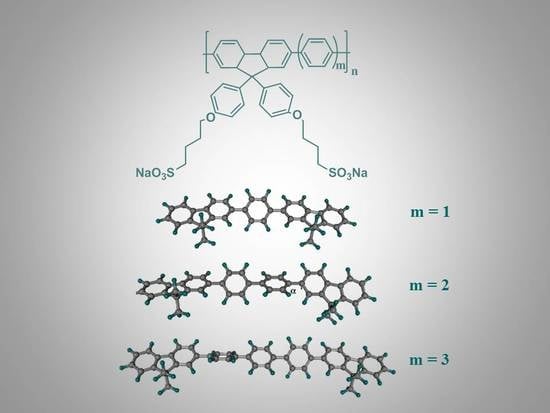
Effects of Charge Density on Photophysics and Aggregation Behavior of Anionic Fluorene-Arylene Conjugated Polyelectrolytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation and Methods
3. Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoven, C.V.; Garcia, A.; Bazan, G.C.; Nguyen, T.-Q. Recent Applications of Conjugated Polyelectrolytes in Optoelectronic Devices. Adv. Mater. 2008, 20, 3793–3810. [Google Scholar] [CrossRef]
- Jiang, H.; Taranekar, P.; Reynolds, J.R.; Schanze, K.S. Conjugated Polyelectrolytes: Synthesis, Photophysics, and Applications. Angew. Chem. Int. Ed. 2009, 48, 4300–4316. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bazan, G.C. (Eds.) Conjugated Polyelectrolytes: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-33143-7. [Google Scholar]
- Liu, B.; Bazan, G.C. Homogeneous Fluorescence-Based DNA Detection with Water-Soluble Conjugated Polymers. Chem. Mater. 2004, 16, 4467–4476. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Bergstedt, T.S.; Chen, L.; Jones, R.M.; Kumaraswamy, S.; Kushon, S.A.; Ley, K.D.; Lu, L.; McBranch, D.; Mukundan, H.; et al. Fluorescence superquenching of conjugated polyelectrolytes: Applications for biosensing and drug discovery. J. Mater. Chem. 2005, 15, 2648. [Google Scholar] [CrossRef]
- Liu, Y.; Ogawa, K.; Schanze, K.S. Conjugated polyelectrolytes as fluorescent sensors. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 173–190. [Google Scholar] [CrossRef]
- Martelo, L.; Jiménez, A.; Valente, A.J.M.; Burrows, H.D.; Marques, A.T.; Forster, M.; Scherf, U.; Peltzer, M.; Fonseca, S.M. Incorporation of polyfluorenes into poly(lactic acid) films for sensor and optoelectronics applications. Polym. Int. 2012, 61, 1023–1030. [Google Scholar] [CrossRef]
- Fonseca, S.M.; Galvão, R.P.; Burrows, H.D.; Gutacker, A.; Scherf, U.; Bazan, G.C. Selective Fluorescence Quenching in Cationic Fluorene-Thiophene Diblock Copolymers for Ratiometric Sensing of Anions. Macromol. Rapid Commun. 2013, 34, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.J.; Monteserín, M.; Burrows, H.D.; Almeida, J.A.S.; Pais, A.A.C.C.; Pina, J.; Seixas de Melo, J.S.; Jarmelo, S.; Estelrich, J. From molecular modelling to photophysics of neutral oligo- and polyfluorenes incorporated into phospholipid bilayers. Soft Matter 2015, 11, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.C. Harnessing self-assembly strategies for the rational design of conjugated polymer based materials. J. Mater. Chem. C 2013, 27, 4183–4190. [Google Scholar] [CrossRef]
