Anion-Controlled Architecture and Photochromism of Naphthalene Diimide-Based Coordination Polymers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
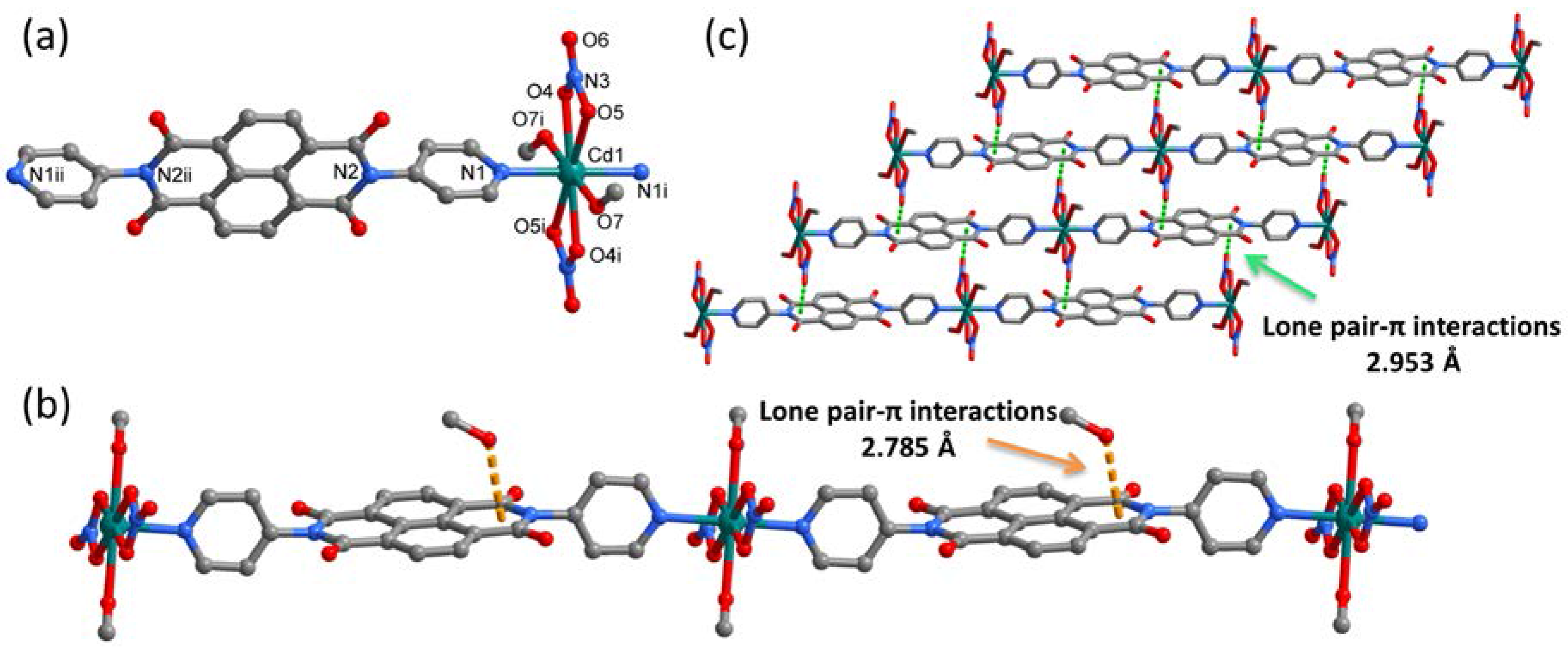
2.2. Synthesis of [Cd(NO3)2(DPNDI)(CH3OH)]·CH3OH (1)
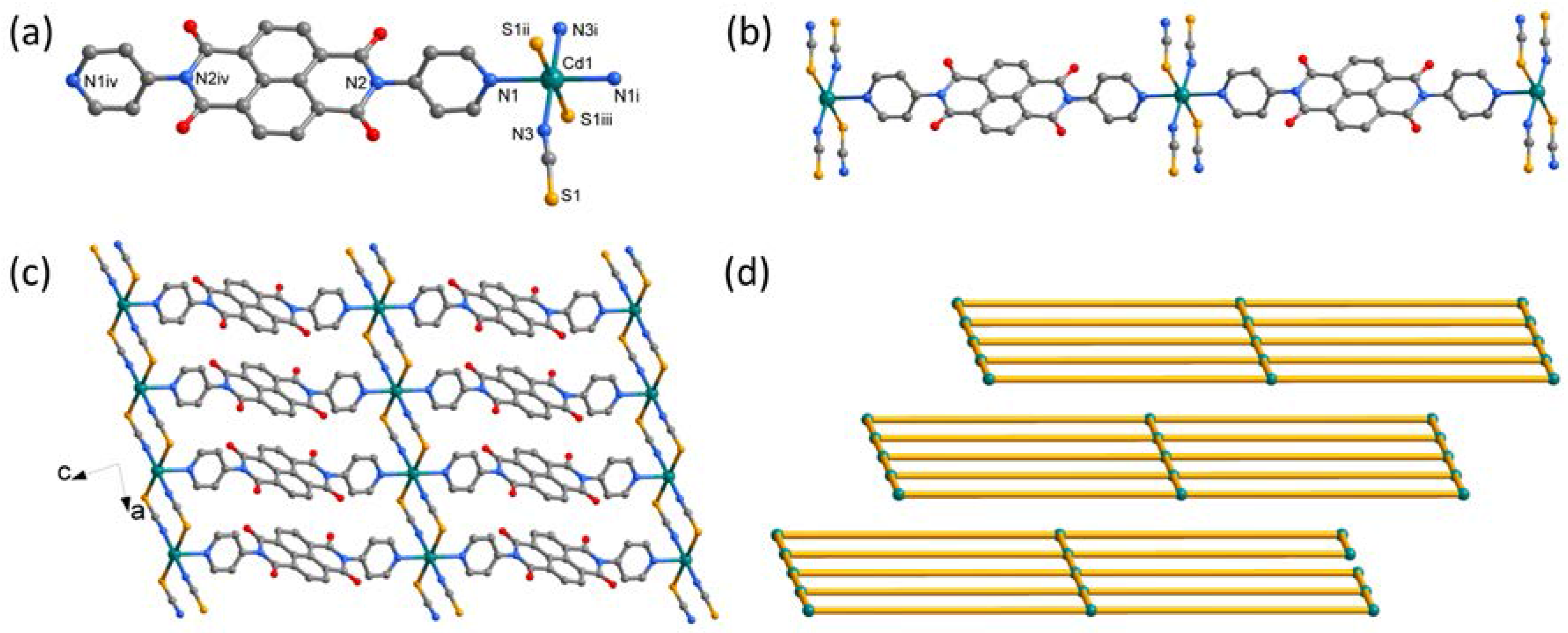
2.3. Synthesis of [Cd(SCN)2(DPNDI)] (2)
2.4. Synthesis of [Cd(DPNDI)2(DMA)2]·2ClO4 (3)
2.5. X-ray Data Collection and Structure Refinement
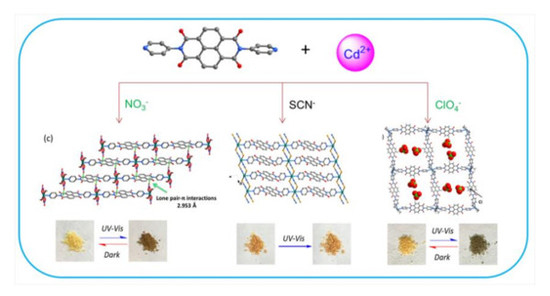
3. Results and Discussion
3.1. Crystal Structure
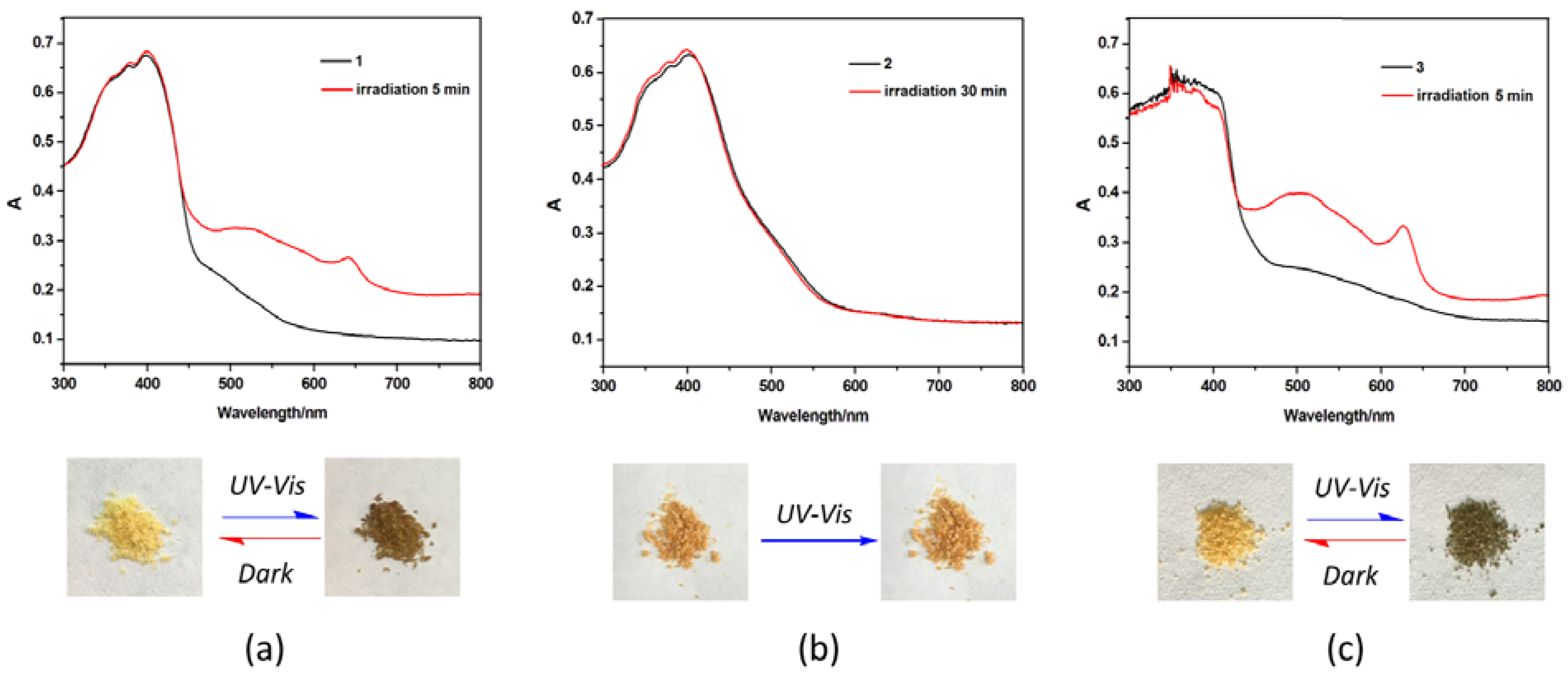
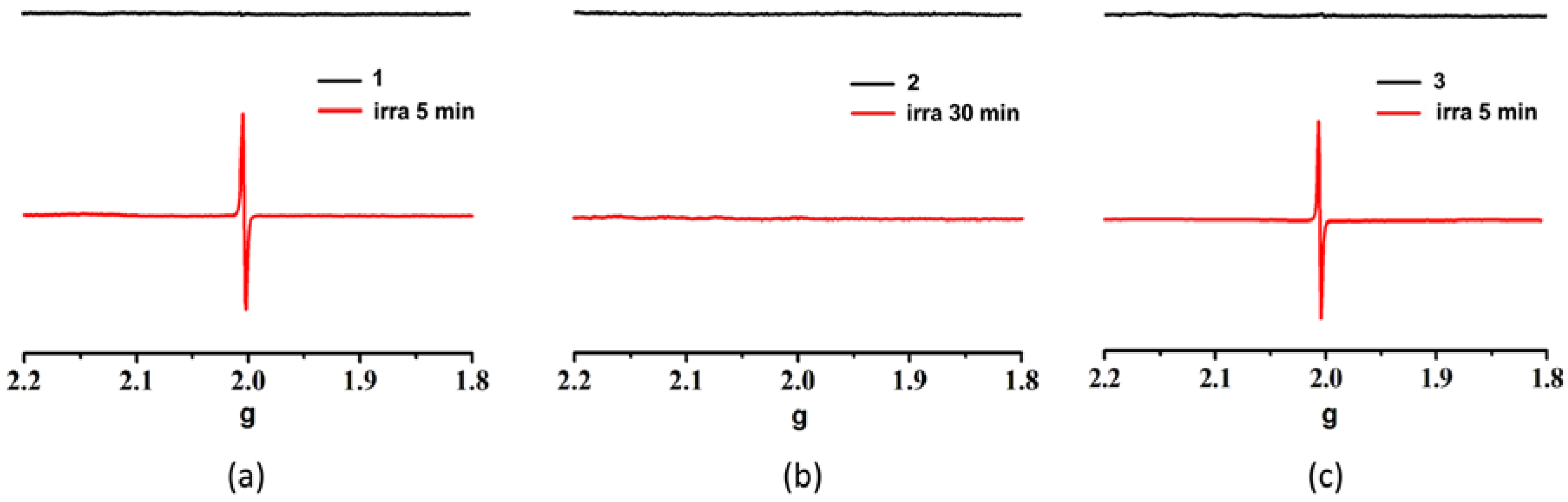
3.2. Photochromic Properties
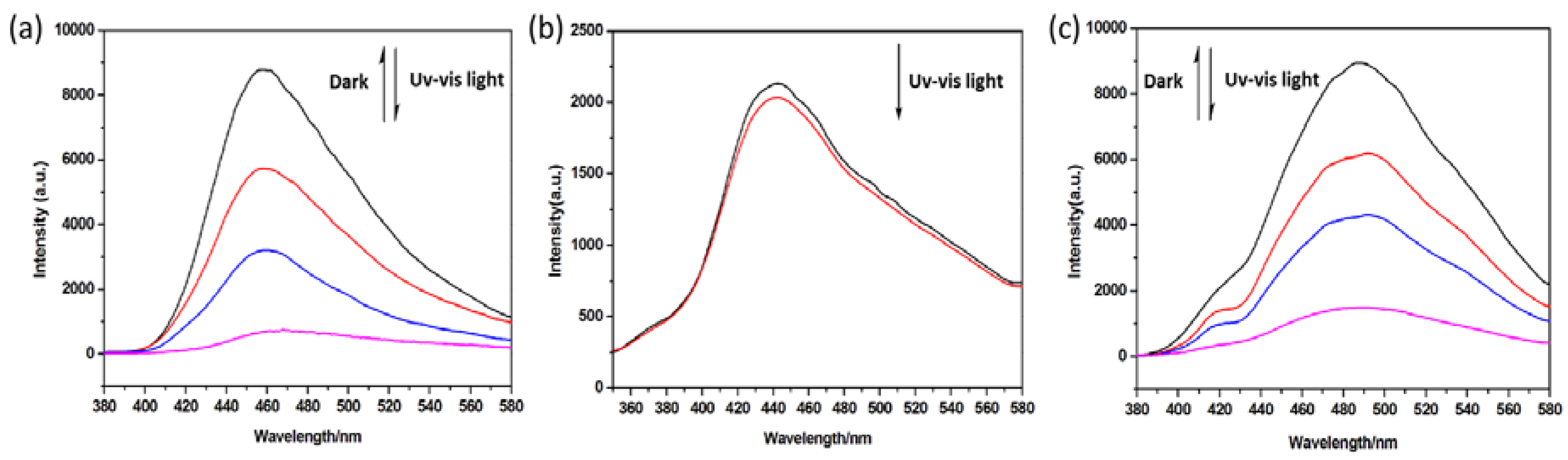
3.3. Luminescence
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Medishetty, R.; Zaręba, J.K.; Mayer, D.; Samoć, M.; Fischer, R.A. Nonlinear optical properties, upconversion and lasing in metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4976–5004. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Furukawa, H.; Zhang, Y.-B.; Yaghi, O.M. High methane storage working capacity in metal organic frameworks with acrylate links. J. Am. Chem. Soc. 2016, 138, 10244–10251. [Google Scholar] [CrossRef] [PubMed]
- De Voorde, B.V.; Bueken, B.; Denayer, J.; Vos, D.D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral metal–organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 2012, 112, 1196–1231. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Duyne, R.P.V.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T., III; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Li, D.; Guo, D.; Zhang, H.; Shi, W.; Cheng, P.; Wojtas, L.; Zaworotko, M.J. Synthesis of a chiral crystal form of MOF-5, CMOF-5, by chiral induction. J. Am. Chem. Soc. 2015, 137, 15406–15409. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-W.; Pan, F.; Zhang, D.; Jin, G.-X.; Ma, J.-P. Self-assembly of new M(II) coordination polymers based on asymmetric 1,3,4-oxadiazole-containing ligands: Effect of counterions and magnetic properties. CrystEngComm 2017, 19, 5864–5872. [Google Scholar] [CrossRef]
- Li, T.; Hong, X.-J.; Liu, X.; Chen, R.; Zhan, Q.-G.; Xu, X.; Cai, Y.-P. Construction of three pH-dependent luminescent metal–organic frameworks with 3-(4-carboxyphen-yl)-1,3-benzoimidazole. CrystEngComm 2014, 16, 3883–3889. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Fang, X.; Zhang, K.-K.; Lin, M.-J.; Gao, D.; Huang, M.-D.; Wang, J.-D. Hydrothermal synthesis of benzothiazole–carboxylic cadmium(II) coordination networks: pH-Controlled topologies and compositional distributions. CrystEngComm 2013, 15, 343–348. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.Y.; Sim, W.; Park, K.-M.; Kim, J.; Lee, S.S. Temperature-dependent 3-D CuI coordination polymers of calix[4]-bis-dithiacrown: Crystal-to-crystal transformation and photoluminescence change on coordinated solvent removal. J. Am. Chem. Soc. 2008, 130, 6902–6903. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Bu, X.-H.; Huang, Z.; Chen, S.-T.; Guo, Y.-M. From metallacyclophanes to 1-D coordination polymers: Role of anions in self-assembly processes of copper(II) and 2,5-bis(3-pyridyl)-1,3,4-oxadiazole. Inorg. Chem. 2003, 42, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, B.; Kurmoo, M.; Green, M.A.; Fujiwara, H.; Otsuka, T.; Kobayashi, H. Synthesis and characterization of a porous magnetic diamond framework, Co3(HCOO)6, and Its N2 sorption characteristic. Inorg. Chem. 2005, 44, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, Z.