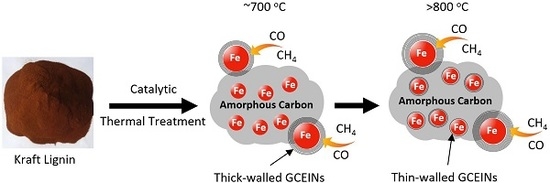
Carbon-Based Nanomaterials from Biopolymer Lignin via Catalytic Thermal Treatment at 700 to 1000 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CBNs
2.3. Characterization
3. Results
3.1. Yield
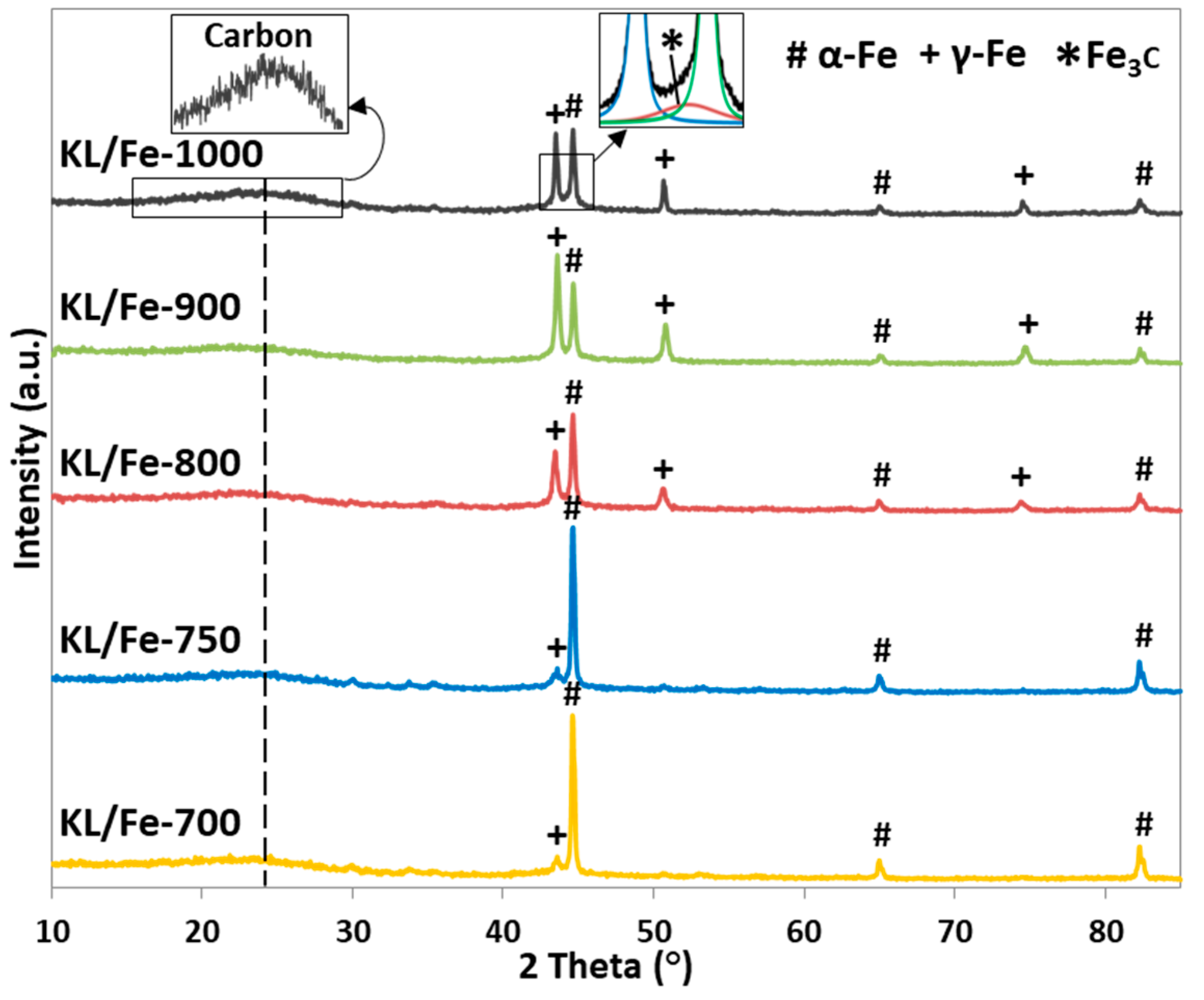
3.2. XRD
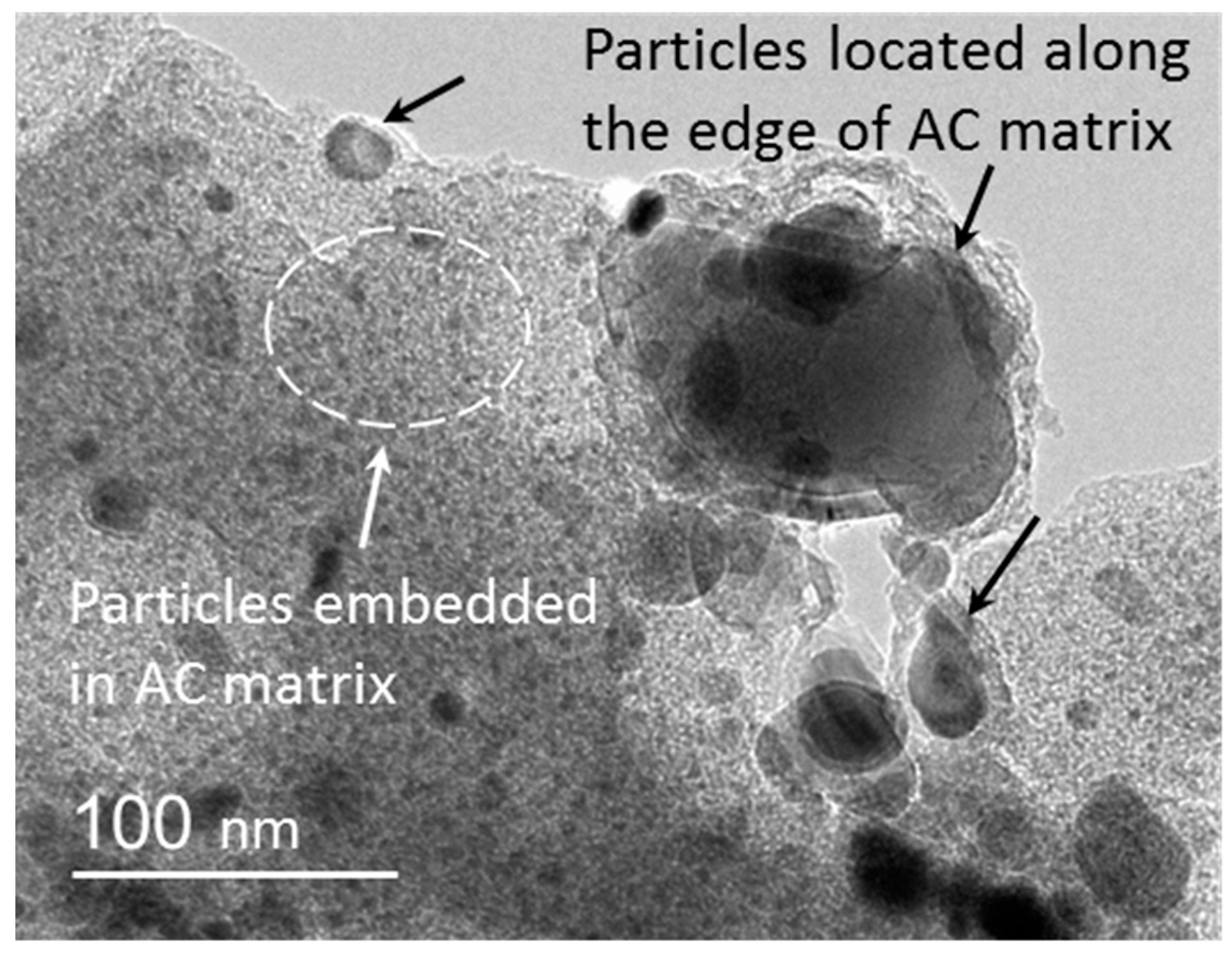
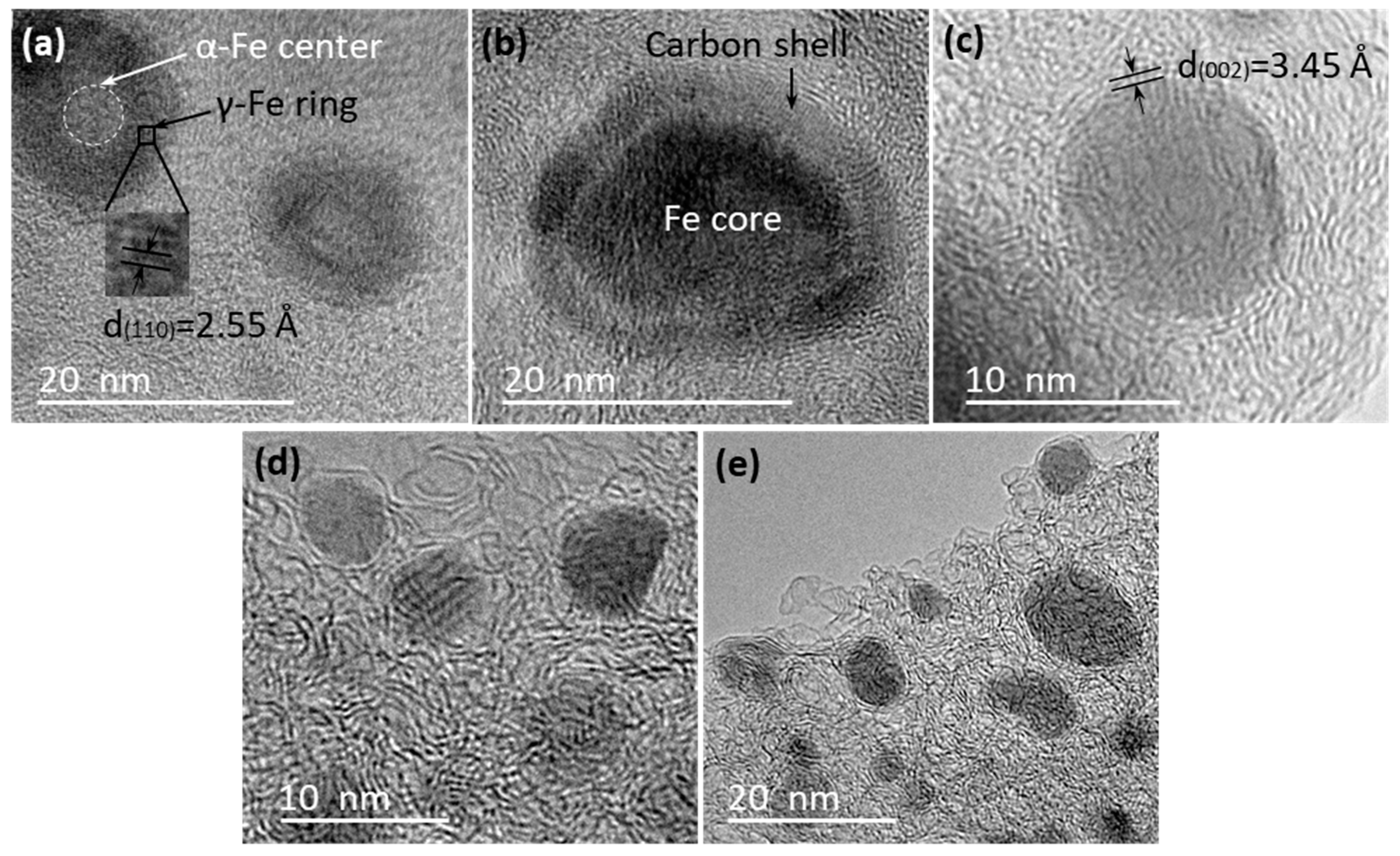
3.3. TEM
4. Carbon Source for GCEINs Formation: AC or Carbonaceous Gases
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, X.; Yan, Q.; Leng, W.; Li, J.; Zhang, J.; Cai, Z.; Hassan, E.B. Carbon nanostructure of kraft lignin thermally treated at 500 to 1000 °C. Materials 2017, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Ben, H.; Ragauskas, A.J. Comparison for the compositions of fast and slow pyrolysis oils by NMR characterization. Bioresour. Technol. 