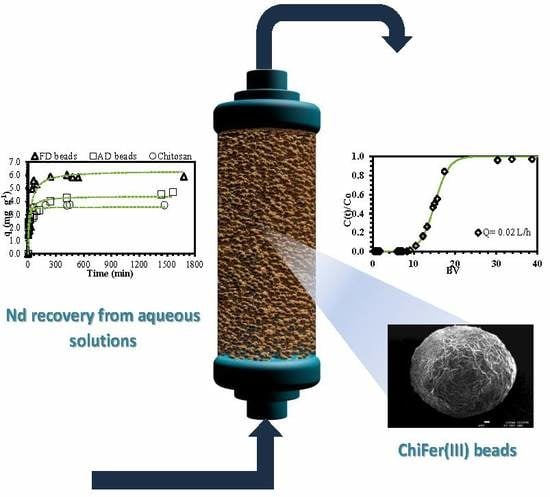
Neodymium Recovery by Chitosan/Iron(III) Hydroxide [ChiFer(III)] Sorbent Material: Batch and Column Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ChiFer(III) Microspheres
2.3. Characterization of Sorbents
2.3.1. Scanning Electron Microscopy
2.3.2. FTIR Analyses
2.4. pH Effect
2.5. Equilibrium Sorption
2.6. Influence of Contact Time
2.7. Dynamic Column Testing
3. Results and Discussion
3.1. Characterization of Sorbent
3.1.1. Scanning Electron Microscopy
3.2. pH Effect
3.3. Equilibrium Studies
3.4. Influence of Contact Time
3.5. Dynamic System
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Cossu, R.; Williams, I.D. Urban mining: Concepts, terminology, challenges. Waste Manag. 2015, 45, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Oddo, G. Die molekularstruktur der radioaktiven atom. Z. Aanorg. Allg. Chem. 1914, 87, 253–268. [Google Scholar] [CrossRef]
- Harkins, W.D. The evolution of the elements and the stability of complex atoms. I. A new periodic system which shows a relation between the abundance of the elements and the structure of the nuclei of atoms. J. Am. Chem. Soc. 1917, 39, 856–879. [Google Scholar] [CrossRef]
- Tu, Y.J.; Lo, S.C.; You, C.F. Selective and fast recovery of neodymium from seawater by magnetic iron oxide Fe3O4. Chem. Eng. J. 2015, 262, 966–972. [Google Scholar] [CrossRef]
- Rademaker, J.H.; Kleijn, R.; Yang, Y.X. Recycling as a strategy against rare earth element criticality: A systemic evaluation of the potential yield of NdFeB magnet recycling. Environ. Sci. Technol. 2013, 47, 10129–10136. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Energy. Critical Materials Strategy, Advanced Research Projects Agency-Energy; U.S. Department of Energy: Washington, DC, USA, 2011.