-H.; Luo, Y.; Zheng, X.-Y.; Kong, X.-J.; Long, L.-S.; Zheng, L.-S. Anion-controlled assembly of a series of heterometallic 3d–4f compounds with 0D cluster, 1D chain, 2D network and 3D frameworks. CrystEngComm 2016, 18, 4142–4149. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Liu, F.; Wang, R.; Sun, D. Anion-controlled formation of two silver lamella frameworks based on in situ ligand reaction. CrystEngComm 2013, 15, 8877–8880. [Google Scholar] [CrossRef]
- Cui, F.; Li, S.; Jia, C.; Mathieson, J.S.; Cronin, L.; Yang, X.-J.; Wu, B. Anion-dependent formation of helicates versus mesocates of triple-stranded M2L3 (M = Fe2+, Cu2+) Complexes. Inorg. Chem. 2012, 51, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wei, K.-J.; Liu, Y.; Huang, X.-C.; Li, D. Silver coordination polymers based on neutral trinitrile ligand: Topology and the role of anion. Cryst. Growth Des. 2010, 10, 3964–3976. [Google Scholar] [CrossRef]
- Tanaka, D.; Kitagawa, S. Template effects in porous coordination polymers. Chem. Mater. 2008, 20, 922–931. [Google Scholar] [CrossRef]
- Liu, J.-J.; Guan, Y.-F.; Lin, M.-J.; Huang, C.-C.; Dai, W.-X. Anion-mediated architecture and photochromism of rigid bipyridinium-based coordination polymers. Cryst. Growth Des. 2016, 16, 2836–2842. [Google Scholar] [CrossRef]
