Electrospun Poly(ε-caprolactone) Nanofibrous Mesh for Imiquimod Delivery in Melanoma Therapy
Abstract
:1. Introduction
2. Materials and Methods
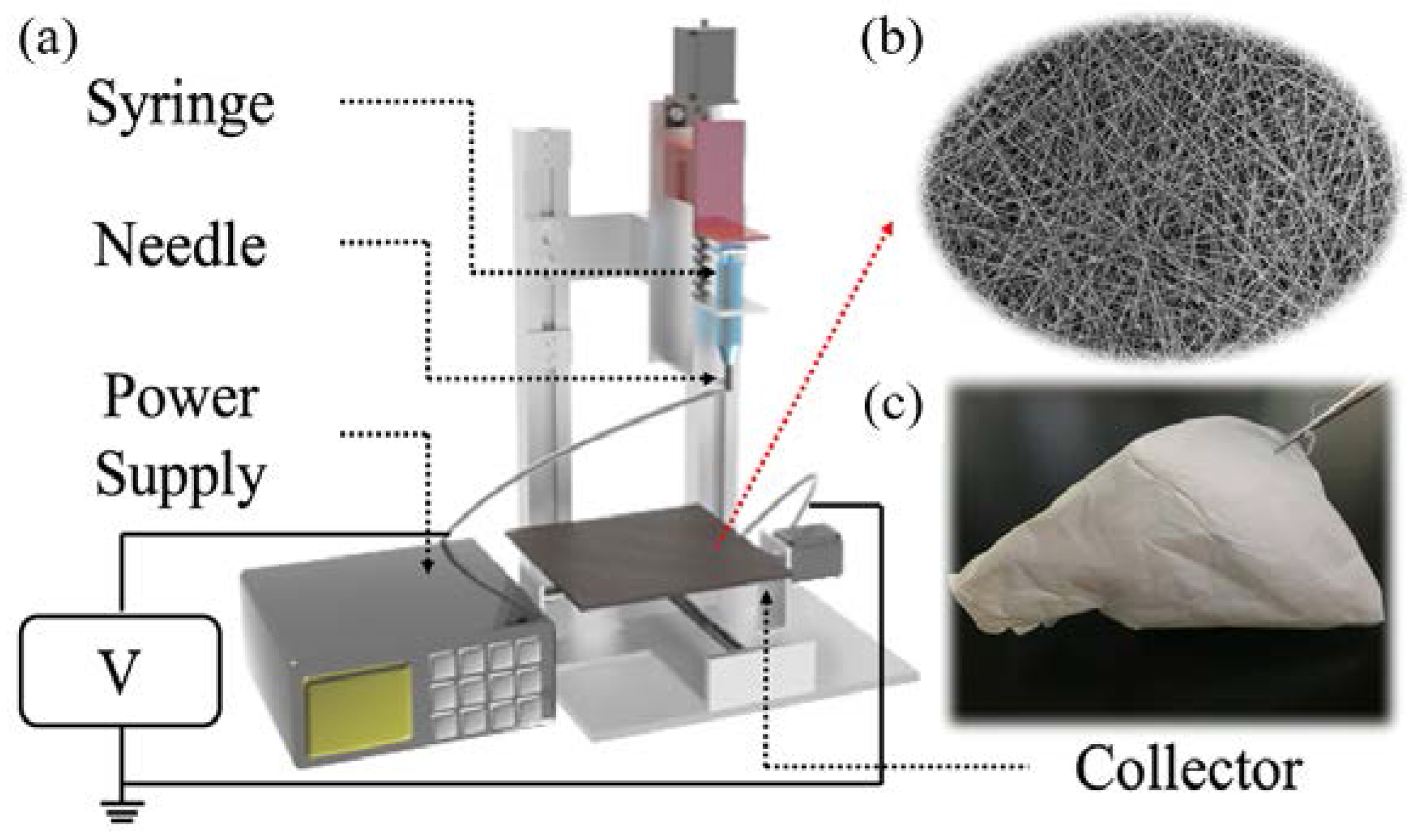
2.1. Prepration of the PCL-Imiquimod Fibrous Meshes
2.1.1. Preparation of the PCL-Imiquimod Solution
2.1.2. Preparation of the PCL Nanofibrous Meshes
2.1.3. Preliminary Study into Biocompatibility of the PCL Nanofibrous Meshes
2.2. Characteristics of the Imiquimod-Nanofibrous Meshes
2.3. Evalulation of the In Vitro Imiquimod Release Profiles
2.4. Cell Cultures and In Vitro Cytotoxicity Assays
2.5. Simulation of the Imiquimod Release Profile
3. Results
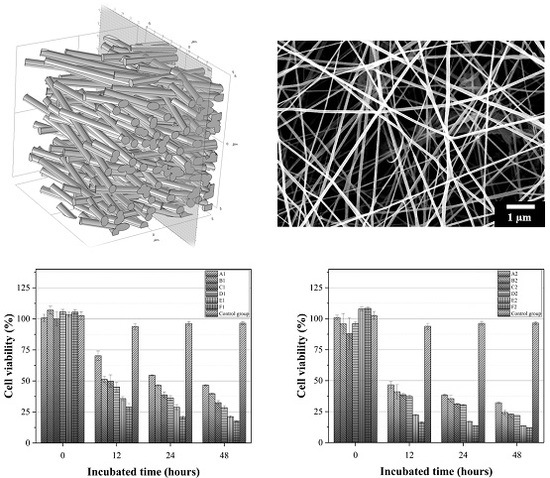
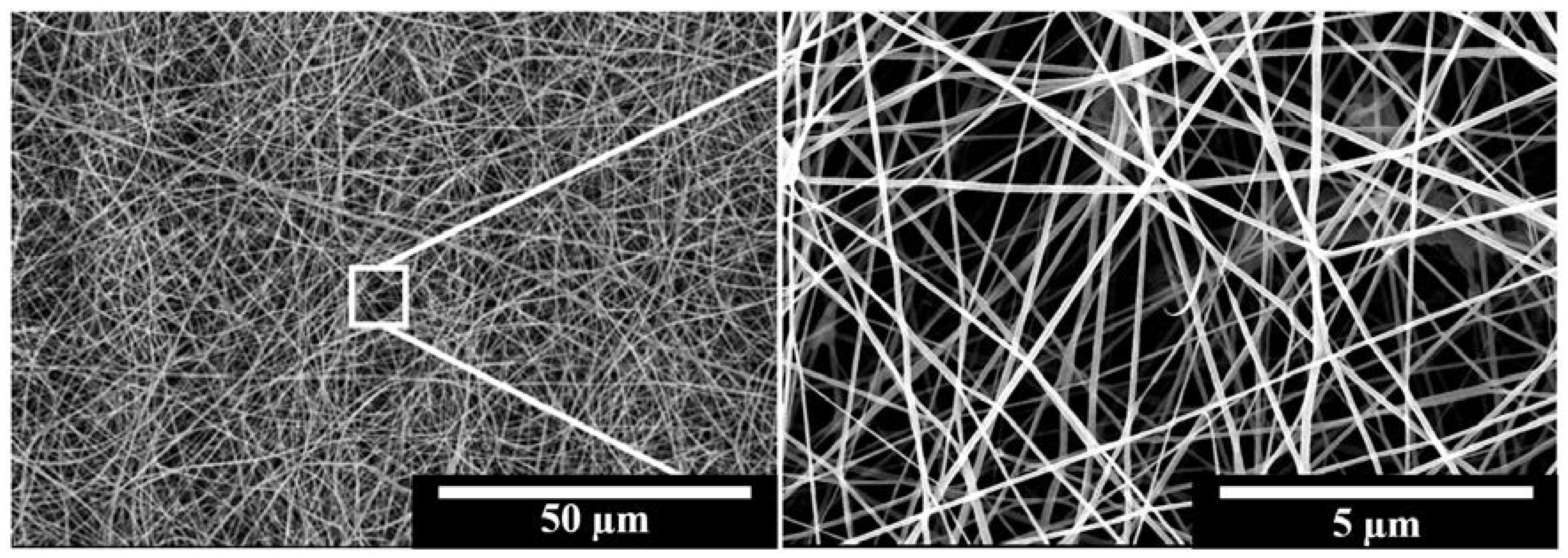
3.1. Characterisation of the Fabricated IMQ-PCL Nanofibrous Mesh
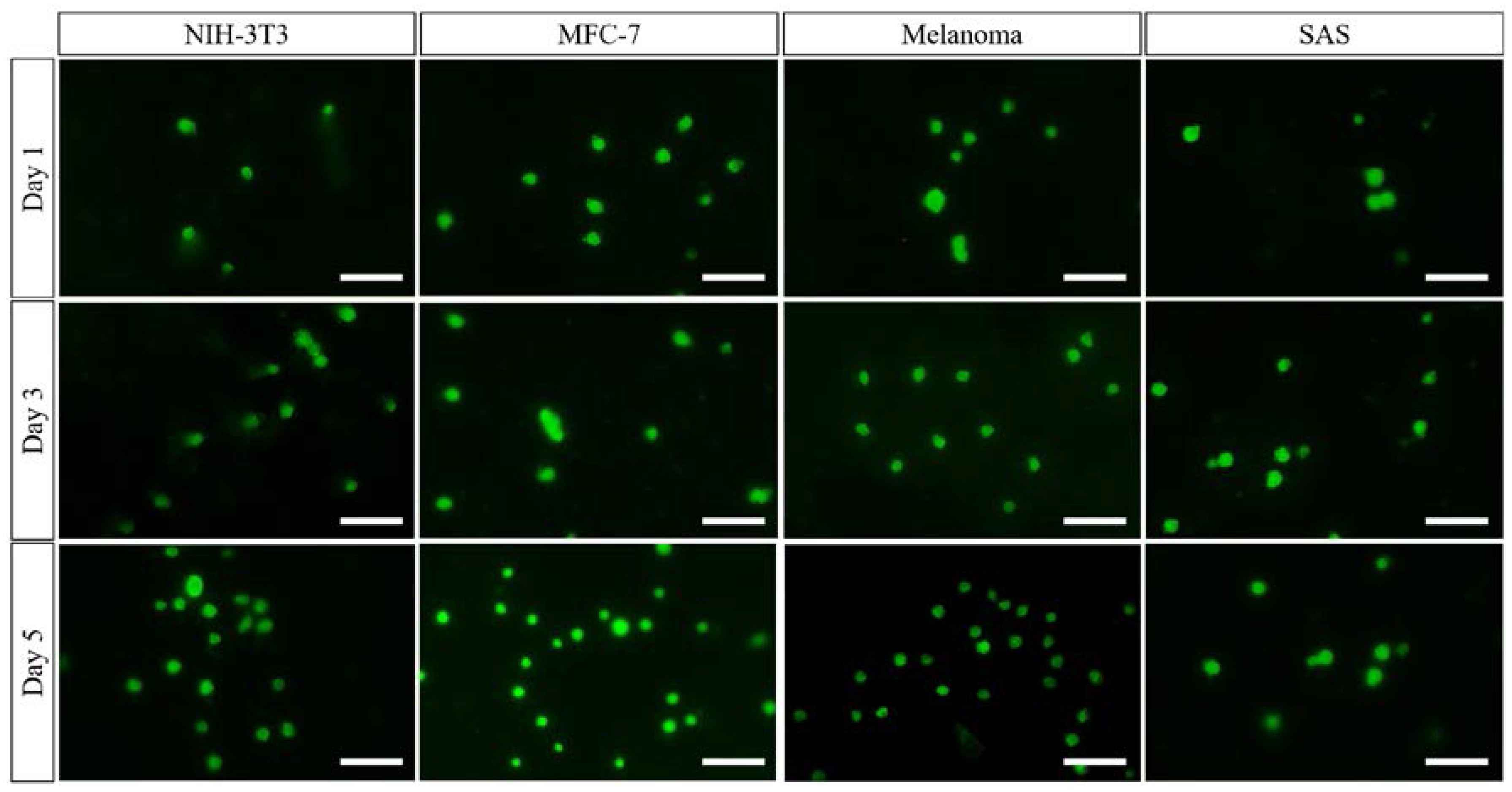
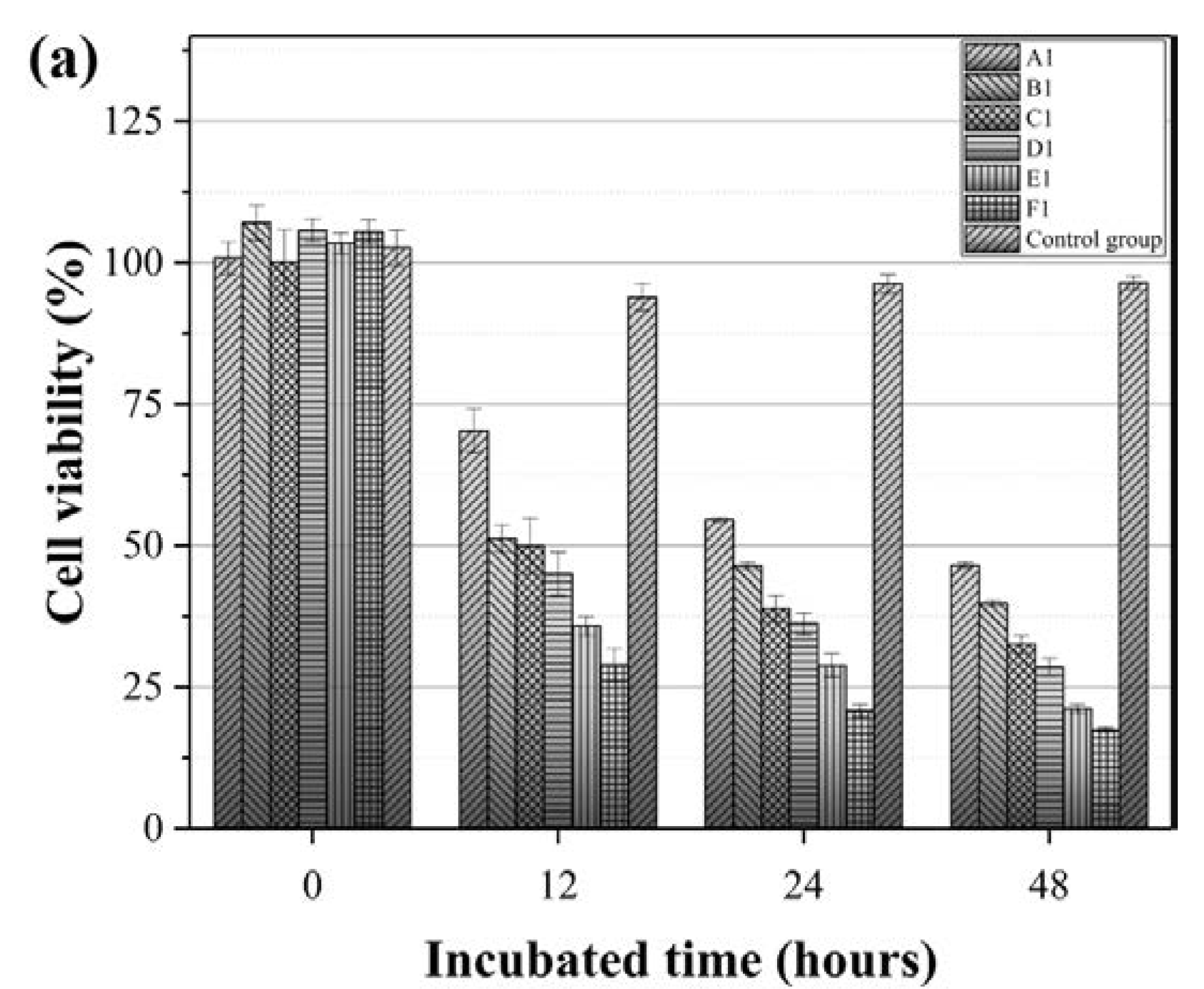
3.2. Preliminary Study into Biocompatibility of the PCL Nanofibrous Meshes
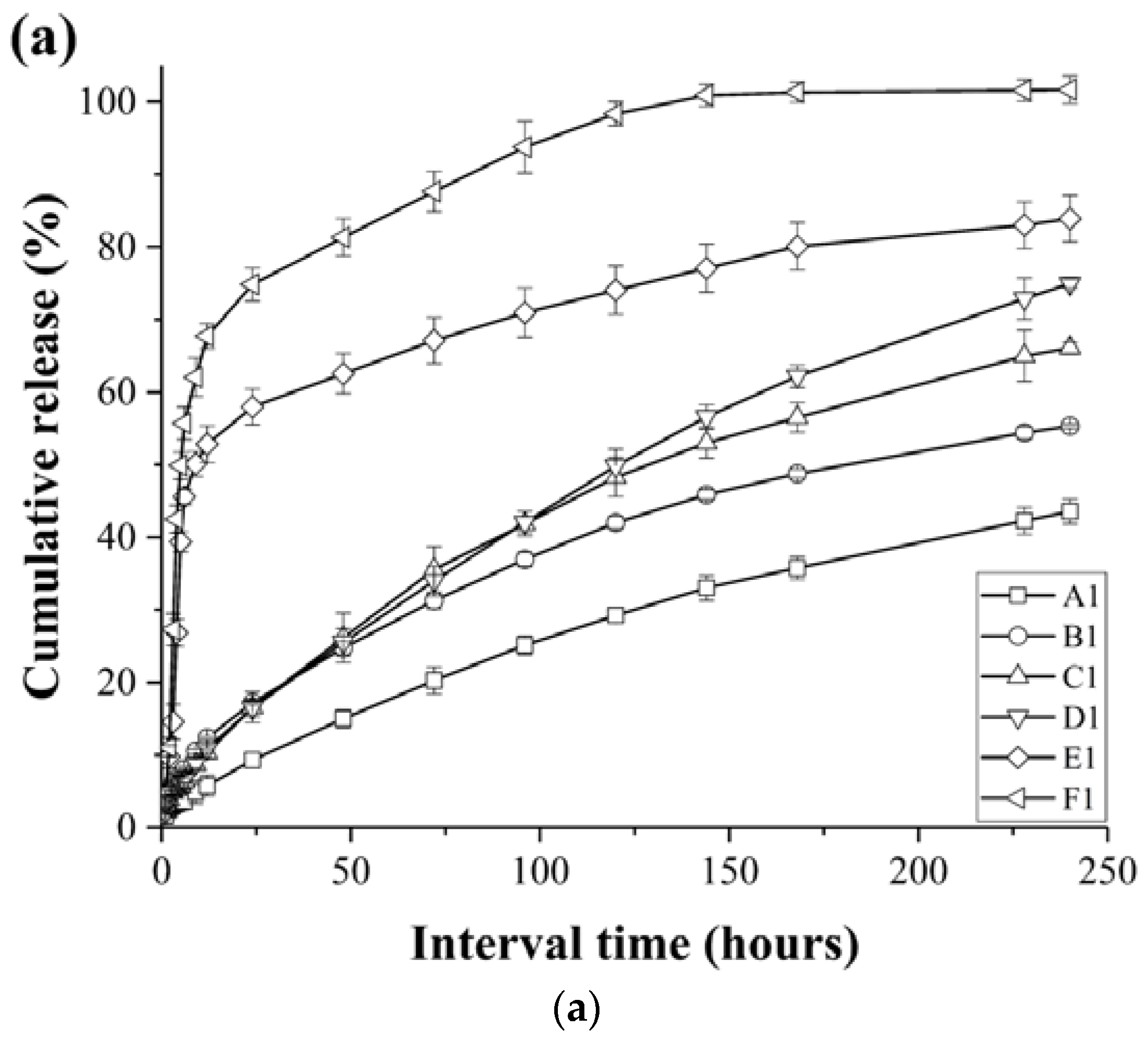
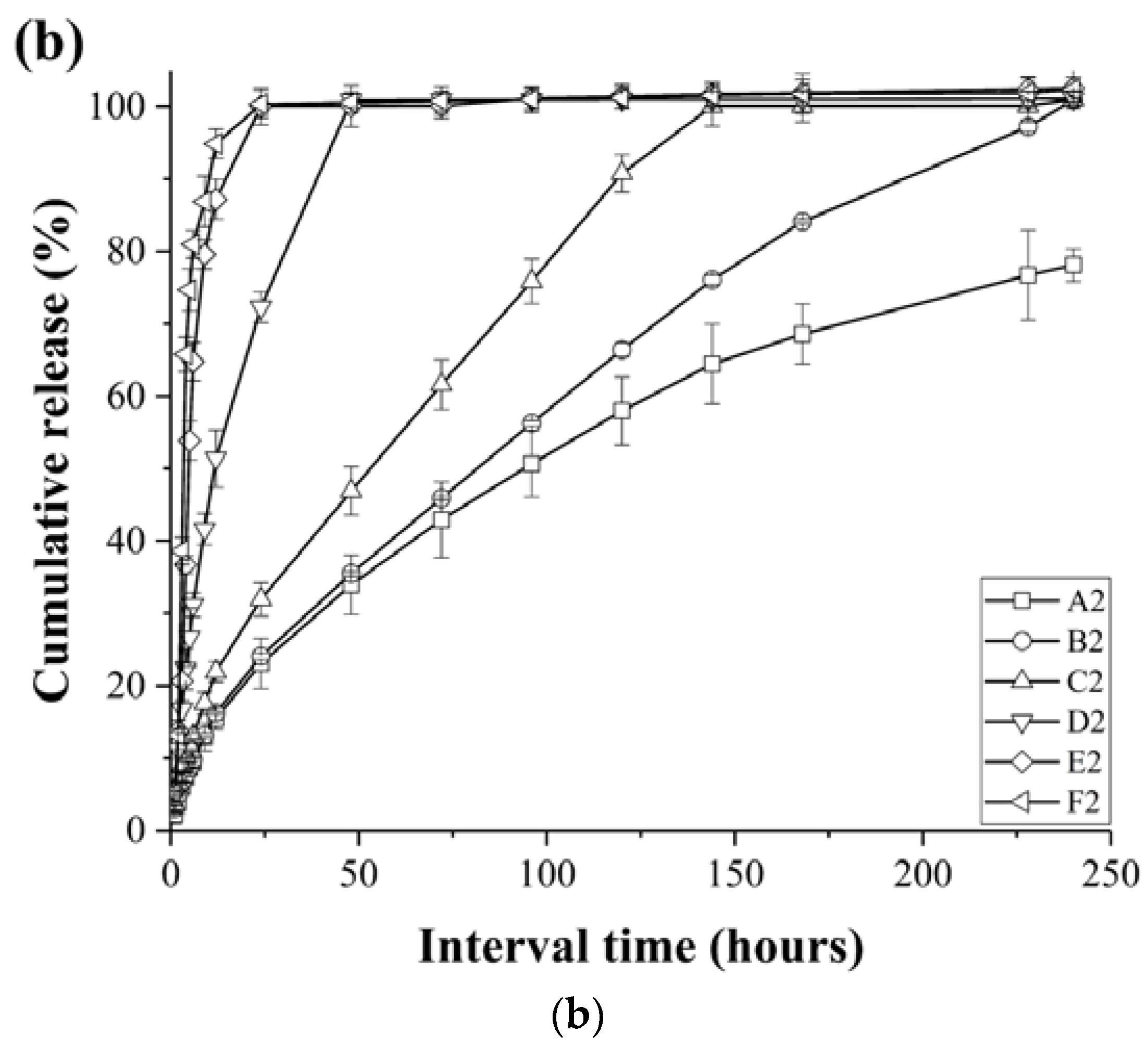
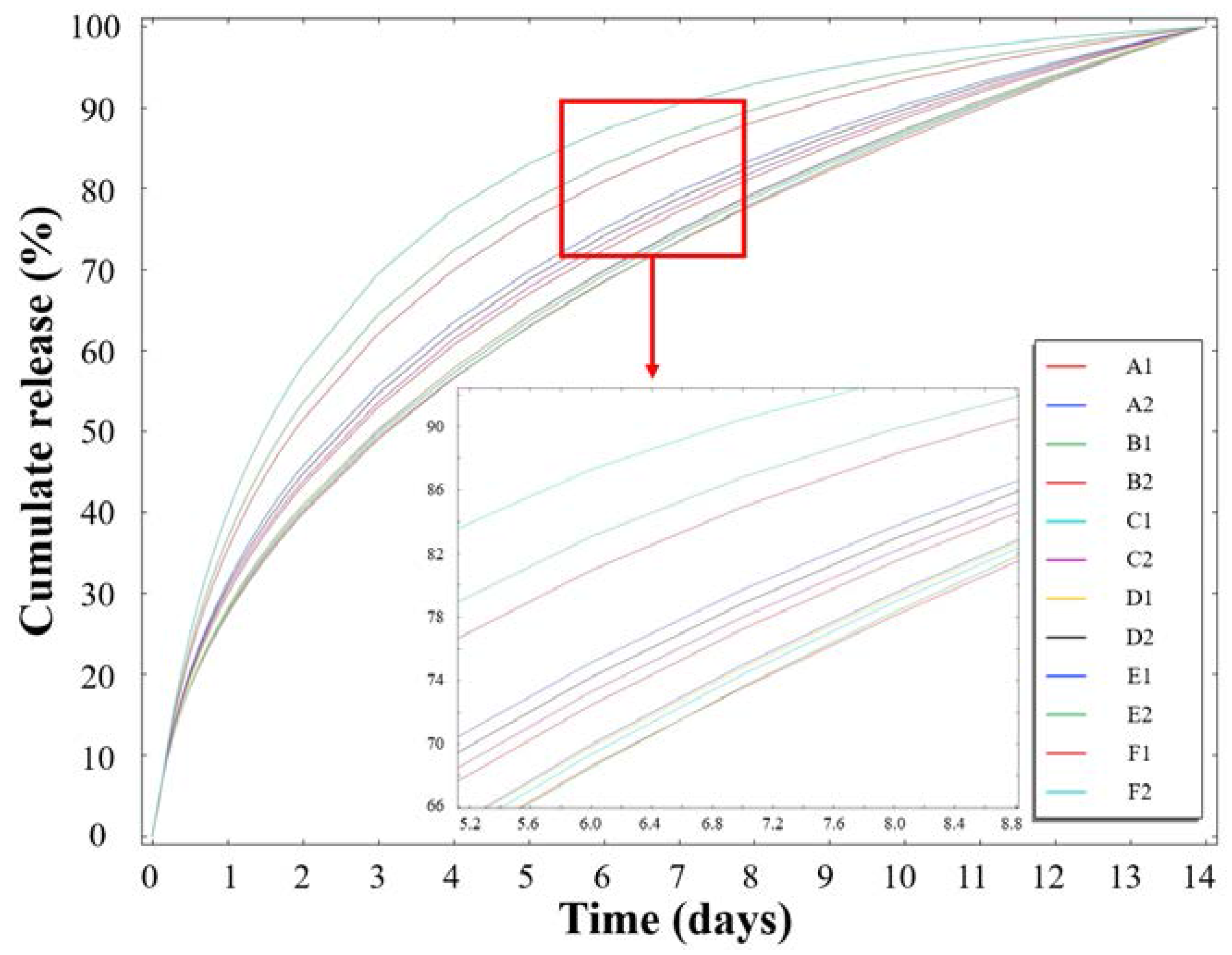
3.3. Release Profiles of the Loaded Imiquimod
3.4. Effect of the IMQ-PCL Nanofibrous Mesh on the Proliferation of Melanoma Cells
3.5. Simulation of the Imiquimod Release Profile