- Yang, R.; Xu, Y.; Dang, X.-D.; Nguyen, T.-Q.; Cao, Y.; Bazan, G.C. Conjugated Oligoelectrolyte Electron Transport/Injection Layers for Organic Optoelectronic Devices. J. Am. Chem. Soc. 2008, 130, 3282–3283. [Google Scholar] [CrossRef] [PubMed]
- Edman, L.; Liu, B.; Vehse, M.; Swensen, J.; Bazan, G.C.; Heeger, A.J. Single-component light-emitting electrochemical cell fabricated from cationic polyfluorene: Effect of film morphology on device performance. J. Appl. Phys. 2005, 98, 44502. [Google Scholar] [CrossRef]
- Meazzini, I.; Blayo, C.; Arlt, J.; Marques, A.-T.; Scherf, U.; Burrows, H.D.; Evans, R.C. Ureasil organic-inorganic hybrids as photoactive waveguides for conjugated polyelectrolyte luminescent solar concentrators. Mater. Chem. Front. 2017, 1, 2271–2282. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Burrows, H.D.; Valente, A.J.M.; Costa, T.; Stewart, B.; Tapia, M.J.; Scherf, U. What conjugated polyelectrolytes tell us about aggregation in polyelectrolyte/surfactant systems. J. Mol. Liq. 2015, 210, 82–99. [Google Scholar] [CrossRef]
- Burrows, H.D.; Fonseca, S.M.; Dias, F.B.; de Melo, J.S.; Monkman, A.P.; Scherf, U.; Pradhan, S. Singlet Excitation Energy Harvesting and Triplet Emission in the Self-Assembled System Poly{1,4-phenylene-[9,9-bis (4-phenoxy-butylsulfonate)]fluorene-2,7-diyl} copolymer/tris(bipyridyl)ruthenium(II)in Aqueous Solution. Adv. Mater. 2009, 21, 1155–1159. [Google Scholar] [CrossRef]
- Scherf, U.; List, E.J.W. Semiconducting Polyfluorenes—Towards Reliable Structure-Property Relationships. Adv. Mater. 2002, 14, 477–487. [Google Scholar] [CrossRef]
- Monkman, A.; Rothe, C.; King, S.; Dias, F. Polyfluorene Photophysics. In Polyfluorenes; Springer: Berlin/Heidelberg, Germany, 2008; pp. 187–225. [Google Scholar]
