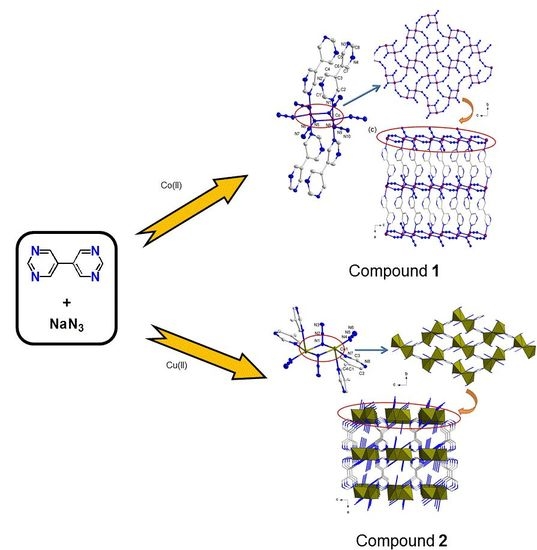
Two New Three-Dimensional Pillared-Layer Co(II) and Cu(II) Frameworks Involving a [M2(EO-N3)2] Motif from a Semi-Flexible N-Donor Ligand, 5,5′-Bipyrimidin: Syntheses, Structures and Magnetic Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of [Co2(N3)4(bpym)2]n (1)
2.3. Synthesis of [Cu2(N3)4(bpym)]n (2)
2.4. X-ray Crystallography
2.5. Physical Measurements
3. Results and Discussion
3.1. Syntheses and Characterization of Compounds 1 and 2
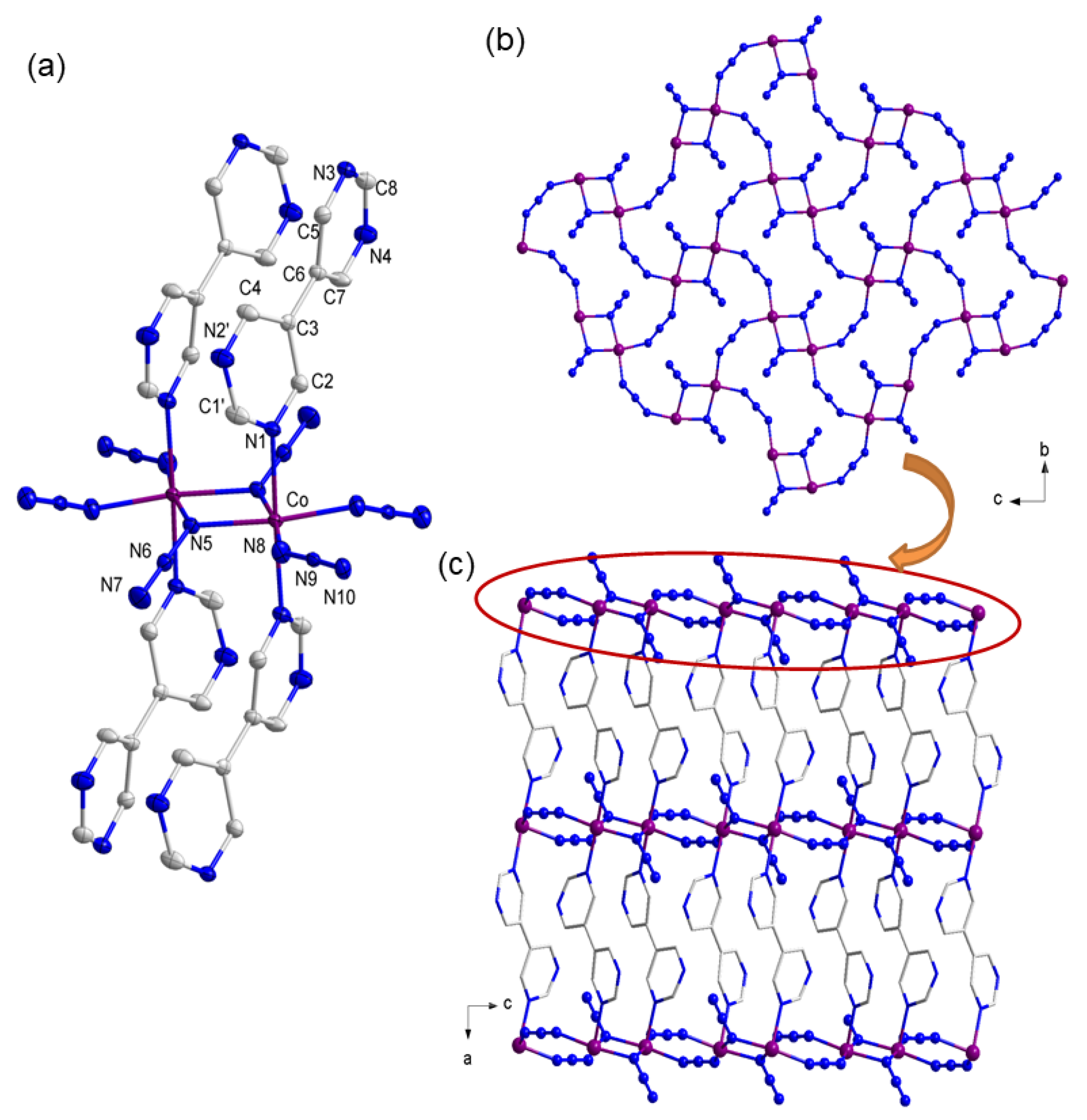
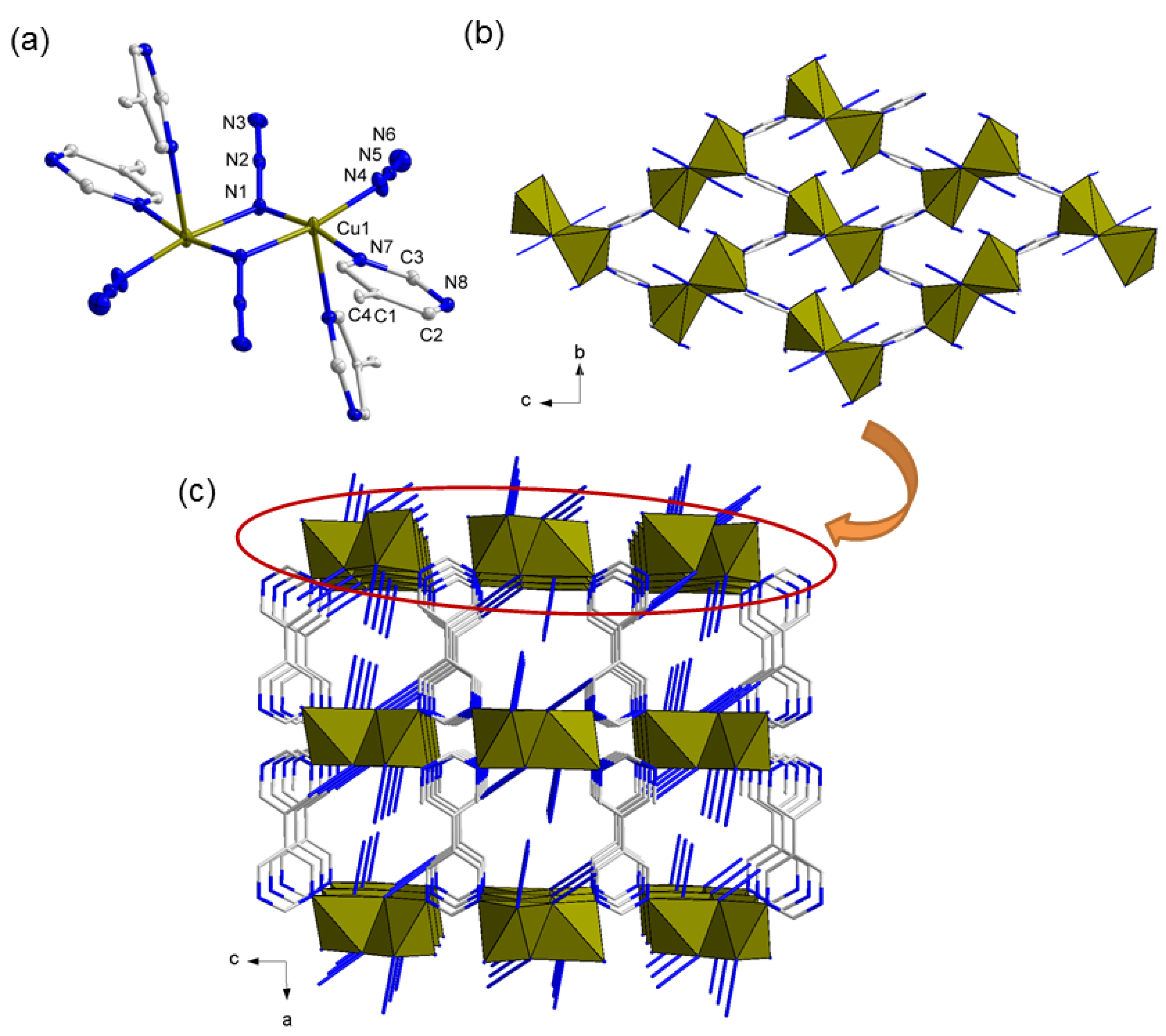
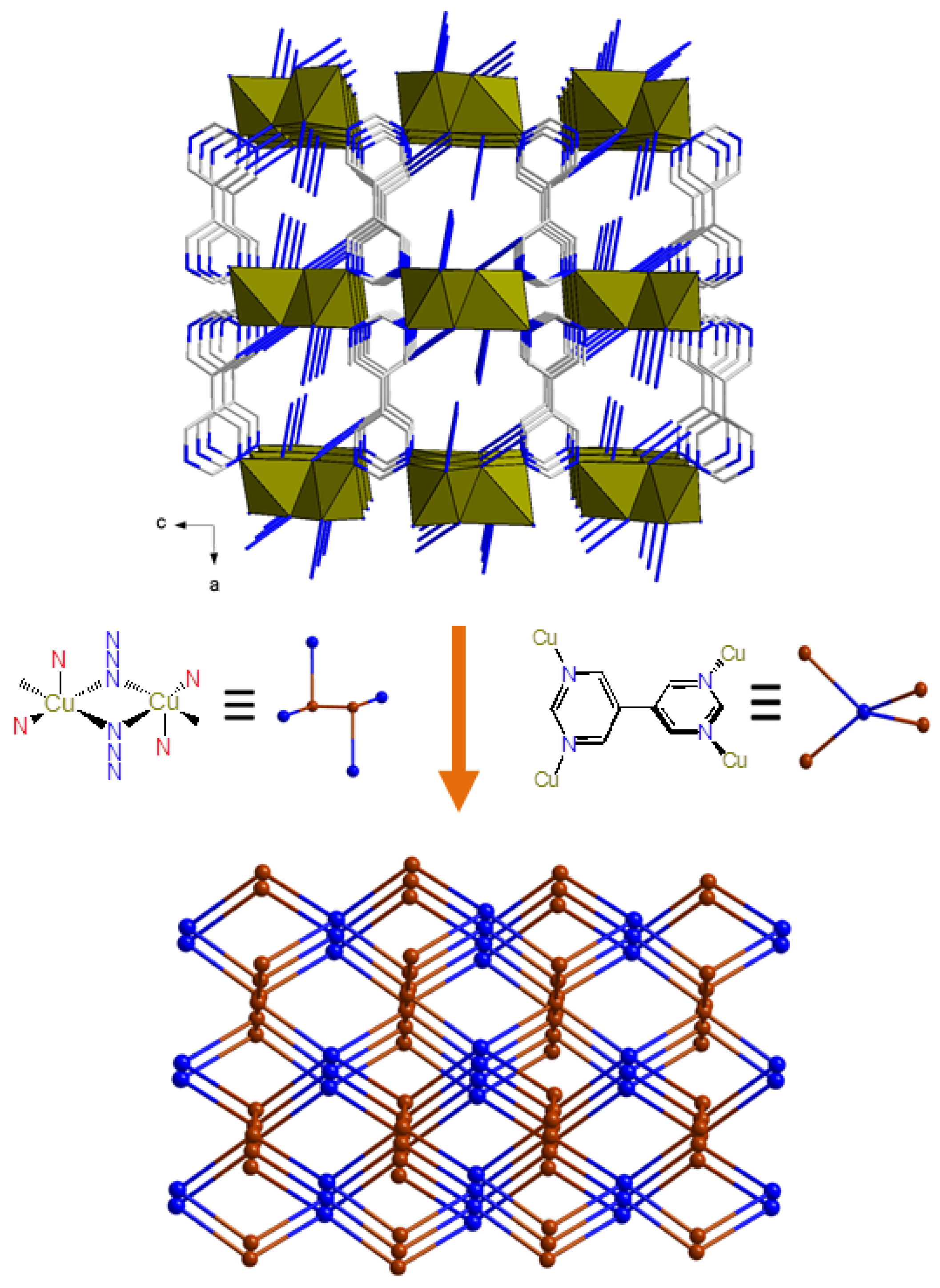
3.2. Description of Structure
3.2.1. Crystal Structures of Compound 1
3.2.2. Crystal Structures of Compound 2
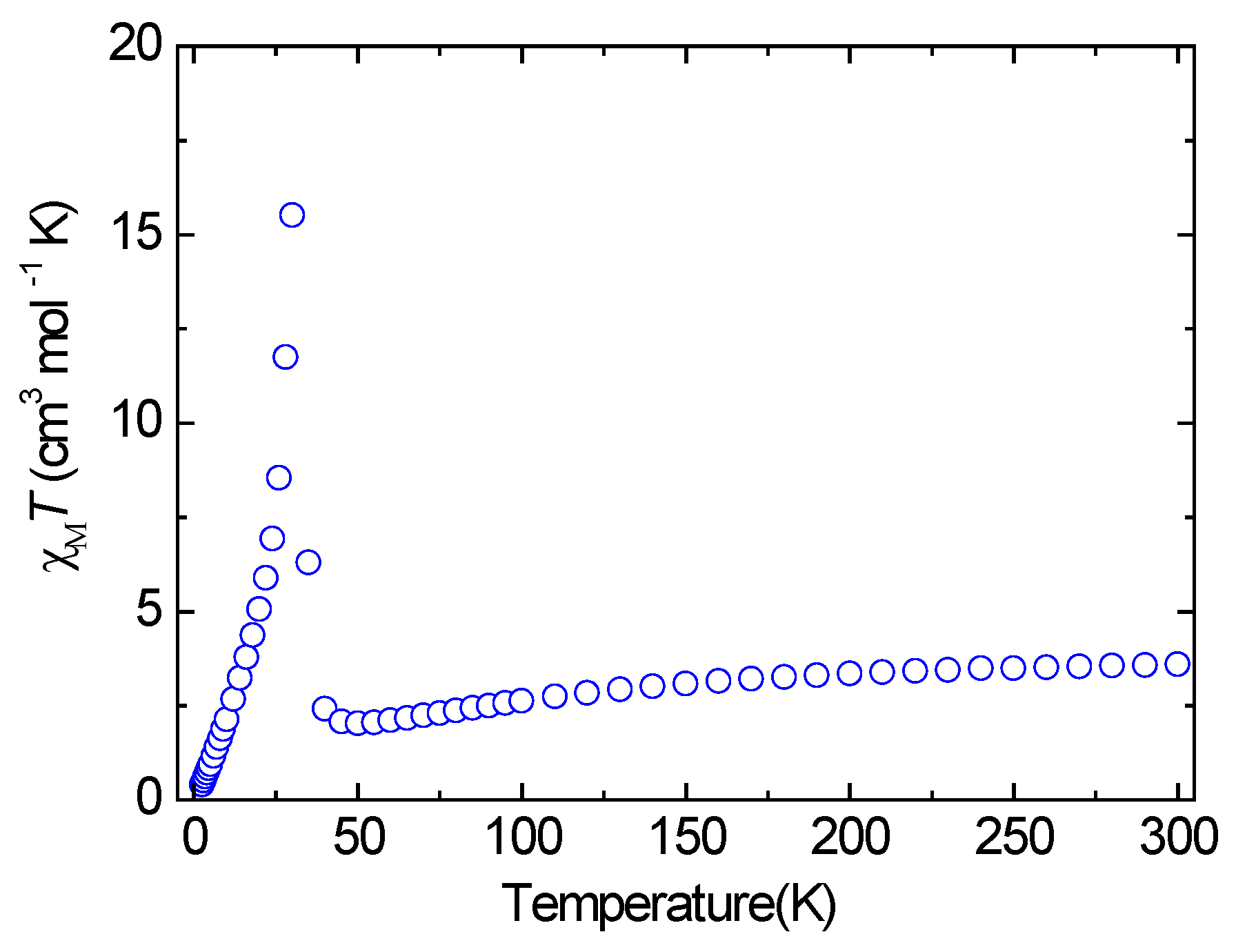
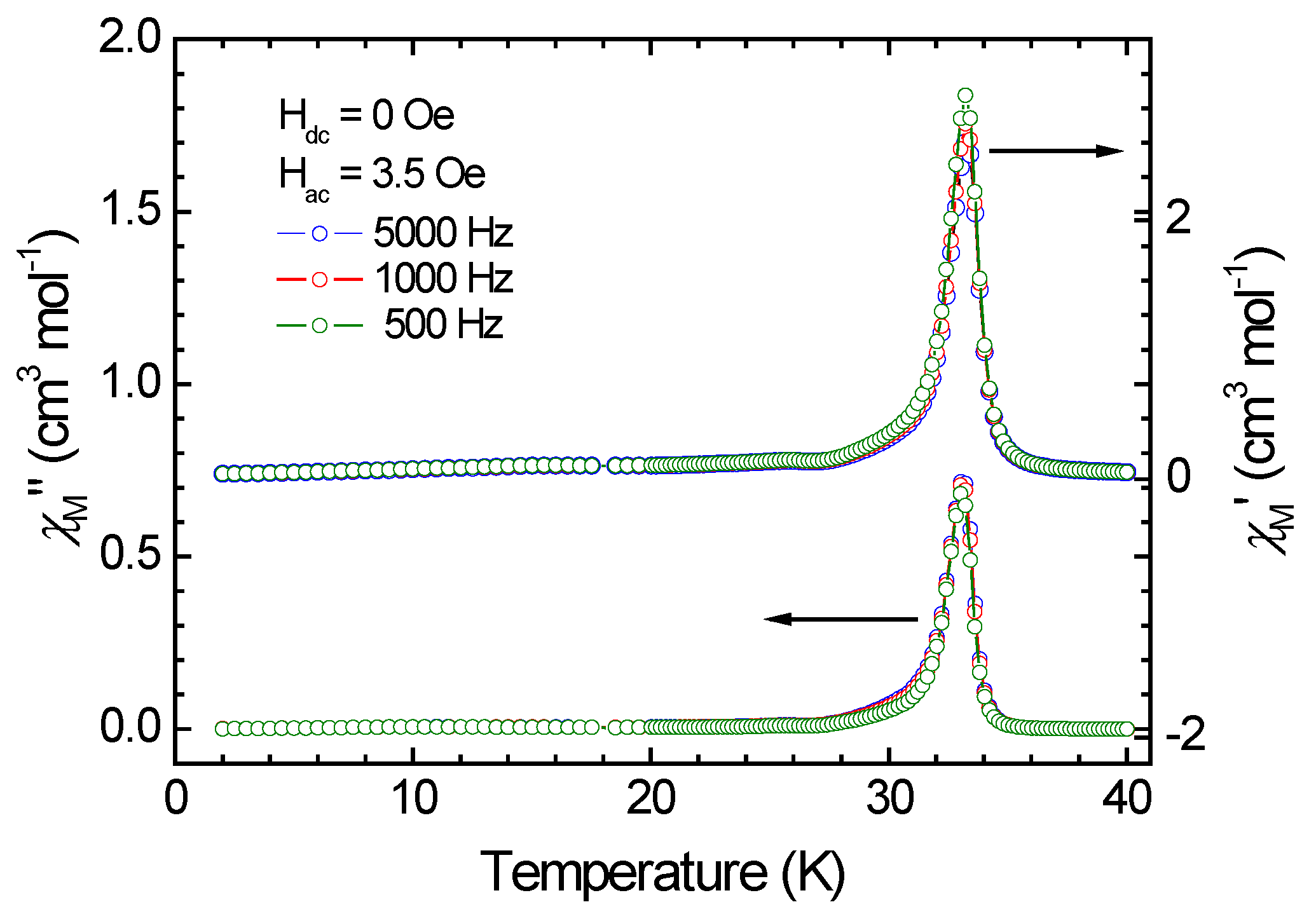
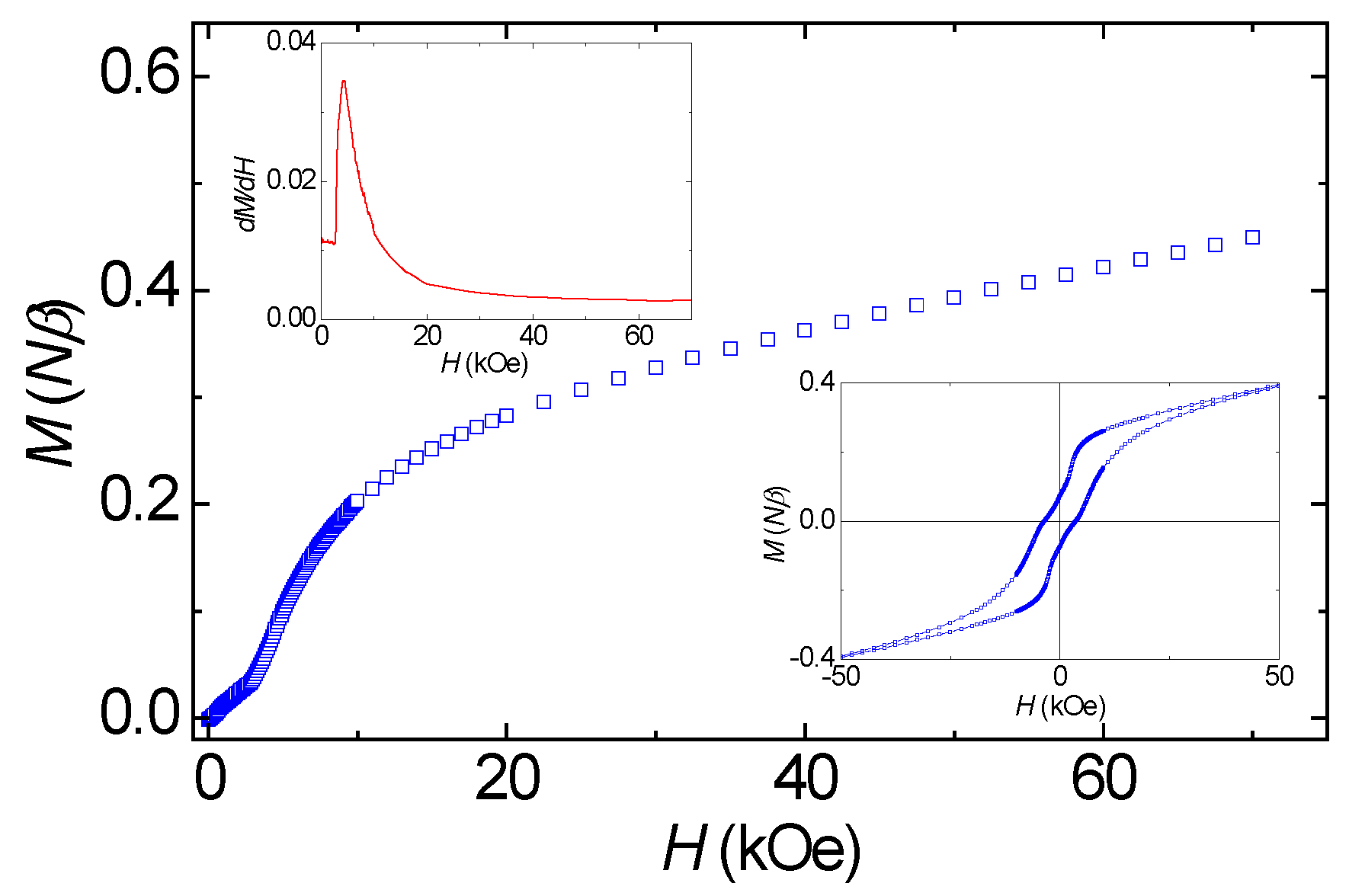
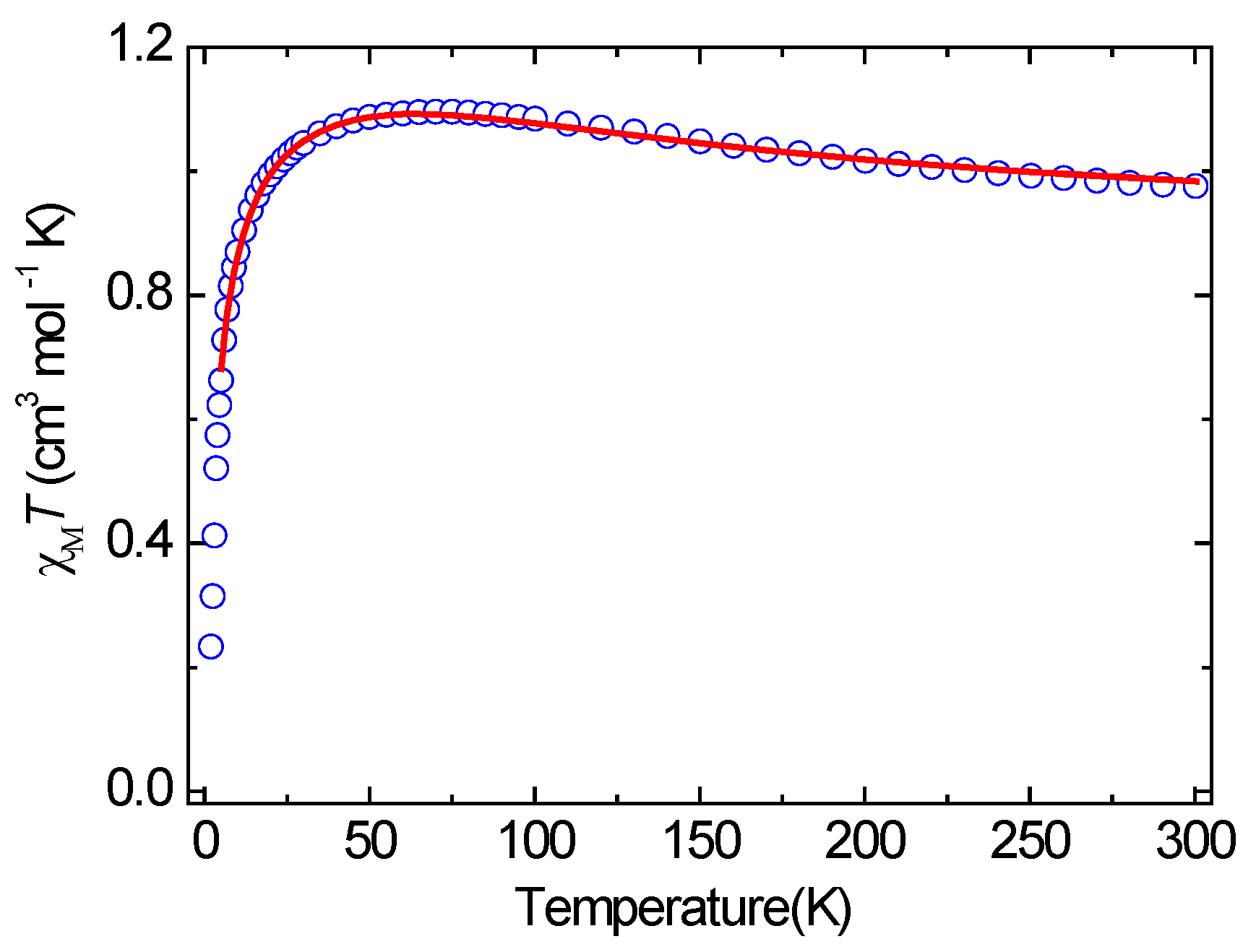
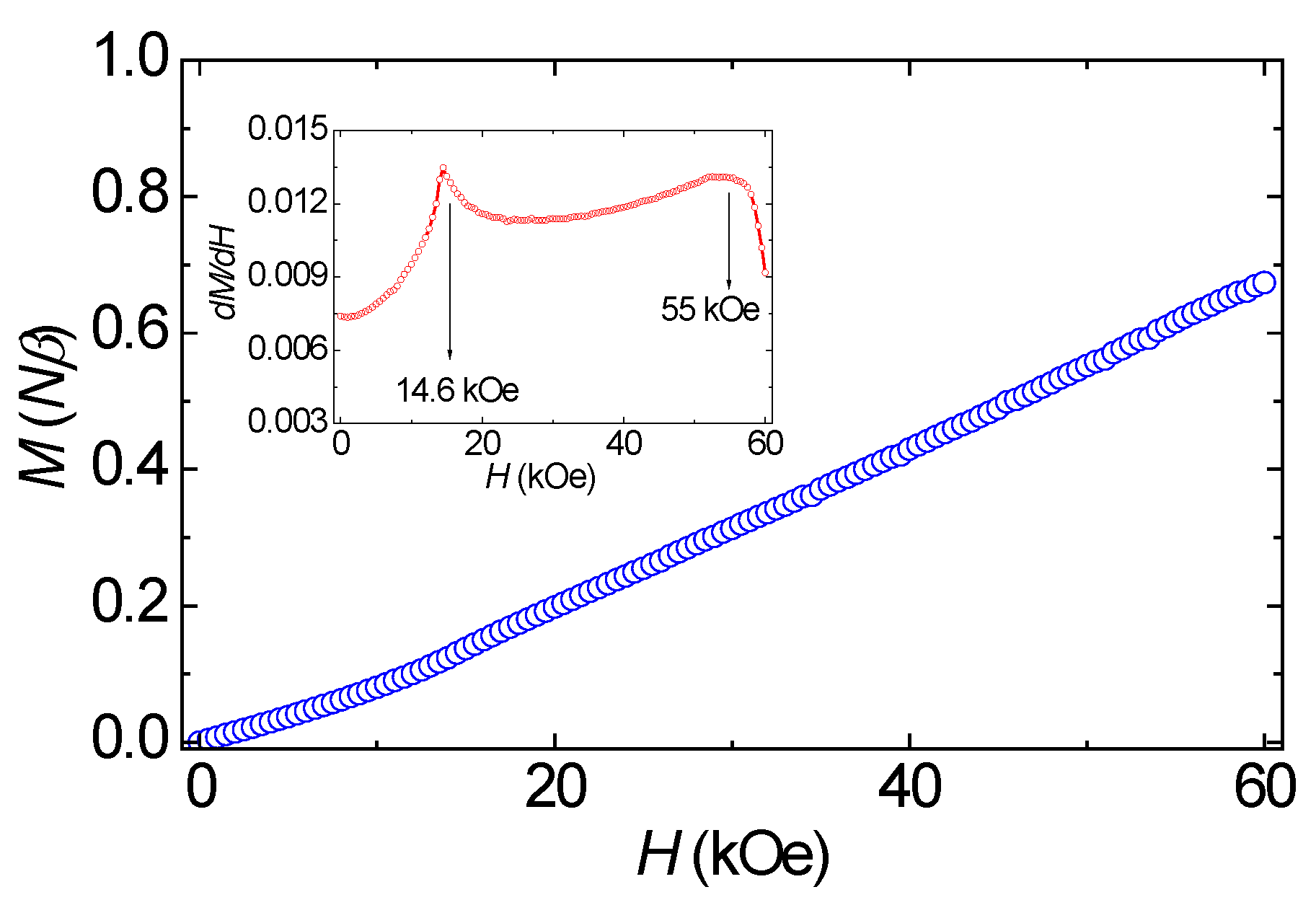
3.3. Magnetic Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiang, S.; Wu, X.; Zhang, J.; Fu, R.; Hu, S.; Zhang, X. A 3D Canted Antiferromagnetic Porous Metal–Organic Framework with Anatase Topology through Assembly of an Analogue of Polyoxometalate. J. Am. Chem. Soc. 2005, 127, 16352–16353. [Google Scholar] [CrossRef] [PubMed]
- Palii, A.V.; Reu, O.S.; Ostrovsky, S.M.; Klokishner, S.I.; Tsukerblat, B.S.; Sun, Z.-M.; Mao, J.-G.; Prosvirin, A.V.; Zhao, H.-H.; Dunbar, K.R. A highly anisotropic cobalt(II)-based single-chain magnet: Exploration of spin canting in an antiferromagnetic array. J. Am. Chem. Soc. 2008, 130, 14729–14738. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of metal-organic frameworks featuring multi-functional sites. Coord. Chem. Rev. 2016, 307, 106–129. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Hu, Z.; Wang, G.; Uvdal, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination polymers, metal–organic frameworks and the need for terminology guidelines. CrystEngComm 2012, 14, 3001. [Google Scholar] [CrossRef]
- Bradshaw, D.; Claridge, J.B.; Cussen, E.J.; Prior, T.J.; Rosseinsky, M.J. Design, Chirality, and Flexibility in Nanoporous Molecule-Based Materials. Acc. Chem. Res. 2005, 38, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366. [Google Scholar] [CrossRef]
- Toma, L.M.; Ruiz-Pérez, C.; Pasán, J.; Wernsdorfer, W.; Lloret, F.; Julve, M. Molecular Engineering to Control the Magnetic Interaction between Single-Chain Magnets Assembled in a Two-Dimensional Network. J. Am. Chem. Soc. 2012, 134, 15265–15268. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional metal–organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O̅kawa, H.; Shigematsu, A.; Sadakiyo, M.; Miyagawa, T.; Yoneda, K.; Ohba, M.; Kitagawa, H. Oxalate-Bridged Bimetallic Complexes {NH(prol)3}[MCr(ox)3] (M = MnII, FeII , CoII; NH(prol)3+ = Tri(3-hydroxypropyl)ammonium) Exhibiting Coexistent Ferromagnetism and Proton Conduction. J. Am. Chem. Soc. 2009, 131, 13516–13522. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Colacio, E.; Lloret, F.; Navarrete, M.; Romerosa, A.; Stoeckli-Evans, H.; Suarez-Varela, J. 2D and 3D coordination polymers based on 2,2′-bipyrimidine and cyanide bridging ligands incorporating coordinated and guest ammonia molecules. Synthesis, crystal structures, magnetic properties and thermal analysis of {[Ni(CN)4]2[(Ni(NH3)2)2(bpym)]·2H2O}n and {[Cu2(CN)2(bpym)]·NH3}n. New J. Chem. 2005, 29, 1189. [Google Scholar]
- De Munno, G.; Viterbo, D.; Caneschi, A.; Lloret, F.; Julve, M. A Giant Antiferromagnetic Interaction through the Bihydroxide Bridge (H3O2−). Inorg. Chem. 1994, 33, 1585–1586. [Google Scholar] [CrossRef]
- Kar, P.; Drew, M.G.B.; Gómez-García, C.J.; Ghosh, A. Coordination Polymers Containing Manganese(II)-Azido Layers Connected by Dipyridyl-tetrazine and 4,4′-Azobis(pyridine) Linkers. Inorg. Chem. 2013, 52, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Cortes, R.; Urtiaga, K.; Lezama, L.; Pizarro, J.L.; Goni, A.; Arriortua, M.I.; Rojo, T. Crystal Structure and Spectroscopic and Magnetic Properties of Two cis-Azido Catenas of Nickel (II): cis-catena-(μ-N3)[Ni(bipy)2](X)(X = ClO4, PF6). Inorg. Chem. 1994, 33, 4009–4015. [Google Scholar] [CrossRef]
- Cheng, X.N.; Xue, W.; Huang, J.H.; Chen, X.M. Spin canting and/or metamagnetic behaviours of four isostructural grid-type coordination networks. Dalton Trans. 2009, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Cui, H.; Zhang, C.; Zhang, L.; Su, C.-Y. Porous Metal–Organic Framework Catalyzing the Three-Component Coupling of Sulfonyl Azide, Alkyne, and Amine. Inorg. Chem. 2013, 52, 9053–9059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, F.; Liu, T.; Yuan, M.; Wang, Z.