2013, 147, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, Q.; Hassan, E.B.; Li, J.; Cai, Z.; Zhang, J. Temperature effects on formation of carbon-based nanomaterials from kraft lignin. Mater. Lett. 2017, 203, 42–45. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Li, J.; Zhang, J.; Cai, Z. Effects of physical and chemical states of iron-based catalysts on formation of carbon-encapsulated iron nanoparticles from kraft lignin. Materials 2018, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Aorigele; Sun, Y. Preparation of Iron Oxide Particle-Decorated Lignin-Based Carbon Nanofibers as Electrode Material for Pseudocapacitor. J. Wood Chem. Technol. 2017, 37, 423–432. [Google Scholar] [CrossRef]
- Qin, H.; Zhou, Y.; Bai, J.; Zhu, B.; Ni, Z.; Wang, L.; Liu, W.; Zhou, Q.; Li, X. Lignin-derived thin-walled graphitic carbon-encapsulated iron nanoparticles: Growth, characterization, and applications. ACS Sustain. Chem. Eng. 2017, 5, 1917–1923. [Google Scholar] [CrossRef]
- Qin, H.; Wang, B.; Zhang, C.; Zhu, B.; Zhou, Y.; Zhou, Q. Lignin based synthesis of graphitic carbon-encapsulated iron nanoparticles as effective catalyst for forming lower olefins via Fischer-Tropsch synthesis. Catal. Commun. 2017, 96, 28–31. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Huczko, A.; Lange, H. Arc plasma route to carbon-encapsulated magnetic nanoparticles for biomedical applications. Sens. Actuators B Chem. 2005, 109, 81–85. [Google Scholar] [CrossRef]
- Tessonnier, J.-P.; Su, D.S. Recent progress on the growth mechanism of carbon nanotubes: A review. ChemSusChem 2011, 4, 824–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of chemical vapor deposition of graphene and related applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Barnes, H.M.; Cai, Z.; Zhang, J. Temperature and copper concentration