- Mirbagheri, S.A.; Hosseini, S.N. Pilot plan investigation on petrochemical wastewater treatment for the removal of copper and chromium with the objective of reuse. Desalination 2005, 171, 85–93. [Google Scholar] [CrossRef]
- Özverdi, A.; Erdem, M. Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J. Hazard. Mater. 2006, 137, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Aji, B.A.; Yavuz, Y.; Koparal, A.S. Electrocoagulation of heavy metals containing model wastewater using monopolar iron electrodes. Sep. Purif. Technol. 2012, 86, 248–254. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Blöcher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.K.; Matis, K.A. Hybrid flotation-membrane filtration process for the removal of heavy metal ions from wastewater. Water Res. 2003, 37, 4018–4046. [Google Scholar] [CrossRef]
- Samper, E.; Rodriguez, M.; De la Rubia, M.A.; Prats, D. Removal of metal ions at low concentration by micellar-enhanced ultrafiltration (MEUF) using sodium dodecyl sulfate (SDS) and linear alkylbenzene sulfonate (LAS). Sep. Purif. Technol. 2009, 65, 337–342. [Google Scholar] [CrossRef]
- Obon, E.; Fortuny, A.; Coll, M.T.; Sastre, A.M. Mathematical modeling of neodymium, terbium and dysprosium solvent extraction from chloride media using methyl-tri(octyl/decyl)ammonium oleate ionic liquid as extractant. Hydrometallurgy 2017, 173, 84–90. [Google Scholar] [CrossRef]
- Edebali, S.; Pehlivan, E. Evaluation of chelate and cation exchange resins to remove copper ions. Powder Technol. 2016, 301, 520–525. [Google Scholar] [CrossRef]
- Kang, S.Y.; Lee, J.U.; Moon, S.H.; Kim, K.W. Competitive adsorption characteristics of Co2+, Ni2+, and Cr3+ by IRN-77 cation exchange resin in synthesized wastewater. Chemosphere 2004, 56, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K.Z.; Guibal, E. Selective removal of Hg(II) from aqueous solution by functionalized magnetic-macromolecular hybrid material. Chem. Eng. J. 2015, 281, 345–359. [Google Scholar] [CrossRef]
- Mahfouz, M.G.; Galhoum, A.A.; Gomaa, N.A.; Abdel-Rehem, S.S.; Atia, A.A.; Vincent, T.; Guibal, E. Uranium extraction using magnetic nano-based particles of diethylenetriamine-functionalized chitosan: Equilibrium and kinetic studies. Chem. Eng. J. 2015, 262, 198–209. [Google Scholar] [CrossRef]
- Demey, H.; Tria, S.A.; Soleri, R.; Guiseppi-Elie, A.; Bazin, I. Sorption of his-tagged Protein G and Protein G onto chitosan/divalent metal ion sorbent used for detection of microcystin-LR. Environ. Sci. Pollut. Res. Int. 2017, 24, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280. [Google Scholar] [CrossRef]
- Pavon, S.; Kutucu, M.; Coll, M.T.; Fortuny, A.; Sastre, A.M. Comparison of Cyanex 272 and Cyanex 572 on the separation of Neodymium from a Nd/Tb/Dy mixture by pertraction. J. Chem. Technol. Biotechnol. 2017. [Google Scholar] [CrossRef]
- Yurekli, Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J. Hazard. Mater. 2016, 309, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.A.; Gazi, M. Targeted boron removal from highly-saline and boron-spiked seawater using magnetic nanobeads: Chemometric optimisation and modelling studies. Chem. Eng. Res. Des. 2017, 121, 329–338. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Ruiz, M.; Nogueras, M.; Sastre, A.M.; Guibal, E. Boron recovery from seawater with a new low-cost adsorbent material. Chem. Eng. J. 2014, 254, 463–471. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Wei, X.; Huo, F.; Li, W.; Huo, Q.; Liu, H. Adsorption of rare earths (III) by calcium alginate–poly glutamic acid hybrid gels. J. Chem. Technol. Biotechnol. 2014, 89, 969–977. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Mafhouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Atia, A.A.; Vincent, T.; Guibal, E. Cysteine-functionalized chitosan magnetic nano-based particles for the recovery of light and heavy rare earth metals: Uptake kinetics and sorption isotherms. Nanomaterials (Basel) 2015, 5, 154–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Repo, E.; Meng, Y.; Wang, X.; Yin, D.; Sillanpää, M. An EDTA-β-cyclodextrin material for the adsorption of rare earth elements and its applications in preconcentration of rare earth elements in seawater. J. Colloid Interface Sci. 2016, 465, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Repo, E.; Song, Y.; Yin, D.; Hammouda, S.B.; Chen, L.; Kalliola, S.; Tang, J.; Tam, K.C.; Sillanpää, M. Polyethylenimine-cross-linked cellulose nanocrystals for highly efficient recovery of rare earth elements from water and mechanism study. Green Chem. 2017, 19, 4816–4828. [Google Scholar] [CrossRef]
- Wang, G.H.; Liu, J.S.; Wang, X.G.; Xie, Z.Y.; Deng, N.S. Adsorption of uranium (VI) from aqueous solutions onto cross-linked chitosan. J. Hazard. Mater. 2009, 168, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Hosoba, M.; Oshita, K.; Katarina, R.K.; Takayanagi, T.; Oshima, M.; Motomizu, S. Synthesis of novel chitosan resin possessing histidine moiety and its application to the determination of trace silver by ICP-AES coupled with triplet automated-pretreatment system. Anal. Chim. Acta 2009, 639, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Ruiz, M.; Sastre, A.M.; Zikan, M.C.; Guibal, E. Palladium sorption on glutaraldehyde-crosslinked chitosan in fixed-bed systems. J. Appl. Polym. Sci. 2001, 81, 153–165. [Google Scholar] [CrossRef]
- Guibal, E.; Larkin, A.; Vincent, T.; Tobin, J.M. Chitosan sorbents for platinum sorption from dilute solutions. Ind. Eng. Chem. Res. 1999, 38, 401–412. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen. Z. Phys. Chem. 1906, 57A, 385–470. [Google Scholar] [CrossRef]
- El-Korashy, S.A.; Elwakeel, K.Z.; El-Hafeiz, A.A. Fabrication of bentonite/thiourea-formaldehyde composite material for Pb(II), Mn(VII) and Cr(VI) sorption: A combined basic study and industrial application. J. Clean. Prod. 2016, 137, 40–50. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Chen, L.; Kalliola, S.; Tang, J.; Iakovleva, E.; Tam, K.C.; Sillanpää, M. One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solutions. J. Sanit. Eng. Div. ASCE 1963, 89, 31–60. [Google Scholar]
- Lagergreen, S. Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Shi, X.; Li, Q.; Wang, T.; Lackner, K.S. Kinetic analysis of an anion exchange absorbent for CO2 capture from ambient air. PLoS ONE 2017, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Chu, K.H. Fixed bed sorption: Setting the record straight on the Bohart-Adams and Thomas models. J. Hazard. Mater. 2010, 177, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, J.; Zhou, H.; Li, W.; Sui, N.; Lui, H. O-carboxymethyl chitosan entrapped by silica: Preparation and adsorption behavior toward neodymium (III) ions. J. Chem. Technol. Biotechnol. 2013, 88, 317–325. [Google Scholar] [CrossRef]
- Ruiz, M.; Sastre, A.M.; Guibal, E. Pd and Pt recovery using chitosan gel beads: I. Influence of drying process on diffusion properties. Sep. Sci. Technol. 2002, 37, 2143–2166. [Google Scholar] [CrossRef]