- Chen, L.; McBranch, D.W.; Wang, H.-L.; Helgeson, R.; Wudl, F.; Whitten, D.G. Highly sensitive biological and chemical sensors based on reversible fluorescence quenching in a conjugated polymer. Proc. Natl. Acad. Sci. USA 1999, 96, 12287–12292. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Pinto, M.R.; Schanze, K.S. Photophysics, aggregation and amplified quenching of a water-soluble poly(phenylene ethynylene). Chem. Commun. 2002, 446–447. [Google Scholar] [CrossRef]
- Lavigne, J.J.; Broughton, D.L.; Wilson, J.N.; Erdogan, B.; Bunz, U.H.F. “Surfactochromic” Conjugated Polymers: Surfactant Effects on Sugar-Substituted PPEs. Macromolecules 2003, 36, 7409–7412. [Google Scholar] [CrossRef]
- Bockstaller, M.; Köhler, W.; Wegner, G.; Vlassopoulos, D.; Fytas, G. Hierarchical Structures of a Synthetic Rodlike Polyelectrolyte in Water. Macromolecules 2000, 33, 3951–3953. [Google Scholar] [CrossRef]
- Wang, S.; Bazan, G.C. Solvent-dependent aggregation of a water-soluble poly(fluorene) controls energy transfer to chromophore-labeled DNA. Chem. Commun. 2004, 2508–2509. [Google Scholar] [CrossRef] [PubMed]
- Burrows, H.D.; Lobo, V.M.M.; Pina, J.; Ramos, M.L.; De Seixas Melo, J.; Valente, A.J.M.; Tapia, M.J.; Pradhan, S.; Scherf, U. Fluorescence enhancement of the water-soluble poly{1,4-phenylene-[9,9-bis-(4-phenoxybutylsulfonate)]fluorene-2,7-diyl} copolymer in n-dodecylpentaoxyethylene glycol ether micelles. Macromolecules 2004, 37, 7425–7427. [Google Scholar] [CrossRef]
- Wilson, J.S.; Frampton, M.J.; Michels, J.J.; Sardone, L.; Marletta, G.; Friend, R.H.; Samorì, P.; Anderson, H.L.; Cacialli, F. Supramolecular Complexes of Conjugated Polyelectrolytes with Poly(ethylene oxide): Multifunctional Luminescent Semiconductors Exhibiting Electronic and Ionic Transport. Adv. Mater. 2005, 17, 2659–2663. [Google Scholar] [CrossRef]
- Knaapila, M.; Almásy, L.; Garamus, V.M.; Pearson, C.; Pradhan, S.; Petty, M.C.; Scherf, U.; Burrows, H.D.; Monkman, A.P. Solubilization of Polyelectrolytic Hairy-Rod Polyfluorene in Aqueous Solutions of Nonionic Surfactant. J. Phys. Chem. B 2006, 110, 10248–10257. [Google Scholar] [CrossRef] [PubMed]
- Monteserín, M.; Tapia, M.J.; Ribeiro, A.C.F.; Santos, C.I.A.V.; Valente, A.J.M.; Burrows, H.D.; Mallavia, R.; Nilsson, M.; Söderman, O. Multicomponent interdiffusion and self-diffusion of the cationic poly{[9,9-bis(6′-N,N,N-trimethylammonium)hexyl]fluorene-phenylene} dibromide in a dimethyl sulfoxide + water solution. J. Chem. Eng. Data 2010, 55, 1860–1866. [Google Scholar] [CrossRef]