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sill, T.J.; von Recum, H.A. Electro spinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Grafahrend, D.; Klinkhammer, K.; Klee, D.; Möller, M. Electrospinning of polymer melts: Phenomenological observations. Polymer 2007, 48, 6823–6833. [Google Scholar] [CrossRef]
- Xie, J.; Wang, C.H. Electrospun micro- and nanofibers for sustained delivery of paclitaxel to treat C6 glioma in vitro. Pharm. Res. 2006, 23, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; MacKay, J.A.; McDaniel, J.R.; Chilkoti, A.; Clark, R.L. Fabrication of elastin-like polypeptide nanoparticles for drug delivery by electrospraying. Biomacromolecules 2009, 10, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Darrell, H.R.; Iksoo, C. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar]
- Xie, J.; MacEwan, M.R.; Liu, W.; Jesuraj, N.; Li, X.; Hunter, D.; Xia, Y. Nerve guidance conduits based on double-layered scaffolds of electrospun nanofibers for repairing the peripheral nervous system. ACS Appl. Mater. Interfaces 2014, 6, 9472–9480. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, E.; Battin, T.; Payne, R. Epidermal growth factor receptor in human epidermal cells: Direct demonstration in cultured cells. J. Investig. Dermatol. 1982, 78, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, X.; Zhu, X.; Yu, G.; Zhou, S.; Weng, J. Investigation of drug release and matrix degradation of electrospun poly(dl-lactide) fibers with paracetanol inoculation. Biomacromolecules 2006, 7, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Oltra, N.S.; Swift, J.; Mahmud, A.; Rajagopal, K.; Loverde, S.M.; Discher, D.E. Filomicelles in nanomedicine—From flexible, fragmentable, and ligand-targetable drug carrier designs to combination therapy for brain tumors. J. Mater. Chem. B 2013, 1, 5177–5185. [Google Scholar] [CrossRef]
- Bains, A.; Wulff, J.E.; Moffitt, M.G. Microfluidic synthesis of dye-loaded polycaprolactone-block-poly(ethylene oxide) nanoparticles: Insights into flow-directed loading and in vitro release for drug delivery. J. Colloid Interface Sci. 2016, 475, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Chawla, J.S.; Amiji, M.M. Biodegradable poly(ε-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int. J. Pharm. 2002, 249, 127–138. [Google Scholar] [CrossRef]
- An, Y.; Shaw, M.T. Actuating properties of soft gels with ordered iron particles: Basis for a shear actuator. Smart Mater. Struct. 2003, 12, 157. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, Y.; Li, Y.; Zhao, P.; Zhu, K.; Chen, W. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. J. Control. Release 2005, 108, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Hu, M.S.; Maan, Z.N.; Wu, J.C.; Rennert, R.C.; Hong, W.X.; Lai, T.S.; Cheung, A.T.; Walmsley, G.G.; Chung, M.T.; McArdle, A.; et al. Tissue engineering and regenerative repair in wound healing. Ann. Biomed. Eng. 2014, 42, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Wang, X.Y.; Kaplan, D.L.; Garlick, J.A.