-M.; Gao, S. Spin Crossover in a Series of Iron(II) Complexes of 2-(2-Alkyl-2H-tetrazol-5-yl)-1,10-phenanthroline: Effects of Alkyl Side Chain, Solvent, and Anion. Inorg. Chem. 2007, 46, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.-X.; Lin, J.-B.; Zhang, J.-P.; Chen, X.-M. Isomeric Zinc(II) Triazolate Frameworks with 3-Connected Networks: Syntheses, Structures, and Sorption Properties. Inorg. Chem. 2009, 48, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- De Munno, G.; Poerio, T.; Julve, M.; Lloret, F.; Faus, J.; Caneschi, A. Syntheses, crystal structures and magnetic properties of one-, two-and three-dimensional 2, 2′-bipyrimidine-containing copper(II) complexes. J. Chem. Soc. Dalton Trans. 1998, 1679–1686. [Google Scholar] [CrossRef]
- Marino, N.; Armentano, D.; De Munno, G.; Lloret, F.; Cano, J.; Julve, M. Towards a better understanding of honeycomb alternating magnetic networks. Dalton Trans. 2015, 44, 11040–11051. [Google Scholar] [CrossRef] [PubMed]
- Munno, G.D.; Poerio, T.; Julve, M.; Lloretb, F.; Viau, G. Synthesis, crystal structure and magnetic properties of the cobalt(II) chain and the dinuclear compounds [Co(bipym)(H2O)2](NO3)2 and [Co2(bipym)3(H2O)4](NO3)4·2H2O [Co2(bipym)3(H2O)2(SO4)2]·12H2O. New J. Chem. 1998, 299–305. [Google Scholar] [CrossRef]
- Lee, K.; Lee, P.H. Efficient homo-coupling reactions of heterocyclic aromatic bromides catalyzed by Pd(OAc)2 using indium. Tetrahedron Lett. 2008, 49, 4302–4305. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Mulay, L.N.; Boudreaux, E.A. Theory and Applications of Molecular Diamagnetism; Wiley-VCH: New York, NY, USA, 1976; pp. 491–494. [Google Scholar]