- Wägberg, T.; Liu, B.; Orädd, G.; Eliasson, B.; Edman, L. Cationic polyfluorene: Conformation and aggregation in a “good” solvent. Eur. Polym. J. 2009, 45, 3230–3235. [Google Scholar] [CrossRef]
- Burrows, H.D.; Fonseca, S.M.; Silva, C.L.; Pais, A.A.C.C.; Tapia, M.J.; Pradhan, S.; Scherf, U. Aggregation of the hairy rod conjugated polyelectrolyte poly{1,4-phenylene-[9,9-bis(4-phenoxybutylsulfonate)]fluorene-2,7-diyl} in aqueous solution: An experimental and molecular modelling study. Phys. Chem. Chem. Phys. 2008, 10, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Burrows, H.D.; Tapia, M.J.; Fonseca, S.M.; Pradhan, S.; Scherf, U.; Silva, C.L.; Pais, A.A.C.C.; Valente, A.J.M.; Schilén, K.; Alfredsson, V.; et al. Solubilization of poly{1,4-phenylene-[9,9-bis(4-phenoxy-butylsulfonate)] fluorene-2,7-diyl} in water by nonionic amphiphiles. Langmuir 2009, 25, 5545–5556. [Google Scholar] [CrossRef] [PubMed]
- Burrows, H.D.; Knaapila, M.; Monkman, A.P.; Tapia, M.J.; Fonseca, S.M.; Ramos, M.L.; Pyckhout-Hintzen, W.; Pradhan, S.; Scherf, U. Structural studies on cationic poly{9,9-bis [6-(N,N,N-trimethylammonium)alkyl]fluorene-co-1,4-phenylene} iodides in aqueous solutions in the presence of the non-ionic surfactant pentaethyleneglycol monododecyl ether (C12E5 ). J. Phys. Condens. Matter 2008, 20, 104210. [Google Scholar] [CrossRef]
- Monteserín, M.; Burrows, H.D.; Valente, A.J.M.; Pais, A.A.C.C.; Di Paolo, R.E.; Maçanita, A.L.; Tapia, M.J. Fluorescence Enhancement of a Cationic Fluorene-Phenylene Conjugated Polyelectrolyte Induced by Nonionic n-Alkyl Polyoxyethylene Surfactants. Langmuir 2017, 33, 13350–13363. [Google Scholar] [CrossRef] [PubMed]
- Burrows, H.D.; Tapia, M.J.; Silva, C.L.; Pais, A.A.C.C.; Fonseca, S.M.; Pina, J.; Seixas de Melo, J.; Wang, Y.; Marques, E.F.; Knaapila, M.; et al. Interplay of Electrostatic and Hydrophobic Effects with Binding of Cationic Gemini Surfactants and a Conjugated Polyanion: Experimental and Molecular Modeling Studies. J. Phys. Chem. B 2007, 111, 4401–4410. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.M.; Eusébio, M.E.; Castro, R.; Burrows, H.D.; Tapia, M.J.; Olsson, U. Interactions between hairy rod anionic conjugated polyelectrolytes and nonionic alkyloxyethylene surfactants in aqueous solution: Observations from cloud point behaviour. J. Colloid Interface Sci. 2007, 315, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Burrows, H.D.; Tapia, M.J.; Fonseca, S.M.; Valente, A.J.M.; Lobo, V.M.M.; Justino, L.L.G.; Qiu, S.; Pradhan, S.; Scherf, U.; Chattopadhyay, N.; et al. Aqueous solution behavior of anionic fluorene-co-thiophene-based conjugated polyelectrolytes. ACS Appl. Mater. Interfaces 2009, 1, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.J.; Burrows, H.D.; Valente, A.J.M.; Pradhan, S.; Scherf, U.; Lobo, V.M.M.; Pina, J.; Seixas De Melo, J. Interaction between the water soluble poly{1,4-phenylene-[9,9-bis(4-phenoxy butylsulfonate)]fluorene-2,7-diyl} copolymer and ionic surfactants followed by spectroscopic and conductivity measurements. J. Phys. Chem. B 2005, 109, 19108–19115. [Google Scholar] [CrossRef] [PubMed]
- Mougan, M.A.; Coello, A.; Jover, A.; Meijide, F.; Vazquez Tato, J. Spectrofluorimeters as Light-Scattering Apparatus: Application to Polymers Molecular Weight Determination. J. Chem. Educ. 1995, 72, 284. [Google Scholar] [CrossRef]
- Barthel, J.; Feuerlein, F.; Neueder, R.; Wachter, R. Calibration of conductance cells at various temperatures. J. Solut. Chem. 1980, 9, 209–219. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Pina, J.; Seixas de Melo, J.; Burrows, H.D.; Maçanita, A.L.; Galbrecht, F.; Bünnagel, T.; Scherf, U. Alternating Binaphthyl−Thiophene Copolymers: Synthesis, Spectroscopy, and Photophysics and Their Relevance to the Question of Energy Migration versus Conformational Relaxation. Macromolecules 2009, 42, 1710–1719. [Google Scholar] [CrossRef]
- Striker, G.; Subramaniam, V.; Seidel, C.A.M.; Volkmer, A. Photochromicity and Fluorescence Lifetimes of Green Fluorescent Protein. J. Phys. Chem. B 1999, 103, 8612–8617. [Google Scholar] [CrossRef]
- Justino, L.L.G.; Ramos, M.L.; Abreu, P.E.; Carvalho, R.A.; Sobral, A.J.F.N.; Scherf, U.; Burrows, H.D. Conformational Studies of Poly(9,9-dialkylfluorene)s in Solution Using NMR Spectroscopy and Density Functional Theory Calculations. J. Phys. Chem. B 2009, 113, 11808–11821. [Google Scholar] [CrossRef] [PubMed]