effects on the formation of graphene-encapsulated copper nanoparticles from kraft lignin. Materials 2017, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Sanchís, C.; Valdés-Solís, T.; Morallón, E.; Fuertes, A.B. Direct synthesis of graphitic carbon nanostructures from saccharides and their use as electrocatalytic supports. Carbon 2008, 46, 931–939. [Google Scholar] [CrossRef]
- Mun, S.P.; Cai, Z.; Zhang, J. Fe-catalyzed thermal conversion of sodium lignosulfonate to graphene. Mater. Lett. 2013, 100, 180–183. [Google Scholar] [CrossRef]
- Kubo, S.; Uraki, Y.; Sano, Y. Catalytic graphitization of hardwood acetic acid lignin with nickel acetate. J. Wood Sci. 2003, 49, 188–192. [Google Scholar] [CrossRef]
- Thompson, E.; Danks, A.E.; Bourgeois, L.; Schnepp, Z. Iron-catalyzed graphitization of biomass. Green Chem. 2014, 17, 551–556. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Klingeler, R.; Gemming, T.; Büchner, B.; Rümmeli, M.H. Synthesis of carbon-encapsulated iron nanoparticles by pyrolysis of iron citrate and poly(vinyl alcohol): A critical evaluation of yield and selectivity. Nanotechnology 2011, 22, 315606. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Lecea, C.S.M.; Valdés-Solís, T.; Morallón, E.; Fuertes, A.B. Solid-phase synthesis of graphitic carbon nanostructures from iron and cobalt gluconates and their utilization as electrocatalyst supports. Phys. Chem. Chem. Phys. 2008, 10, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Song, H.; Chen, X.; Lian, W. Large-scale synthesis of onion-like carbon nanoparticles by carbonization of phenolic resin. Acta Mater. 2007, 55, 6144–6150. [Google Scholar] [CrossRef]