- Borisova, A.; De Bruyn, M.; Budarin, V.L.; Shuttleworth, P.S.; Dodson, J.R.; Segatto, M.L.; Clark, J.H. A sustainable freeze-drying route to porous polysaccharide with tailored hierarchical meso-and macroporosity. Macromol. Rapid Commun. 2015, 36, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Gazi, M.; Shahmohammadi, S. Removal of trace boron from aqueous solutions using iminobis-(propylene glycol) modified chitosan beads. React. Funct. Polym. 2012, 72, 680–686. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Ruiz, M.; Sastre, A.M.; Guibal, E. Development of a new chitosan/Ni(OH)2-based sorbent for boron removal. Chem. Eng. J. 2014, 244, 576–586. [Google Scholar] [CrossRef]
- Shinde, T.J.; Gadkari, A.B.; Vasambekar, P.N. Influence of Nd+3 substitution on structural, electrical and magnetic properties of nanocrystalline nickel ferrites. J. Alloy Compd. 2012, 513, 80–95. [Google Scholar] [CrossRef]
- Sakai, Y.; Hayano, K.; Yoshioka, H.; Yoshioka, H. A novel method of dissolving chitosan in water for industrial applications. Polym. J. 2001, 33, 640–642. [Google Scholar] [CrossRef]
- Sorlier, P.; Viton, C.; Domard, A. Relation between solution properties and degree of acetylation of chitosan: Role of aging. Biomacromolecules 2002, 3, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- El-Sonbati, A.Z.; El-Deen, I.M.; El-Bindary, M.A. Adsorption of hazardous azorhodanine dye from an aqueous solution using rice straw fly ash. J. Dispers. Sci. Technol. 2016, 37, 715–722. [Google Scholar] [CrossRef]
- Vlachou, A.; Symeopoulos, B.D.; Koutinas, A.A. A comparative study of neodymium sorption by yeast cells. Radiochim. Acta 2009, 97, 437–441. [Google Scholar] [CrossRef]
- Guo, J.; Cai, J.; Su, Q. Ion imprinted polymer particles of neodymium: Synthesis, characterization and selective recognition. J. Rare Earth 2009, 27, 22–27. [Google Scholar] [CrossRef]
- Benettayeb, A.; Guibal, E.; Morsli, A.; Kessas, R. Chemical modification of alginate for enhanced sorption of Cd(II), Cu(II) and Pb(II). Chem. Eng. J. 2017, 316, 704–714. [Google Scholar] [CrossRef]
- Rorrer, G.L.; Hsien, T.Y.; Way, J.D. Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from waste water. Ind. Eng. Chem. Res. 1993, 32, 2170–2178. [Google Scholar] [CrossRef]
- Chu, K.H. Improved fixed bed models for metal biosorption. Chem. Eng. J. 2004, 97, 233–239. [Google Scholar] [CrossRef]
- Muhamad, H.; Doan, H.; Lohi, A. Batch and continuous fixed-bed column biosorption of Cd2+ and Cu2+. Chem. Eng. J. 2010, 158, 369–377. [Google Scholar] [CrossRef]
- Kleinübing, S.J.; Da Silva, E.A.; Da Silva, M.G.C.; Guibal, E. Equilibrium of Cu(II) and Ni(II) biosorption by marine alga Sargassum filipendula in a dynamic system: Competitiveness and selectivity. Bioresour. Technol. 2011, 7, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; Poch, J.; Villaescusa, I. Modelling of breakthrough curves of single and binary mixtures of Cu(II), Cd(II), Ni(II) and Pb(II) sorption onto grape stalks waste. Chem. Eng. J. 2013, 217, 129–138. [Google Scholar] [CrossRef]
- Sag, Y.; Kutsal, T. Recent trends in the biosorption of heavy metals: A review. Biotechnol. Bioprocess Eng. 2001, 6, 376–385. [Google Scholar] [CrossRef]
- Shiewer, S.; Volesky, B. Ionic strength and electrostatic effects in biosorption of divalent metal ions and protons. Environ. Sci. Technol. 1997, 31, 2478–2485. [Google Scholar] [CrossRef]