- Belletête, M.; Beaupré, S.; Bouchard, J.; Blondin, P.; Leclerc, M.; Durocher, G. Theoretical and Experimental Investigations of the Spectroscopic and Photophysical Properties of Fluorene-Phenylene and Fluorene-Thiophene Derivatives: Precursors of Light-Emitting Polymers. J. Phys. Chem. B 2000, 104, 9118–9125. [Google Scholar] [CrossRef]
- Justino, L.L.G.; Luísa Ramos, M.L.; Abreu, P.E.; Charas, A.; Morgado, J.; Scherf, U.; Minaev, B.F.; Ågren, H.; Burrows, H.D. Structural and Electronic Properties of Poly(9,9-dialkylfluorene)-Based Alternating Copolymers in Solution: An NMR Spectroscopy and Density Functional Theory Study. J. Phys. Chem. C 2013, 117, 17969–17982. [Google Scholar] [CrossRef]
- Marques, A.T.; Burrows, H.D.; Seixas de Melo, J.S.; Valente, A.J.M.; Justino, L.L.G.; Scherf, U.; Fron, E.; Rocha, S.; Hofkens, J.; Snedden, E.W.; et al. Spectroscopic properties, excitation, and electron transfer in an anionic water-soluble poly(fluorene-alt-phenylene)-perylenediimide copolymer. J. Phys. Chem. B 2012, 116, 7548–7559. [Google Scholar] [CrossRef] [PubMed]
- Attar, H.A.A.; Monkman, A.P. Effect of Surfactant on FRET and Quenching in DNA Sequence Detection Using Conjugated Polymers. Adv. Funct. Mater. 2008, 18, 2498–2509. [Google Scholar] [CrossRef]
- Davies, M.L.; Douglas, P.; Burrows, H.D.; da Graça Miguel, M.; Douglas, A. Effect of Aggregation on the Photophysical Properties of Three Fluorene-Phenylene-Based Cationic Conjugated Polyelectrolytes. J. Phys. Chem. B 2011, 115, 6885–6892. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.B.; Maçanita, A.L.; Seixas de Melo, J.; Burrows, H.D.; Güntner, R.; Scherf, U.; Monkman, A.P. Picosecond conformational relaxation of singlet excited polyfluorene in solution. J. Chem. Phys. 2003, 118, 7119–7126. [Google Scholar] [CrossRef]
- Costa, T.; Marques, A.T.; Seixas de Melo, J.S.; Thomas, A.W.; Garner, L.E.; Scherf, U.; Bazan, G.C.; Burrows, H.D. Self-Assembly of Poly{1,4-phenylene-[9,9-bis(4-phenoxy-butylsulfonate)]fluorene-2,7-diyl} with Oppositely Charged Phenylenevinylene Oligoelectrolytes. J. Phys. Chem. B 2014, 118, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Pina, J.; Seixas de Melo, J.S.; Koenen, N.; Scherf, U. Chain Length