- Shen, J.-J.; Wang, F.; Yu, T.-L.; Zhang, F.-Q.; Tian, L.; Fu, Y.-L. Halogen-dependent photoinduced electron transfer and chromism of three protonated nicotinohydrazide halozincates. Dalton Trans. 2017, 46, 5414–5419. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-K.; Wang, P.; Yao, Q.-X.; Chen, Y.-J.; Li, Z.-H.; Zhang, Y.-F.; Wu, L.-M.; Zhang, J. Solvent- and anion-controlled photochromism of viologen-based metal–organic hybrid materials. J. Mater. Chem. 2012, 22, 12212–12219. [Google Scholar] [CrossRef]
- Kobaisi, M.A.; Bhosale, S.V.; Latham, K.; Raynor, A.M.; Bhosale, S.V. Functional naphthalene diimides: Synthesis, properties, and applications. Chem. Rev. 2016, 116, 11685–11796. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lin, X.-M.; Li, G.-B.; Su, C.-Y. Progress in the study of metal–organic materials applying naphthalene diimide (NDI) ligands. Coord. Chem. Rev. 2011, 255, 1921–1936. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Jani, C.H.; Langford, S.J. Chemistry of naphthalene diimides. Chem. Soc. Rev. 2008, 37, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Domoto, Y.; Orentas, E.; Beuchat, C.; Emery, D.; Mareda, J.; Sakai, N.; Matile, S. Catalysis with Anion-π interactions. Angew. Chem. Int. Ed. 2013, 52, 9940–9943. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Qin, L.; Xu, L.; Zhou, Y.; Sun, J.; Zou, X. A novel photochromic calcium-based metal–organic framework derived from a naphthalene diimide chromophore. Chem. Commun. 2013, 49, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-X.; Zhao, W.-N.; Li, G.-C.; Liu, P.-F.; Han, L. A Naphthalenediimide-based metal–organic framework and thin film exhibiting photochromic and electrochromic properties. Inorg. Chem. 2016, 55, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-B.; Liu, J.