloysite: A Low-Cost Alternative. Aust. J. Chem. 2003, 56, 723. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Sukhorukov, G.B.; Price, R.R.; Lvov, Y.M. Halloysite nanotubes as biomimetic nanoreactors. Small 2005, 1, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Lun, H.L.; Jing, O.Y.; Yang, H.M. Halloysite Nanotubes as Biomimetic Nanoreactors. RSC Adv. 2014, 4, 44197–44202. [Google Scholar] [CrossRef]
- Veerabadran, N.G.; Mongayt, D.; Torchilin, V.; Price, R.R.; Lvov, Y.M. Organized Shells on Clay Nanotubes for Controlled Release of Macromolecules. Macromol. Rapid Commun. 2009, 30, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.M.; Shchukin, D.G.; Mohwald, H.; Price, R.R. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.B.; Minullina, R.; Abdullayev, E.; Fakhrullin, R.; Millsa, D.; Lvov, Y. Enhanced efficiency of antiseptics with sustained release from clay nanotubes. RSC Adv. 2014, 4, 488–494. [Google Scholar] [CrossRef]
- Fix, D.; Andreeva, D.V.; Lvov, Y.M.; Shchukin, D.G.; Mӧhwald, H. Application of Inhibitor-Loaded Halloysite Nanotubes in Active Anti-Corrosive Coatings. Adv. Funct. Mater. 2009, 19, 1720–1727. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, J.; Wang, A. In situ generation of sodium alginate/hydroxyapatite/halloysite nanotubes nanocomposite hydrogel beads as drug-controlled release matrices. J. Mater. Chem. B 2013, 1, 6261–6270. [Google Scholar] [CrossRef]
- Abdullayeva, E.; Lvov, Y.M. Clay nanotubes for corrosion inhibitor encapsulation: Release control with end stoppers. J. Mater. Chem. 2010, 20, 6681–6687. [Google Scholar] [CrossRef]
- Suh, Y.J.; Kil, D.S.; Chung, K.S.; Abdullayev, E.; Lvov, Y.M.; Mongayt, D. Natural Nanocontainer for the Controlled Delivery of Glycerol as a Moisturizing Agent. J. Nanosci. Nanotechnol. 2011, 11, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Skorb, E.V.; Mohwald, H. 25th Anniversary Article: Dynamic Interfaces for Responsive Encapsulation Systems. Adv. Mater. 2013, 25, 5029–5042. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Guo, R.; Shen, M.W.; Cao, X.Y.; Zhang, L.Q.; Shi, X.Y. Electrospun poly(lactic-co-glycolic acid)/halloysite nanotube composite nanofibers for drug encapsulation and sustained release. J. Mater. Chem. 2010, 20, 10622–10629. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Abdullayev, E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Chiu, F.-C. Halloysite nanotube- and organoclay-filled biodegradable poly (butylene succinate-co-adipate)/maleated polyethylene blend-based nanocomposites with enhanced rigidity. Compos. Part B Eng. 2017, 110, 193–203. [Google Scholar] [CrossRef]
- Rousakis, T.C.; Kouravelou, K.B.; Karachalios, T.K. Effects of carbon nanotube enrichment of epoxy resins on hybrid FRP–FR confinement of concrete. Compos. B 2014, 57, 210–218. [Google Scholar] [CrossRef]
- Irshidat, M.R.; Al-Saleh, M.H. Effect of using carbon nanotube modified epoxy on bond-slip behavior between concrete and FRP sheets. Constr. Build. Mater. 2016, 105, 511–518. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle Polymer Composites: Where Two Small Worlds Meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Li, J.F.; Zhang, X.Y.; Ouyang, Z.F.; Li, Q.; Su, Z.Q.; Wei, G. Synthesis, characterization and drug release application of carbon nanotube-polymer nanosphere composites. RSC Adv. 2013, 3, 9304–9310. [Google Scholar] [CrossRef]
- You, Y.Z.; Hong, C.Y.; Pan, C.Y. Preparation of Smart Polymer/Carbon Nanotube Conjugates via Stimuli-Responsive Linkages. Adv. Funct. Mater. 2007, 17, 2470–2477. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Sanzillo, V. Modified Halloysite Nanotubes: Nanoarchitectures for Enhancing the Capture of Oils from Vapor and Liquid Phases. ACS Appl. Mater. Interfaces 2014, 6, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulychev, N.; Dervaux, B.; Dirnberger, K.; Zubov, V.; Du Prez, F.E.; Eisenbach, C.D. Structure of Adsorption Layers of Amphiphilic Copolymers on Inorganic or Organic Particle Surfaces. Macromol. Chem. Phys. 2010, 211, 971–976. [Google Scholar] [CrossRef]
- Yu, D.F.; Yang, H.; Wang, H.; Cui, Y.X.; Yang, G.; Zhang, J.; Wang, J.B. Interactions between Colloidal Particles in the Presence of an Ultrahighly Charged Amphiphilic Polyelectrolyte. Langmuir 2014, 30, 14512–14521. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, P.; Shi, X.F.; Yu, D.F.; Wang, J.B.; Yan, H.K.; Ji, G. Environmentally Responsive Polymeric Materials: Effect of the Topological Structure on Self-Assembly. Soft Matter 2014, 10, 6749–6757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, X.; Wu, Y.; Song, H.; Ba, X. High-efficiency grafting of halloysite nanotubes by using π-conjugated polyfluorenes via “click” chemistry. J. Mater. Sci. 2015, 50, 4387–4395. [Google Scholar] [CrossRef]
- Kierys, A. Synthesis of Aspirin-loaded Polymer–Silica Composites and their Release Characteristics. ACS Appl. Mater. Interfaces 2014, 6, 14369–14376. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, J.; Zhang, P.; Yan, H. Surface-Engineered Nanocontainers Based on Molecular Self-Assembly and Their Release of Methenamine. Polymers 2018, 10, 163. https://doi.org/10.3390/polym10020163
Zhang M, Wang J, Zhang P, Yan H. Surface-Engineered Nanocontainers Based on Molecular Self-Assembly and Their Release of Methenamine. Polymers. 2018; 10(2):163. https://doi.org/10.3390/polym10020163
Chicago/Turabian StyleZhang, Minghui, Jinben Wang, Pei Zhang, and Haike Yan. 2018. "Surface-Engineered Nanocontainers Based on Molecular Self-Assembly and Their Release of Methenamine" Polymers 10, no. 2: 163. https://doi.org/10.3390/polym10020163