Dependent Excited-State Decay Processes of Diluted PF2/6 Solutions. J. Phys. Chem. B 2013, 117, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Carless, J.; Challis, R.; Mulley, B. Nonionic surface-active agents. Part V. The effect of the alkyl and the polyglycol chain length on the critical micelle concentration of some monoalkyl polyethers. J. Colloid Sci. 1964, 19, 201–212. [Google Scholar] [CrossRef]
- Thalberg, K.; van Stam, J.; Lindblad, C.; Almgren, M.; Lindman, B. Time-Resolved Fluorescence and Self-Diffusion Studies in Systems of a Cationic Surfactant and an Anionic Polyelectrolyte. J. Phys. Chem. 1991, 95, 8975–8982. [Google Scholar] [CrossRef]
- Nirmesh, J.; Trabelsi, S.; Guillot, S.; McLoughlin, D.; Langevin, D.; Letellier, P.; Turmine, M. Critical Aggregation Concentration in Mixed Solutions of Anionic Polyelectrolytes and Cationic Surfactants. Langmuir 2004, 20, 8496–8503. [Google Scholar] [CrossRef]
- Dias, F.B.; Morgado, J.; Maçanita, A.L.; da Costa, F.P.; Burrows, H.D.; Monkman, A.P. Kinetics and Thermodynamics of Poly(9,9-dioctylfluorene) β-Phase Formation in Dilute Solution. Macromolecules 2006, 39, 5854–5864. [Google Scholar] [CrossRef]
- Bright, D.W.; Dias, F.B.; Galbrecht, F.; Scherf, U.; Monkman, A.P. The Influence of Alkyl-Chain Length on Beta-Phase Formation in Polyfluorenes. Adv. Funct. Mater. 2009, 19, 67–73. [Google Scholar] [CrossRef]
- Guggenheim, E.A. On the determination of the velocity constant of a unimolecular reaction. Philos. Mag. 1926, 2, 538–543. [Google Scholar] [CrossRef]
- Frost, A.A.; Pearson, R.G. Kinetics and Mechanism; John Wiley: New York, NY, USA, 1961. [Google Scholar]
- Wilkinson, F. Chemical Kinetics and Reaction Mechanism; Van Nostrand Reinhold: Wokingham, UK, 1980. [Google Scholar]
- Ubbelohde, A.R. Melting and Crystal Structure; Oxford University Press: Oxford, UK, 1965. [Google Scholar]
- Guzmán, E.; Llamas, L.; Maestro, A.; Fernández-Peña, L.; Akanno, A.; Miller, R.; Ortega, F.; Rubio, R.G. Polymer-Surfactant Systems in Bulk and at Fluid Interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. [Google Scholar] [CrossRef] [PubMed]
- Kamtekar, K.Y.; Monkman, A.P.; Bryce, M.R. White Organic Light-Emitting Materials and Devices. Adv. Mater. 2010, 22, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: London, UK, 1985. [Google Scholar]