-M.; Cai, Y.-P.; Su, C.-Y. Structural diversity of a series of Mn(II), Cd(II), and Co(II) complexes with pyridine donor diimide ligands. Cryst. Growth Des. 2011, 11, 2763–2772. [Google Scholar] [CrossRef]
- Mallick, A.; Garai, B.; Addicoat, M.A.; Petkov, P.S.; Heine, T.; Banerjee, R. Solid state organic amine detection in a photochromic porous metal organic framework. Chem. Sci. 2015, 6, 1420–1425. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Xiang, B. Solvothermal syntheses, crystal structures, and luminescent properties of two new cadmium(II) coordination polymers based on rigid/flexible dicarboxylate and N,N′-Bis(4-pyridyl)-1,4,5,8-naphthalenetetracarboxydiimide. Z. Anorg. Allg. Chem. 2015, 641, 853–857. [Google Scholar] [CrossRef]
- Liao, J.-Z.; Dui, X.-J.; Zhang, H.-L.; Wu, X.-Y.; Lu, C.-Z. Polyoxometalate anion-π interaction-directed assembly of a three-dimensional hydrogen-bonded supramolecular framework with nanoscale porosity. CrystEngComm 2014, 16, 10530–10533. [Google Scholar] [CrossRef]
- Guha, S.; Goodson, F.S.; Corson, L.J.; Saha, S. Boundaries of anion/naphthalenediimide interactions: From anion-π interactions to anion-induced charge-transfer and electron-transfer phenomena. J. Am. Chem. Soc. 2012, 134, 13679–13691. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Hong, Y.-J.; Guan, Y.-F.; Lin, M.-J.; Huang, C.-C.; Dai, W.-X. Lone pair-π interaction-induced generation of non-interpenetrated and photochromic cuboid 3-D naphthalene diimide coordination networks. Dalton Trans. 2015, 44, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Liao, J.