- Sevilla, M.; Sanchís, C.; Valdés-Solís, T.; Morallón, E.; Fuertes, A.B. Synthesis of graphitic carbon nanostructures from sawdust and their application as electrocatalyst supports. J. Phys. Chem. C 2007, 111, 9749–9756. [Google Scholar] [CrossRef]
- Du, Y.; Wang, C.; Toghiani, H.; Cai, Z.; Liu, X.; Zhang, J.; Yan, Q. Synthesis of carbon-encapsulated metal nanoparticles from wood char. For. Prod. J. 2010, 60, 527–533. [Google Scholar] [CrossRef]
- Mun, S.P.; Cai, Z.; Zhang, J. Preparation of Fe-cored carbon nanomaterials from mountain pine beetle-killed pine wood. Mater. Lett. 2015, 142, 45–48. [Google Scholar] [CrossRef]
- Müller-Hagedorn, M.; Bockhorn, H.; Krebs, L.; Müller, U. A comparative kinetic study on the pyrolysis of three different wood species. J. Anal. Appl. Pyrolysis 2003, 68, 231–249. [Google Scholar] [CrossRef]
- Huo, J.; Song, H.; Chen, X. Preparation of carbon-encapsulated iron nanoparticles by co-carbonization of aromatic heavy oil and ferrocene. Carbon 2004, 42, 3177–3182. [Google Scholar] [CrossRef]