- Tsao, J.-Y.; Tsai, J.-D.; Yang, C.-I. Azide-bridged Cu(II), Mn(II) and Co(II) coordination polymers constructed with a bifunctional ligand of 6-(1H-tetrazol-5-yl)-2,2′-bipyridine. Dalton Trans. 2016, 45, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Bellitto, A.; Federici, F.; Colapietro, M.; Portalone, G.; Caschera, D. X-ray single-crystal structure and magnetic properties of Fe[CH3PO3)]·xH3O: A layered weak ferromagnet. Inorg. Chem. 2002, 41, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-D.; Yang, C.-I. Utilization of ligand containing 2,2′-bipyridyl and tetrazolate groups to construct of a 2D Co(II) coordination polymer: Spin canting and metamagnetism. Dalton Trans. 2014, 43, 15576–15582. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shang, R.; Hu, K.-L.; Wang, Z.-M.; Gao, S. A new series of chiral metal formate frameworks of [HONH3][M(II)(HCOO)3] (M = Mn, Co, Ni, Zn, and Mg): Synthesis, structures, and properties. Inorg. Chem. 2012, 51, 13363–13372. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, H.; Nakata, K.; Lecren, L.; Coulon, C.; Nakazawa, Y.; Fujisaki, T.; Sugiura, K.-I.; Yamashita, M.; Clérac, R. Two-Dimensional Networks Based on Mn4 Complex Linked by Dicyanamide Anion: From Single-Molecule Magnet to Classical Magnet Behavior. J. Am. Chem. Soc. 2006, 128, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Y.; Wang, X.-Y.; Liu, T.; Xu, H.-B.; Zhao, F.; Wang, Z.-M.; Gao, S. Synthesis, structure, and magnetism of three azido-bridged Co2+ compounds with a flexible coligand 1,2-(tetrazole-1-yl)ethane. Inorg. Chem. 2008, 47, 8134–8142. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-C.; Wang, S.-L.; Lee, S.-F.; Lii, K.-H. Metamagnetism in cobalt phosphates with pillared layer structures: [Co3(pyz)(HPO4)2F2] and [Co3(4,4′-bpy)(HPO4)2F2]·xH2O. Inorg. Chem. 2004, 43, 2564–2571. [Google Scholar]
- Shao, D.; Zhou, Y.; Pi, Q.; Shen, F.-X.; Yang, S.-R.; Zhang, S.-L.; Wang, X.-Y. Two-dimensional frameworks formed by pentagonal bipyramidal cobalt(II) ions and hexacyanometallates: Antiferromagnetic ordering, metamagnetism and slow magnetic relaxation. Dalton Trans. 2017, 46, 9088–9096. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-Y.; Shi, W.; Gao, H.-L.; Cui, J.-Z.; Cheng, P. Spin canting and metamagnetism in 3D pillared-layer homospin cobalt(II) molecular magnetic materials constructed via a mixed ligands approach. Inorg. Chem. Front. 2014, 1, 242–248. [Google Scholar] [CrossRef]
- Yang, B.-P.; Prosvirin, A.V.; Guo, Y.-Q.; Mao, J.-G. Co[HO2C(CH2)3NH(CH2PO3H)2]2: A New Canted Antiferromagnet. Inorg. Chem. 2008, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Balanda, M.; Falk, K.; Griesar, K.; Tomkowicz, Z.; Haase, W. Characterization of magnetic ordering in porphyrin-based molecular magnets [Mn(R)4TPP][TCNE] (R = OC12H25, F, CN). J. Magn. Magn. Mater. 1999, 205, 14–26. [Google Scholar] [CrossRef]
- Lazari, G.; Stamatatos, T.C.; Raptopoulou, C.P.; Psycharis, V.; Pissas, M.; Perlepes, S.P.; Boudalis, A.K. A metamagnetic 2D copper(II)-azide complex with 1D ferromagnetism and a hysteretic spin-flop transition. Dalton Trans. 2009, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