- Evans, D.F.; Wennerström, H. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet; VCH Publishers: New York, NY, USA, 1994. [Google Scholar]

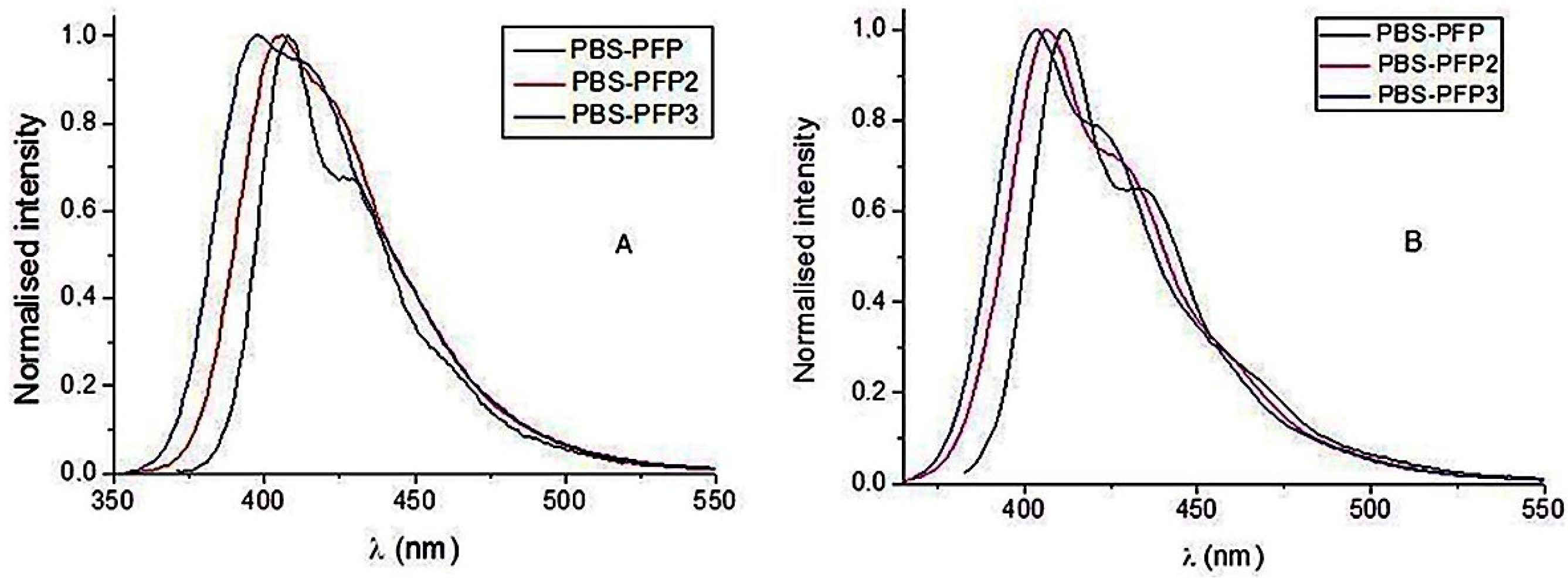
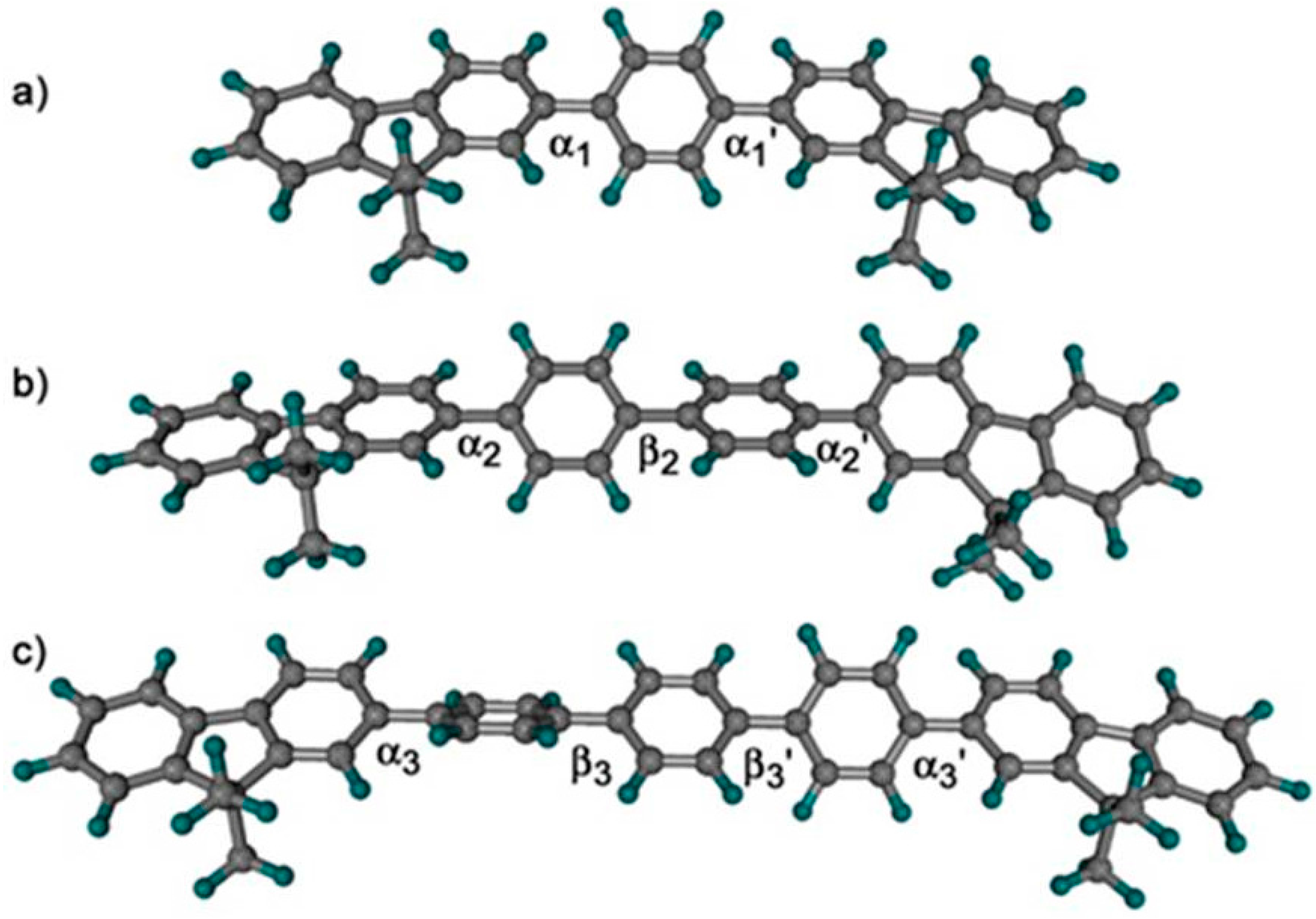
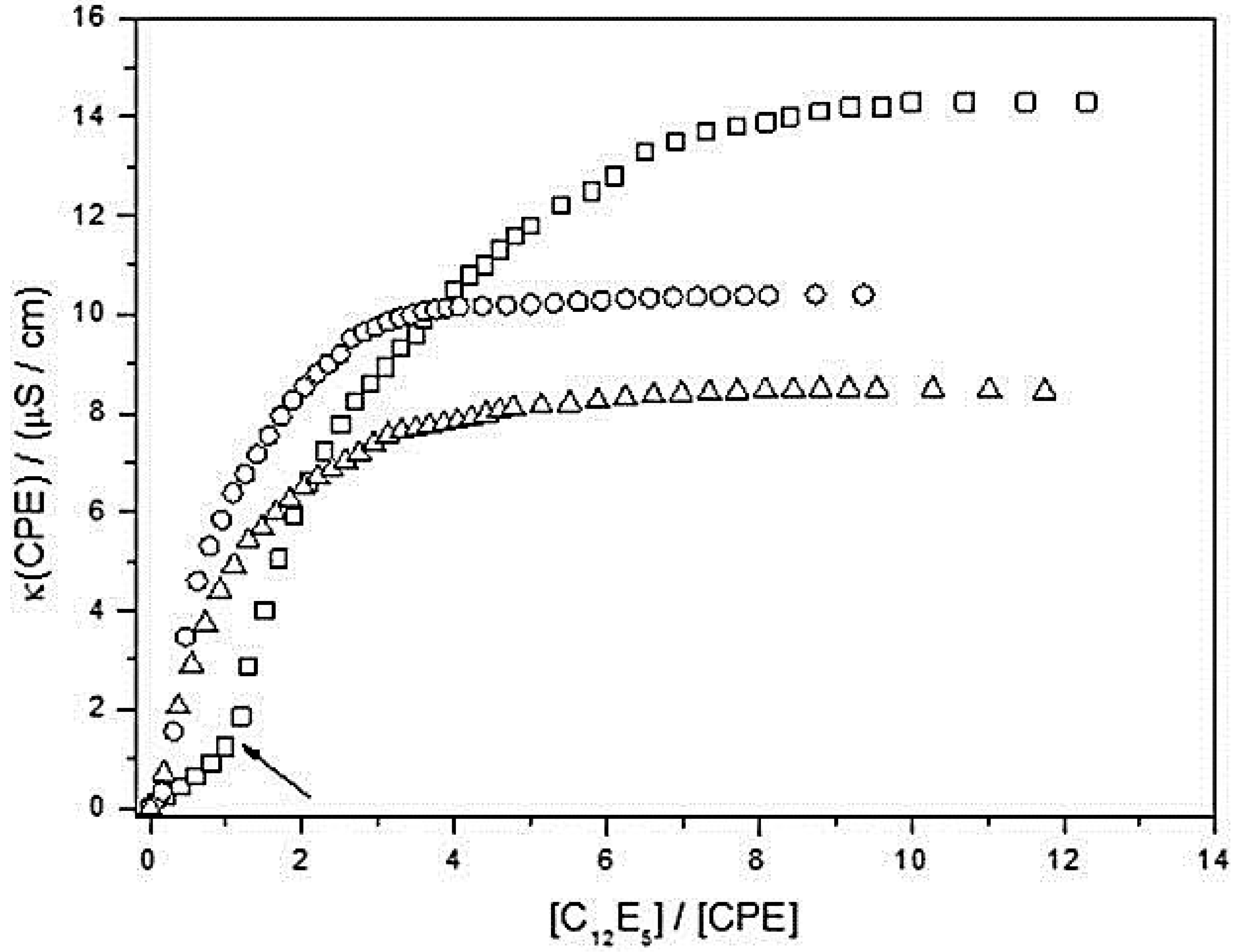
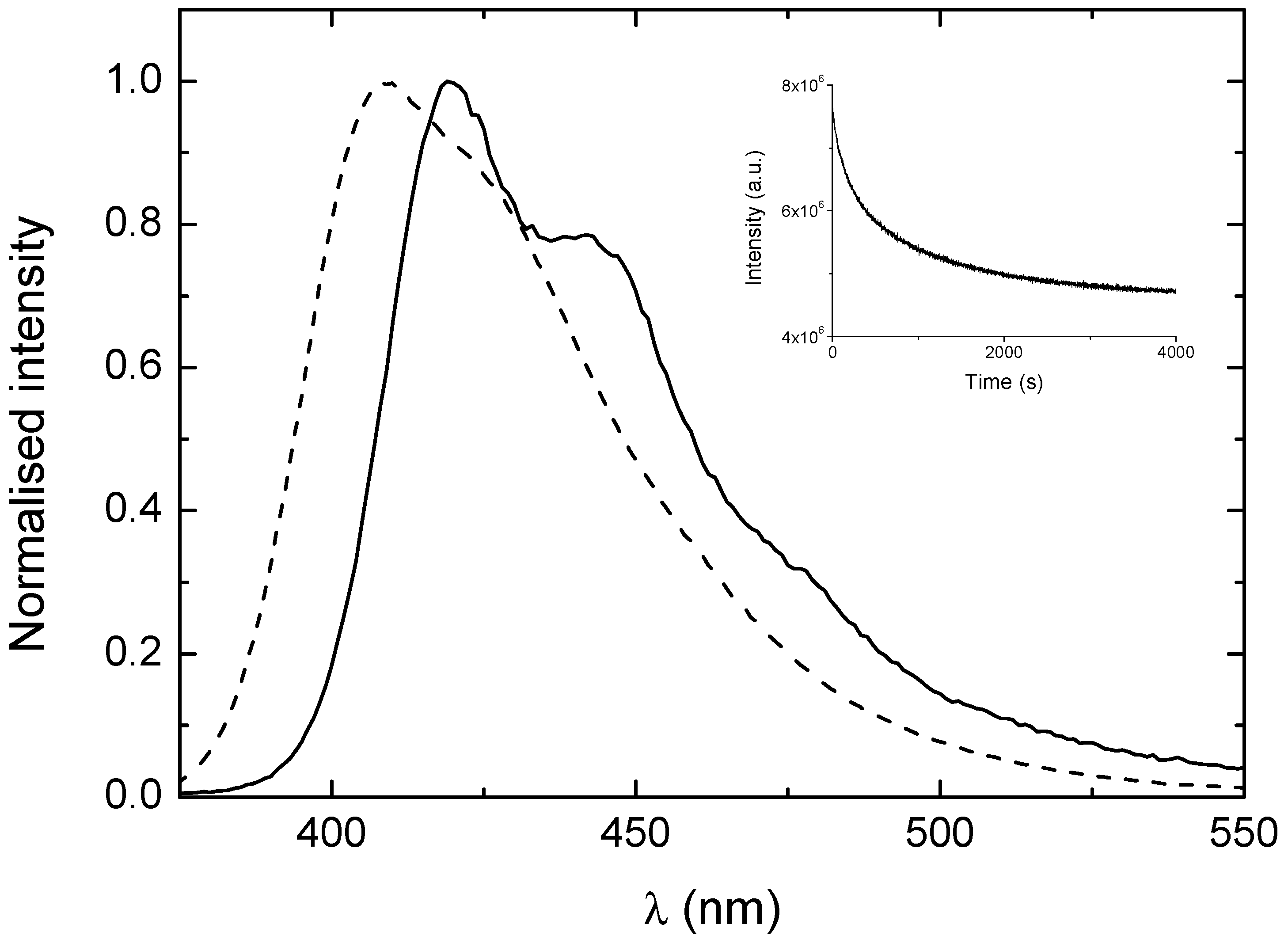
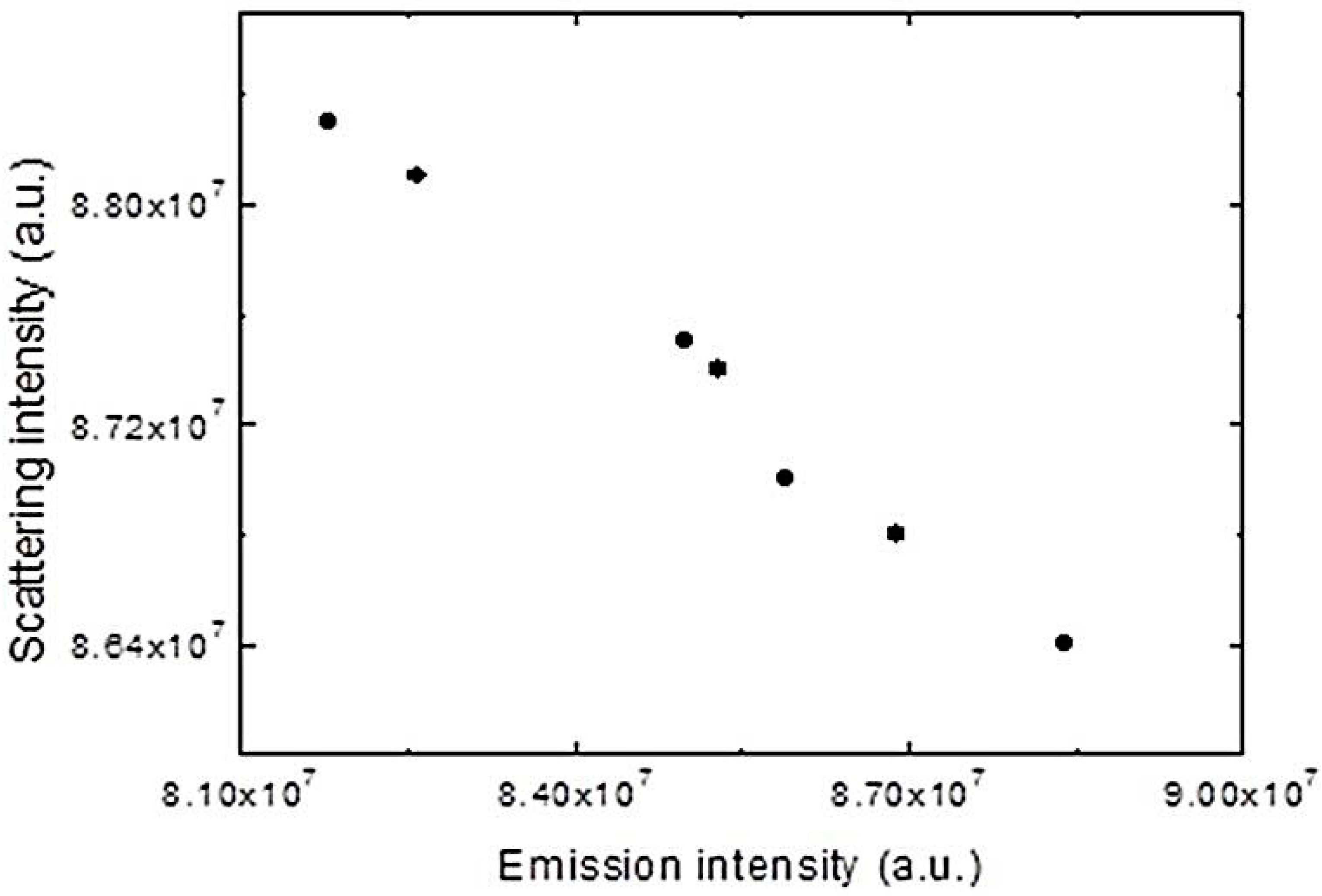
| CPEs | C12E5/H2O | 1:1 MeOH/H20 |
|---|---|---|
| PBS-PFP | 0.60 | 0.59 |
| PBS-PFP2 | 0.66 | 0.64 |
| PBS-PFP3 | 0.61 | 0.60 |
| τ1 (ns) | τ2 (ns) | τ3 (ns) | a1 | a2 | a3 | |
|---|---|---|---|---|---|---|
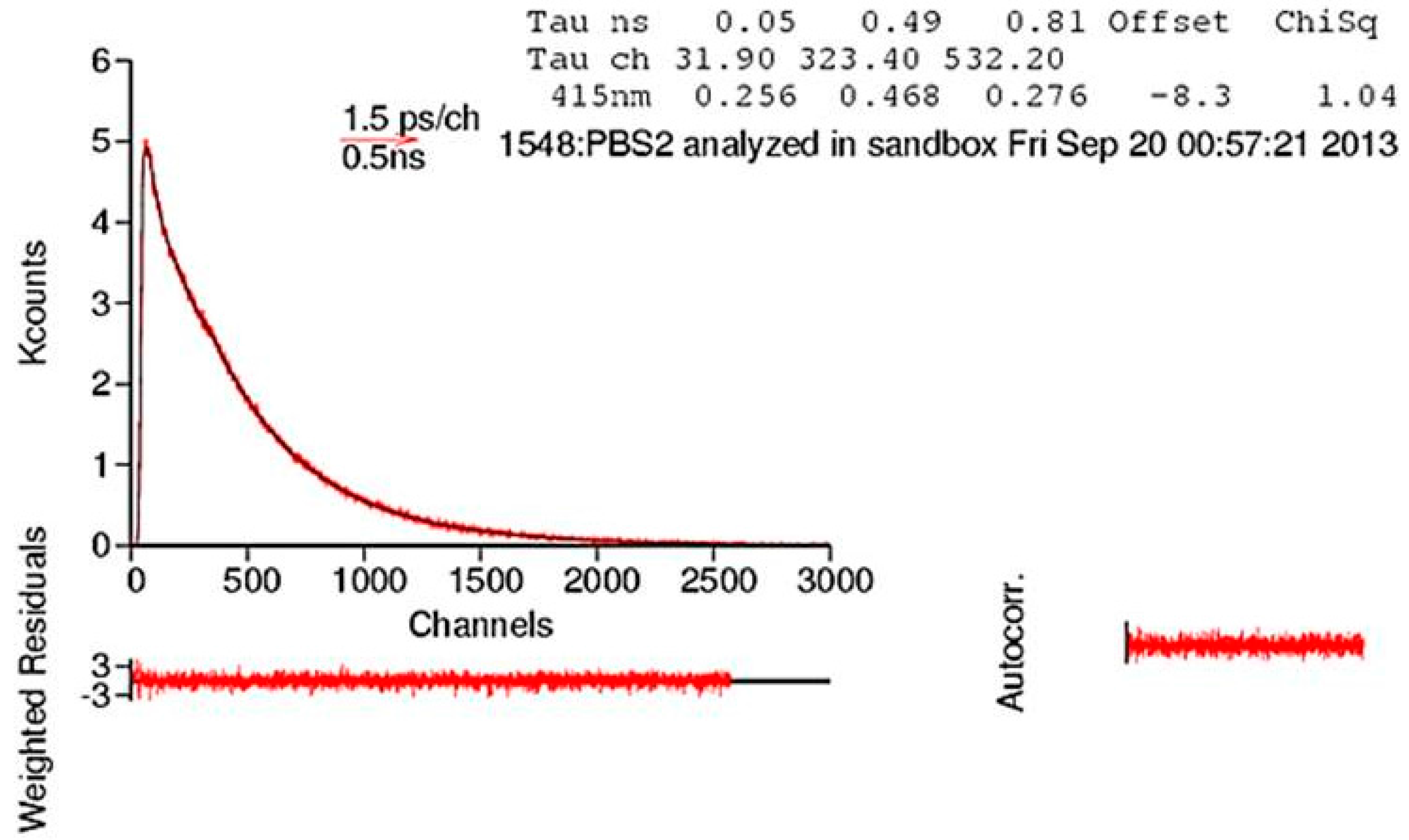
| PBS-PFP | 1.80 | 0.66 | 0.05 | 0.328 | 0.625 | 0.046 |
| PBS-PFP2 | 0.80 | 0.49 | 0.05 | 0.291 | 0.454 | 0.255 |
| PBS-PFP3 | 1.26 | 0.53 | 0.08 | 0.305 | 0.388 | 0.307 |
| PBS-PFP2 | PBS-PFP3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect of salt— [NaCl] (M) | Effect of temperature (°C) | Effect of concentration (×10−5 M) | |||||||||
| Polyelectrolyte | PBS-PFP | 0 | 0.01 | 0.1 | 25.5 | 35.5 | 45.0 | 1.63 | 2.48 | 3.31 | 4.98 |
| k (×10−3 s−1) | 0.90 | 1.4 | 1.6 | 1.9 | 0.90 | 1.3 a | 1.3 a | 2.0 | 1.9 | 1.7 | 1.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martelo, L.M.; Fonseca, S.M.; Marques, A.T.; Burrows, H.D.; Valente, A.J.M.; Justino, L.L.G.; Scherf, U.; Pradhan, S.; Song, Q. Effects of Charge Density on Photophysics and Aggregation Behavior of Anionic Fluorene-Arylene Conjugated Polyelectrolytes. Polymers 2018, 10, 258. https://doi.org/10.3390/polym10030258
Martelo LM, Fonseca SM, Marques AT, Burrows HD, Valente AJM, Justino LLG, Scherf U, Pradhan S, Song Q. Effects of Charge Density on Photophysics and Aggregation Behavior of Anionic Fluorene-Arylene Conjugated Polyelectrolytes. Polymers. 2018; 10(3):258. https://doi.org/10.3390/polym10030258
Chicago/Turabian StyleMartelo, Liliana M., Sofia M. Fonseca, Ana T. Marques, Hugh D. Burrows, Artur J. M. Valente, Licínia L. G. Justino, Ullrich Scherf, Swapna Pradhan, and Qiu Song. 2018. "Effects of Charge Density on Photophysics and Aggregation Behavior of Anionic Fluorene-Arylene Conjugated Polyelectrolytes" Polymers 10, no. 3: 258. https://doi.org/10.3390/polym10030258