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Yoon, G.H.; Lee, H.C.; Shin, H.S. Chitosan nanoparticle/pcl nanofiber composite for wound dressing and drug delivery. J. Biomater. Sci. Polym. Ed. 2015, 26, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Ding, G.; Shi, X.; Guo, W.; Ni, Z.; Fu, H.; Fu, Z. A bioengineered drug-eluting scaffold accelerated cutaneous wound healing in diabetic mice. Colloids Surf. B Biointerfaces 2016, 145, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Hedrick, J.L.; Ong, Z.Y.; Yang, C.; Ee, P.L.; Hammond, P.T.; Yang, Y.Y. The effects of polymeric nanostructure shape on drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG–PLA and PEG–PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Tigli, R.S.; Kazaroglu, N.M.; Mavis, B.; Gumusderelioglu, M. Cellular behavior on epidermal growth factor (EGF)-immobilized PCL/gelatin nanofibrous scaffolds. J. Biomater. Sci. Polym. Ed. 2011, 22, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Pal, N.; Mishra, P.K.; Srivastava, P.; Mohanty, S.; Maiti, P. 5-florouracil-loaded poly(lactic acid)-poly(caprolactone) hybrid scaffold: Potential chemotherapeutic implant. J. Biomed. Mater. Res. Part A 2014, 102, 2600–2612. [Google Scholar] [CrossRef] [PubMed]