| Compound | 1 | 2 |
|---|---|---|
| Formula | C8H6CoN10 | C4H8CuN8 |
| Fw | 301.16 | 226.69 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | C2/c |
| a/Å | 9.9130(7) | 16.0377(18) |
| b/Å | 8.1550(6) | 6.1184(7) |
| c/Å | 13.1987(10) | 14.2463(16) |
| α/° | 90 | 90 |
| β/° | 90.1186(14) | 92.031(2) |
| γ/° | 90 | 90 |
| V/Å3 | 1066.99(14) | 1397.0(3) |
| Z | 4 | 3 |
| T/K | 150(2) | 150(2) |
| Dc/g cm−3 | 1.875 | 2.156 |
| μ/mm−1 | 1.613 | 3.084 |
| (Δρ)max, min/e Å−3 | 0.559, −0.572 | 0.429, −0.413 |
| Measured/independent (Rint) reflections | 9357/2454(0.0326) | 4917/1668(0.0213) |
| Observed reflections (I > 2σ(I)) | 9357 | 4917 |
| Goodness-of-fits on F2 | 1.225 | 1.063 |
| R1 1, wR2 2 (all data) | 0.0445, 0.0831 | 0.0273, 0.0577 |
| R1 1, wR2 2 (I > 2σ(I)) | 0.0403, 0.0814 | 0.0230, 0.0554 |
| 1 a | |||
| Co-N(1) | 2.161(2) | Co-N(3) 3 | 2.163(2) |
| Co-N(5) | 2.147(2) | Co-N(8) | 2.113(2) |
| Co-N(5) 2 | 2.158(2) | Co-N(10) 1 | 2.119(2) |
| N(8)-Co-N(10) 1 | 97.50(9) | N(5)-Co-N(1) | 90.58(8) |
| N(8)-Co-N(5) | 92.98(8) | N(5) 2-Co-N(1) | 92.07(8) |
| N(10) 1-Co-N(5) | 169.21(8) | N(8)-Co-N(3) 3 | 87.73(9) |
| N(8)-Co-N(5) 2 | 171.71(8) | N(10) 1-Co-N(3) 3 | 91.62(8) |
| N(10)1-Co-N(5) 2 | 90.79(8) | N(5)-Co-N(3) 3 | 91.37(8) |
| N(5)-Co-N(5) 2 | 78.73(8) | N(5) 2-Co-N(3) 3 | 92.50(8) |
| N(8)-Co-N(1) | 87.91(9) | N(1)-Co-N(3) 3 | 175.31(8) |
| N(10) 1-Co-N(1) | 87.24(8) | N(6)-N(5)-Co 2 | 123.57(17) |
| Co-N(5)-Co 2 | 101.27(8) | ||
| 2 b | |||