- Smith, E.; Ghiassi, K. Chromate removal by an iron sorbent: Mechanism and modeling. Water Environ. Res. 2006, 78, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Grossl, P.R.; Eick, M.J.; Sparks, D.L.; Goldberg, S.; Ainsworth, C.C. Arsenate and chromate retention mechanisms on goethite. 2. Kinetic evaluation using a pressure-jump relaxation technique. Environ. Sci. Technol. 1997, 31, 321–326. [Google Scholar] [CrossRef]
- Ruiz, M.; Tobalina, C.; Demey-Cedeño, H.; Barron-Zambrano, J.A.; Sastre, A.M. Sorption of boron on calcium alginate gel beads. React. Funct. Polym. 2013, 73, 653–657. [Google Scholar] [CrossRef]









| Experimental | Langmuir | Freundlich | Sips | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sorbent | qexp (mg·g−1) | qmax (mg·g−1) | b (L·mg−1) | r2 | KF (mg1−1/n g−1·L1/n) | n | r2 | qmax (mg·g−1) | Ks (L·mg−1) | ns | r2 |
| Chitosan | 3.77 | 3.54 | 1.91 | 0.971 | 2.78 | 20.44 | 0.957 | 3.51 | 1.75 | 0.80 | 0.966 |
| ChiFer(III) | 11.51 | 13.76 | 0.03 | 0.973 | 1.65 | 2.63 | 0.922 | 13.80 | 0.03 | 1.00 | 0.969 |
| Sorbent | pH Range | qmax (mg·g−1) | Authors |
|---|---|---|---|
| C. colliculosa yeast | 1.5 | 10.0 | Vlachou et al. [52] |
| K. marxianus yeast | 1.5 | 12.0 | Vlachou et al. [52] |
| D. hansenii yeast | 1.5 | 10.0 | Vlachou et al. [52] |
| ChiFer(III) beads | 4.0–5.0 | 13.8 | This work |
| Cysteine-functionalized chitosan magnetic particles | 6.0 | 15.3–17.1 | Galhoum et al. [25] |
| Magnetic iron oxide (Fe3O4) | 8.2 | 24.8 | Tu et al. [4] |
| Ion imprinted polymer | 7.0–7.5 | 35.2 | Guo et al. [53] |
| SiO2/Carboxymethyl chitosan (CMCH) | 6.9 | 53.0 | Wang et al. [42] |
| Experimental | Pseudo-First Order Rate Equation (PFORE) | Pseudo-Second Order Rate Equation (PSORE) | Weber and Morris Equation (W&M) | |||||
|---|---|---|---|---|---|---|---|---|
| Sorbent | qexp (mg·g−1) | K1 (min−1) | q1 (mg·g−1) | r2 | K2 (g·mg−1·min−1) | q2 (mg·g−1) | r2 | Kp (mg·g−1·min−1/2) |
| Air-dried, AD (C0 = 10 mg·g−1) | 4.50 | 5.95 × 10−2 | 3.96 | 0.455 | 1.69 × 10−2 | 4.39 | 0.619 | 0.14 |
| Freeze-dried, FD (C0 = 10 mg·g−1) | 5.89 | 2.78 × 10−2 | 5.93 | 0.908 | 6.16 × 10−3 | 6.34 | 0.887 | 1.24 |
| Chitosan (C0 =10 mg·g−1) | 3.74 | 0.177 | 3.44 | 0.757 | 0.11 | 3.56 | 0.851 | 2.81 × 10−2 |
| FD (C0 =20 mg·g−1) | 7.39 | 0.129 | 6.88 | 0.778 | 3.9 × 10−2 | 7.13 | 0.885 | 0.64 |
| Experimental | Thomas Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Sorbate | Q (L·h−1) | qexp (mg·g−1) | Sorption Efficiency (%) | qBP (mg·g−1) | Bed-Volume (BVBP) | qT (mg·g−1) | KT (L·h−1·mg−1) | r2 |
| Nd | 0.02 | 4.02 | 24.97 | 2.12 | 9.06 | 3.46 | 1.98 × 10−2 | 0.984 |
| Nd | 0.01 | 6.40 | 79.17 | 4.79 | 19.03 | 6.55 | 5.27 × 10−3 | 0.981 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demey, H.; Lapo, B.; Ruiz, M.; Fortuny, A.; Marchand, M.; Sastre, A.M. Neodymium Recovery by Chitosan/Iron(III) Hydroxide [ChiFer(III)] Sorbent Material: Batch and Column Systems. Polymers 2018, 10, 204. https://doi.org/10.3390/polym10020204
Demey H, Lapo B, Ruiz M, Fortuny A, Marchand M, Sastre AM. Neodymium Recovery by Chitosan/Iron(III) Hydroxide [ChiFer(III)] Sorbent Material: Batch and Column Systems. Polymers. 2018; 10(2):204. https://doi.org/10.3390/polym10020204
Chicago/Turabian StyleDemey, Hary, Byron Lapo, Montserrat Ruiz, Agustin Fortuny, Muriel Marchand, and Ana M. Sastre. 2018. "Neodymium Recovery by Chitosan/Iron(III) Hydroxide [ChiFer(III)] Sorbent Material: Batch and Column Systems" Polymers 10, no. 2: 204. https://doi.org/10.3390/polym10020204