- Can, E.; Udenir, G.; Kanneci, A.I.; Kose, G.; Bucak, S. Investigation of PLLA/PCL blends and paclitaxel release profiles. AAPS PharmSciTech 2011, 12, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Bahner, J.D.; Bordeaux, J.S. Non-melanoma skin cancers: Photodynamic therapy, cryotherapy, 5-fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin. Dermatol. 2013, 31, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.A.; Breen, D.; Culleton, S.; Zhang, L.; Kamra, J.; Tsao, M.; Balogh, J. Palliative radiotherapy for non-melanoma skin cancer. Clin. Oncol. 2010, 22, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Hamdoon, Z.; Jerjes, W.; Upile, T.; Hopper, C. Optical coherence tomography-guided photodynamic therapy for skin cancer: Case study. Photodiagn. Photodyn. Ther. 2011, 8, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Sauder, D.N. Imiquimod: Modes of action. Br. J. Dermatol. 2003, 149, 5–8. [Google Scholar] [CrossRef]
- Schon, M.P.; Schon, M. Imiquimod: Mode of action. Br. J. Dermatol. 2007, 157 (Suppl. 2), 8–13. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.A. Imiquimod and the imidazoquinolones: Mechanism of action and therapeutic potential. Clin. Exp. Dermatol. 2002, 27, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Beutner, K.R.; Geisse, J.K.; Helman, D.; Fox, T.L.; Ginkel, A.; Owens, M.L. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J. Am. Acad. Dermatol. 1999, 41, 1002–1007. [Google Scholar] [CrossRef]