| Cu(1)-N(4) | 1.9661(18) | Cu(1)-N(1) 4 | 2.0312(16) |
| Cu(1)-N(1) | 1.9969(16) | Cu(1)-N(7) | 2.0420(16) |
| N(1)-Cu(1) 4 | 2.0312(16) | ||
| N(4)-Cu(1)-N(1) | 98.93(7) | N(4)-Cu(1)-N(7) | 90.05(7) |
| N(4)-Cu(1)-N(1) 4 | 170.55(8) | N(1)-Cu(1)-N(7) | 169.75(7) |
| N(1)-Cu(1)-N(1) 4 | 79.68(7) | N(1) 4-Cu(1)-N(7) | 92.26(6) |
| N(2)-N(1)-Cu(1) | 123.71(14) | N(5)-N(4)-Cu(1) | 124.42(15) |
| N(2)-N(1)-Cu(1) 4 | 120.02(13) | C(3)-N(7)-Cu(1) | 117.55(12) |
| Cu(1)-N(1)-Cu(1) 4 | 100.32(7) | C(4)-N(7)-Cu(1) | 125.44(13) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-Z.; Chang, H.-T.; Kuo, Y.L.; Lee, G.-H.; Yang, C.-I. Two New Three-Dimensional Pillared-Layer Co(II) and Cu(II) Frameworks Involving a [M2(EO-N3)2] Motif from a Semi-Flexible N-Donor Ligand, 5,5′-Bipyrimidin: Syntheses, Structures and Magnetic Properties. Polymers 2018, 10, 229. https://doi.org/10.3390/polym10030229
Zhang Z-Z, Chang H-T, Kuo YL, Lee G-H, Yang C-I. Two New Three-Dimensional Pillared-Layer Co(II) and Cu(II) Frameworks Involving a [M2(EO-N3)2] Motif from a Semi-Flexible N-Donor Ligand, 5,5′-Bipyrimidin: Syntheses, Structures and Magnetic Properties. Polymers. 2018; 10(3):229. https://doi.org/10.3390/polym10030229
Chicago/Turabian StyleZhang, Zu-Zhen, Han-Ting Chang, Yi Lin Kuo, Gene-Hsiang Lee, and Chen-I Yang. 2018. "Two New Three-Dimensional Pillared-Layer Co(II) and Cu(II) Frameworks Involving a [M2(EO-N3)2] Motif from a Semi-Flexible N-Donor Ligand, 5,5′-Bipyrimidin: Syntheses, Structures and Magnetic Properties" Polymers 10, no. 3: 229. https://doi.org/10.3390/polym10030229