-Z.; Zhang, H.-L.; Wang, S.-S.; Yong, J.-P.; Wu, X.-Y.; Yu, R.; Lu, C.-Z. Multifunctional radical-doped polyoxometalate-based host−guest material: Photochromism and photocatalytic activity. Inorg. Chem. 2015, 54, 4345–4350. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.F.; Chan, B.; Faust, T.B.; D’Alessandro, D.M. Controlling charge separation in a novel donor–acceptor metal–organic framework via redox modulation. Chem. Sci. 2014, 5, 4724–4728. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xin, F.; Liu, Y.; Yin, X.; Ma, W. Photocatalytic reduction of CO2 in methanol to methyl formate over CuO–TiO2 composite catalysts. J. Colloid Interface Sci. 2011, 356, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-Z.; Wu, X.-Y.; Yong, J.-P.; Zhang, H.-L.; Yang, W.-B.; Yu, R.; Lu, C.-Z. Anion-π Interaction-Directed Assembly of Polyoxometalate-Based Host–Guest Compounds and Its Contribution to Photochromism. Cryst. Growth Des. 2015, 15, 4952–4958. [Google Scholar] [CrossRef]
- Liu, J.-J.; Guan, Y.-F.; Chen, Y.; Lin, M.-J.; Huang, C.-C.; Dai, W.-X. The impact of lone pair-π interactions on photochromic properties in 1-D naphthalene diimide coordination networks. Dalton Trans. 2015, 44, 17312–17317. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Mareda, J.; Vauthey, E.; Matile, S. Core-substituted naphthalenediimides. Chem. Commun. 2010, 46, 4225–4237. [Google Scholar] [CrossRef] [PubMed]

| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Chemical formula | C28H20CdN6O14 | C26H12CdN6O4S2 | C56H42CdCl2N10O18 |
| formula weight | 776.91 | 648.94 | 1326.29 |
| crystal system | triclinic | triclinic | monoclinic |