- Geisse, J.; Caro, I.; Lindholm, J.; Golitz, L.; Stampone, P.; Owens, M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: Results from two phase iii, randomized, vehicle-controlled studies. J. Am. Acad. Dermatol. 2004, 50, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Turan, A.; Saricaoglu, H.; Baskan, E.B.; Toker, S.C.; Tunali, S. Treatment of infiltrating basal cell carcinoma with the combination of intralesional IFNα-2b and topical imiquimod 5% cream. Int. J. Dermatol. 2009, 48, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.; Niiyama, E.; Kotsuchibashi, Y.; Uto, K.; Ebara, M. Biodegradable nanofiber for delivery of immunomodulating agent in the treatment of basal cell carcinoma. Fibers 2015, 3, 478–490. [Google Scholar] [CrossRef]
- Kołbuk, D.; Sajkiewicz, P.; Maniura-Weber, K.; Fortunato, G. Structure and morphology of electrospun polycaprolactone/gelatine nanofibres. Eur. Polym. J. 2013, 49, 2052–2061. [Google Scholar] [CrossRef]
- Armour, A.D.; Powell, H.M.; Boyce, S.T. Fluorescein diacetate for determination of cell viability in tissue-engineered skin. Tissue Eng. Part C Methods 2008, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Altman, S.A.; Randers, L.; Rao, G. Comparison of trypan blue dye exclusion and fluorometric assays for mammalian cell viability determinations. Biotechnol. Prog. 1993, 9, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.M.; Gillings, M.R.; Altavilla, N.; Beattie, A.J. Potential problems with fluorescein diacetate assays of cell viability when testing natural products for antimicrobial activity. J. Microbiol. Methods 2001, 46, 261–267. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Zhou, J.; Qi, L.; Gokhale, A.M. Generation of three-dimensional microstructure model for discontinuously reinforced composite by modified random sequential absorption method. J. Eng. Mater. Technol. 2016, 138, 021001. [Google Scholar] [CrossRef]
- Nakielski, P.; Kowalczyk, T.; Kowalewski, T.A. Modeling drug release from materials based on electrospun nanofibers. In Proceedings of the 2013 COMSOL Conference, Rotterdam, The Netherlands, 23–25 October 2013; pp. 23–25. [Google Scholar]
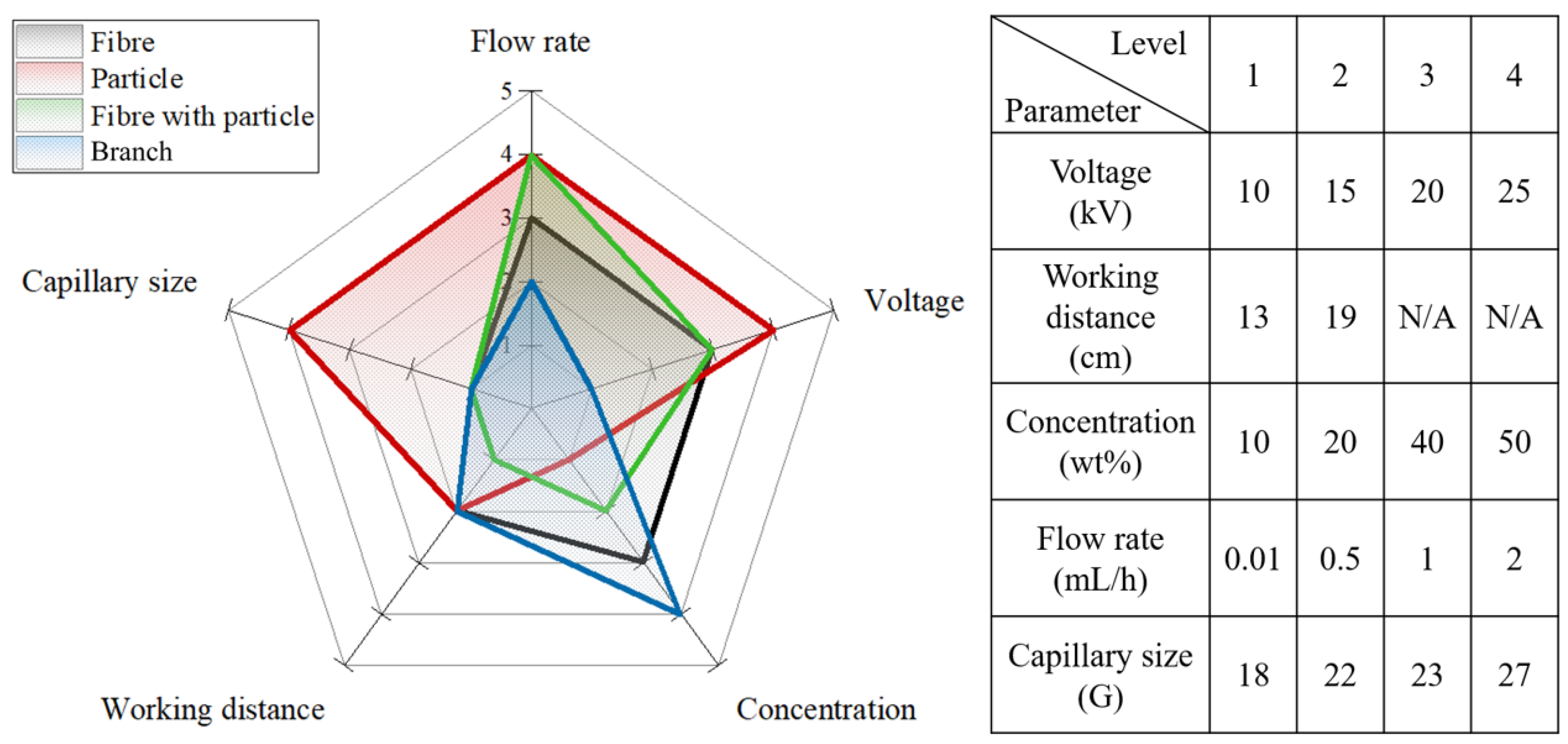

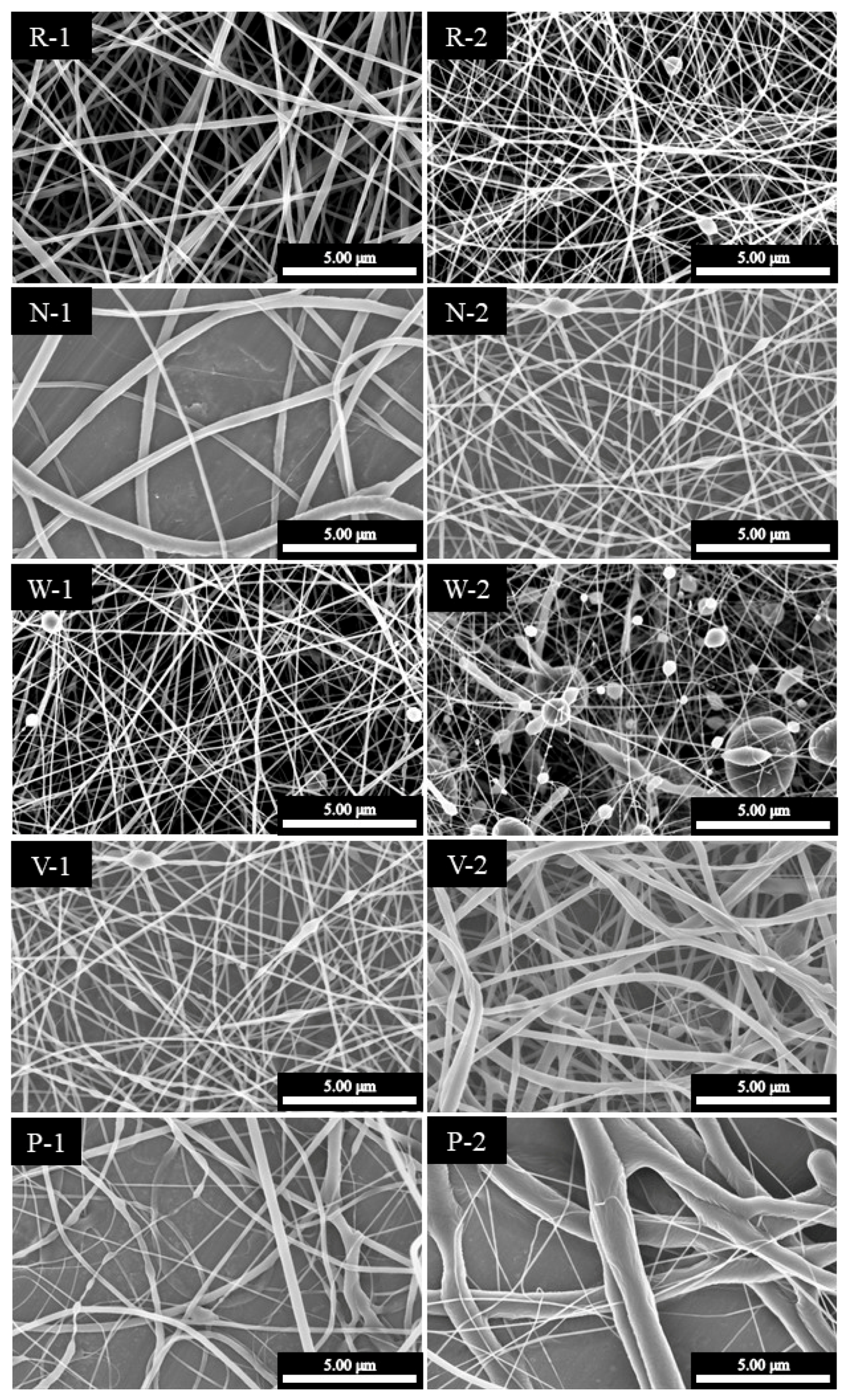

| Recipe | Flow Rate (mL/h) | Capillary Size (G) | Voltage (kV) | Polymeric Solution Concentration (wt %) | Working Distance (cm) | Average Diameter (nm) |
|---|---|---|---|---|---|---|
| R-1 | 0.01 | 23 | 10 | 30 | 13 | 104 ± 40 |
| R-2 | 0.25 | 23 | 10 | 30 | 13 | 172 ± 59 |
| N-1 | 0.01 | 18 | 10 | 30 | 13 | 321 ± 172 |
| N-2 | 0.01 | 23 | 10 | 30 | 13 | 119 ± 35 |
| W-1 | 0.5 | 23 | 10 | 30 | 13 | 101 ± 37 |
| W-2 | 0.5 | 23 | 10 | 30 | 19 | 90 ± 34 |
| V-1 | 0.01 | 23 | 10 | 30 | 13 | 126 ± 32 |
| V-2 | 0.01 | 23 | 20 | 30 | 13 | 249 ± 172 |