- Kim, H.; Sigmund, W. Effect of a graphitic structure on the stability of FCC iron. J. Cryst. Growth 2004, 267, 738–744. [Google Scholar] [CrossRef]
- Wei, B.; Shima, M.; Pati, R.; Nayak, S.K.; Singh, D.J.; Ma, R.; Li, Y.; Bando, Y.; Nasu, S.; Ajayan, P.M. Room-temperature ferromagnetism in doped face-centered cubic Fe nanoparticles. Small 2006, 2, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0-470-55673-3. [Google Scholar]
- Weissker, U.; Hampel, S.; Leonhardt, A.; Büchner, B. Carbon nanotubes filled with ferromagnetic materials. Materials 2010, 3, 4387–4427. [Google Scholar] [CrossRef] [PubMed]
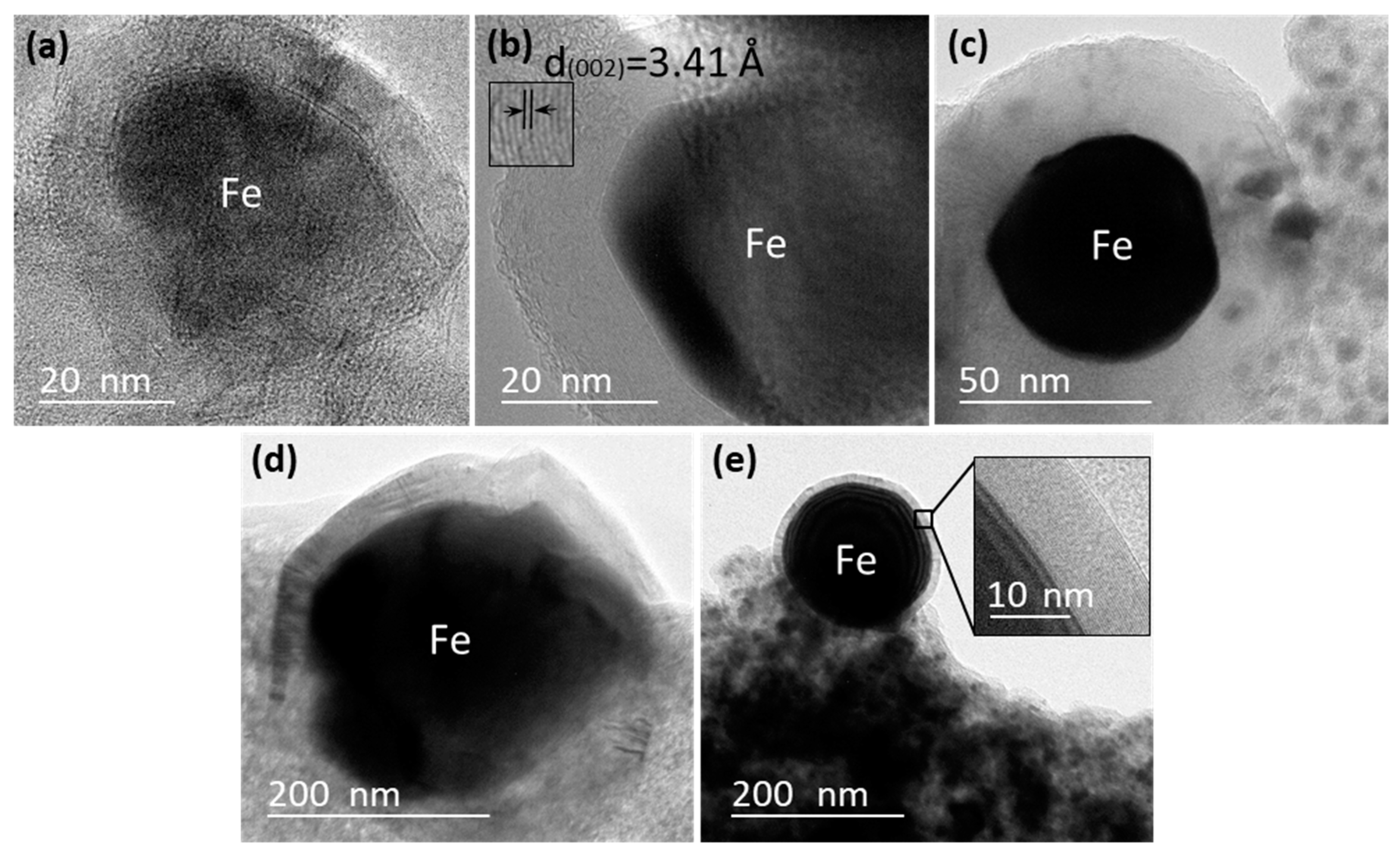
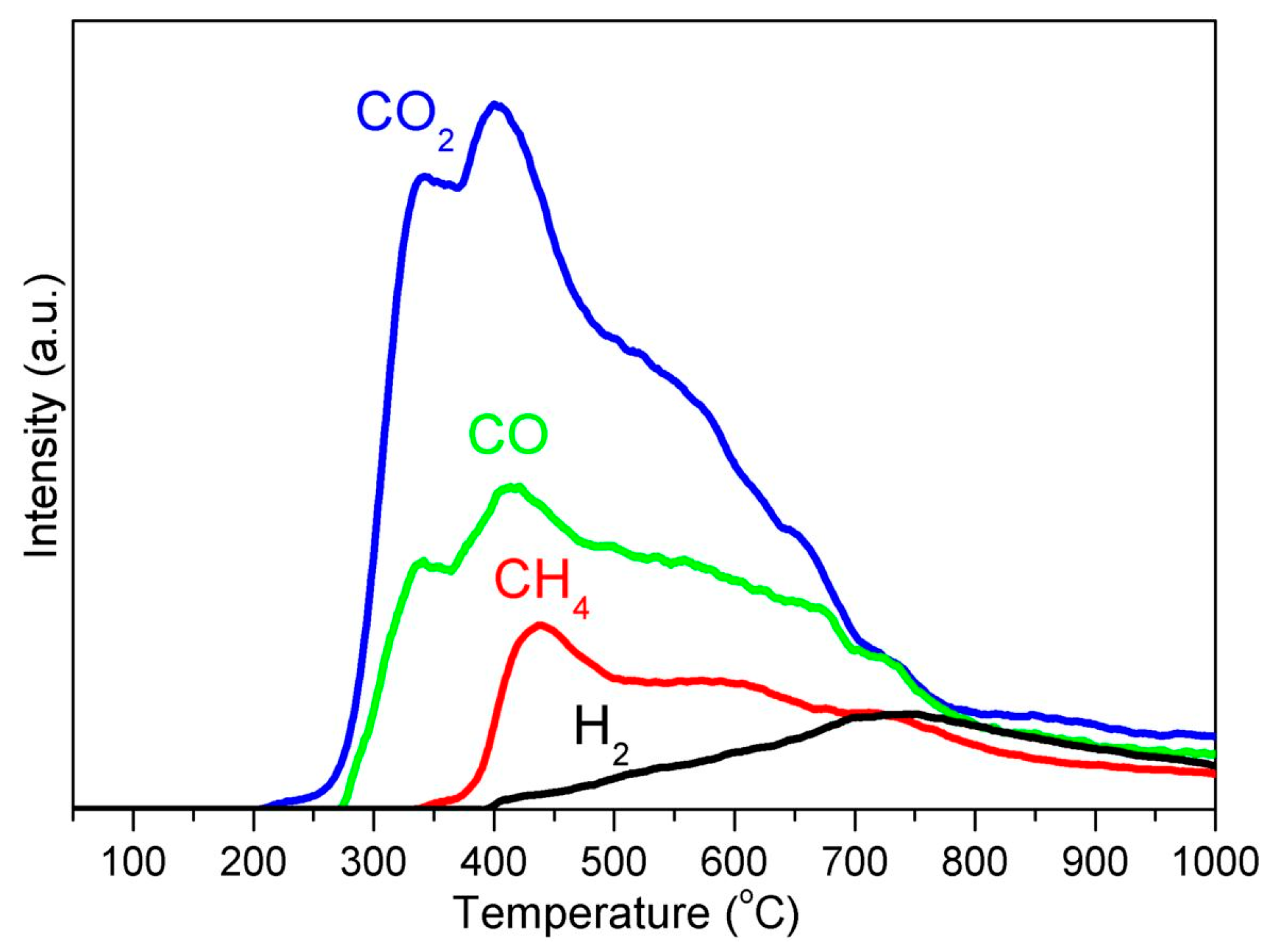
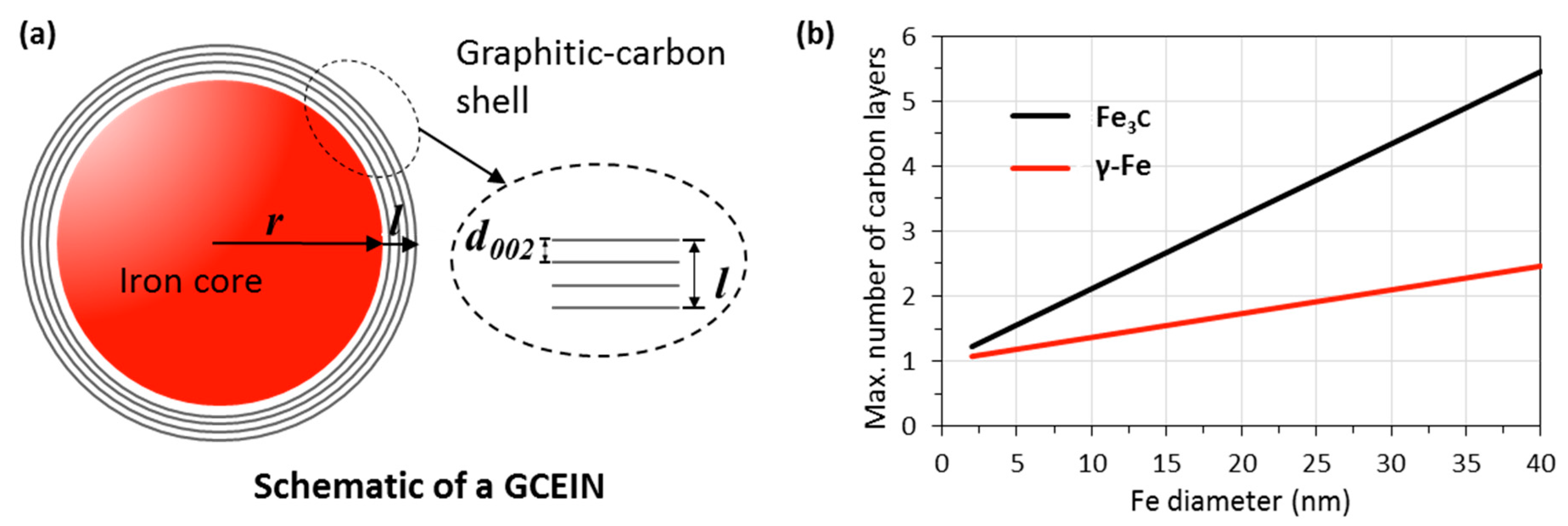
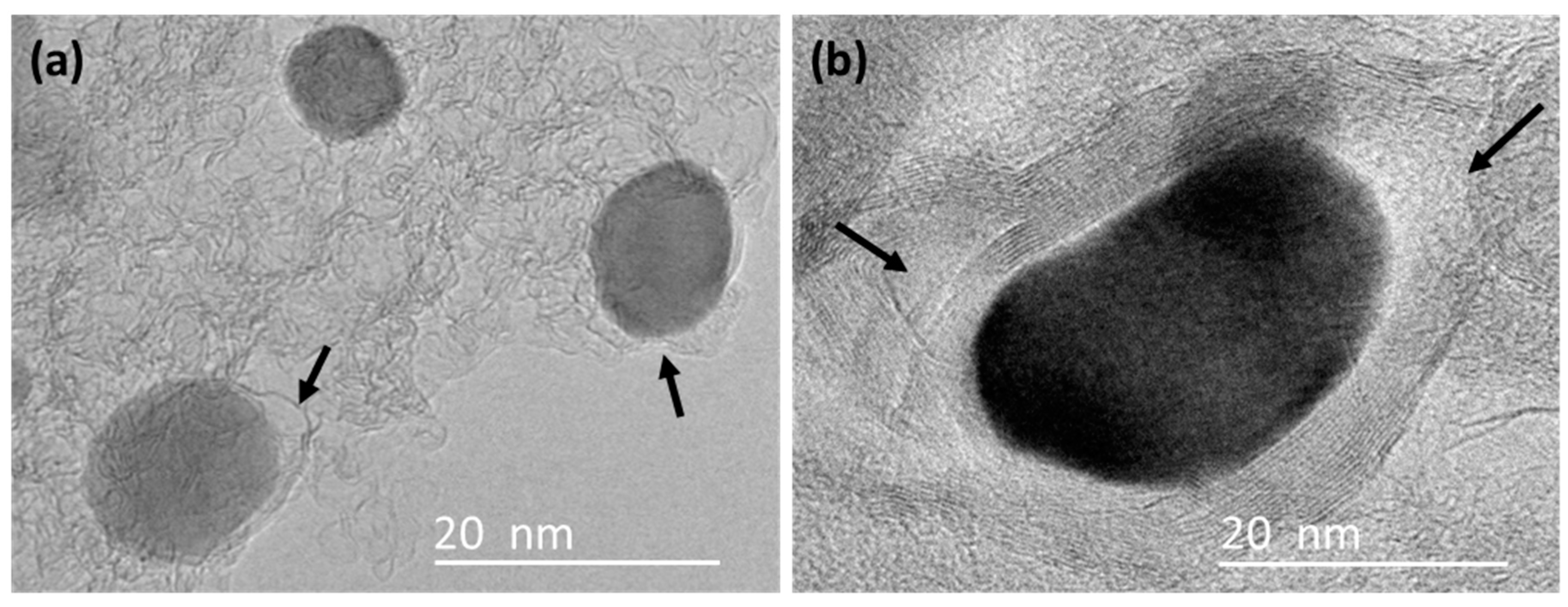
| KL/Fe-700 | KL/Fe-750 | KL/Fe-800 | KL/Fe-900 | KL/Fe-1000 | |
|---|---|---|---|---|---|
| Y, % | 49.28 | 48.96 | 48.77 | 48.62 | 48.55 |
| Yc, % | 47.70 | 47.34 | 47.13 | 46.96 | 46.88 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yan, Q.; Li, J.; Chu, I.-W.; Toghiani, H.; Cai, Z.; Zhang, J. Carbon-Based Nanomaterials from Biopolymer Lignin via Catalytic Thermal Treatment at 700 to 1000 °C. Polymers 2018, 10, 183. https://doi.org/10.3390/polym10020183
Zhang X, Yan Q, Li J, Chu I-W, Toghiani H, Cai Z, Zhang J. Carbon-Based Nanomaterials from Biopolymer Lignin via Catalytic Thermal Treatment at 700 to 1000 °C. Polymers. 2018; 10(2):183. https://doi.org/10.3390/polym10020183
Chicago/Turabian StyleZhang, Xuefeng, Qiangu Yan, Jinghao Li, I-Wei Chu, Hossein Toghiani, Zhiyong Cai, and Jilei Zhang. 2018. "Carbon-Based Nanomaterials from Biopolymer Lignin via Catalytic Thermal Treatment at 700 to 1000 °C" Polymers 10, no. 2: 183. https://doi.org/10.3390/polym10020183