| space group | C2/c | ||
| a (Å) | 7.3845 (4) | 5.7707 (4) | 18.821 (4) |
| b (Å) | 9.2831 (4) | 10.7235 (11) | 27.177 (5) |
| c (Å) | 12.4333 (6) | 16.9651 (16) | 17.704 (4) |
| α (deg) | 73.934 (4) | 87.406 (8) | 90 |
| β (deg) | 87.906 (4) | 82.664 (7) | 108.97 (3) |
| γ (deg) | 68.292 (5) | 81.112 (7) | 90 |
| V (Å3) | 758.87 (7) | 1028.39 (16) | 8564 (3) |
| Z | 1 | 1 | 4 |
| ρcalc(g/cm3) | 1.700 | 1.048 | 1.029 |
| μ (Mo Kα)·(mm−1) | 0.802 | 0.661 | 0.373 |
| F(000) | 390 | 322 | 2696 |
| collected reflns | 5405 | 7524 | 29874 |
| unique reflns/ Rint | 2492/0.0183 | 3509/0.0746 | 7543/0.070 |
| no. of observations | 2433 | 2641 | 6625 |
| GOF | 1.024 | 1.033 | 1.160 |
| R1a, wR2b (I > 2σ(I)) | 0.0315, 0.0907 | 0.0824, 0.2206 | 0.0816, 0.1821 |
| R1a, wR2b (all data) | 0.0322, 0.0912 | 0.0982, 0.2402 | 0.0921, 0.1882 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-J.; Xia, S.-B.; Duan, Y.-L.; Liu, T.; Cheng, F.-X.; Sun, C.-K. Anion-Controlled Architecture and Photochromism of Naphthalene Diimide-Based Coordination Polymers. Polymers 2018, 10, 165. https://doi.org/10.3390/polym10020165
Liu J-J, Xia S-B, Duan Y-L, Liu T, Cheng F-X, Sun C-K. Anion-Controlled Architecture and Photochromism of Naphthalene Diimide-Based Coordination Polymers. Polymers. 2018; 10(2):165. https://doi.org/10.3390/polym10020165
Chicago/Turabian StyleLiu, Jian-Jun, Shu-Biao Xia, Yu-Lian Duan, Teng Liu, Fei-Xiang Cheng, and Cheng-Ke Sun. 2018. "Anion-Controlled Architecture and Photochromism of Naphthalene Diimide-Based Coordination Polymers" Polymers 10, no. 2: 165. https://doi.org/10.3390/polym10020165