| P-1 | 0.05 | 23 | 10 | 20 | 13 | 194 ± 21 |
| P-2 | 0.05 | 23 | 10 | 30 | 13 | 1341 ± 504 |
| Samples | Flow Rate (mL/h) | Imiquimod Loading (w/w %) | Polymeric Solution Concentration (wt %) | Diameter (nm) |
|---|---|---|---|---|
| A1 | 0.225 | 5 | 30 | 268 ± 21 |
| A2 | 10 | 30 | 273 ± 21 | |
| B1 | 0.450 | 5 | 30 | 281 ± 16 |
| B2 | 10 | 30 | 284 ± 19 | |
| C1 | 0.675 | 5 | 30 | 296 ± 42 |
| C2 | 10 | 30 | 291 ± 27 | |
| D1 | 0.900 | 5 | 30 | 318 ± 61 |
| D2 | 10 | 30 | 335 ± 17 | |
| E1 | 1.35 | 5 | 30 | 714 ± 29 |
| E2 | 10 | 30 | 702 ± 33 | |
| F1 | 0.900 | 5 | 40 | 967 ± 48 |
| F2 | 10 | 40 | 959 ± 25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-C.; Yeh, I.-T.; Niyama, E.; Huang, W.-R.; Ebara, M.; Wu, C.-S. Electrospun Poly(ε-caprolactone) Nanofibrous Mesh for Imiquimod Delivery in Melanoma Therapy. Polymers 2018, 10, 231. https://doi.org/10.3390/polym10030231
Lin W-C, Yeh I-T, Niyama E, Huang W-R, Ebara M, Wu C-S. Electrospun Poly(ε-caprolactone) Nanofibrous Mesh for Imiquimod Delivery in Melanoma Therapy. Polymers. 2018; 10(3):231. https://doi.org/10.3390/polym10030231
Chicago/Turabian StyleLin, Wei-Chih, I-Ting Yeh, Eri Niyama, Wan-Rou Huang, Mitsuhiro Ebara, and Chieh-Shan Wu. 2018. "Electrospun Poly(ε-caprolactone) Nanofibrous Mesh for Imiquimod Delivery in Melanoma Therapy" Polymers 10, no. 3: 231. https://doi.org/10.3390/polym10030231