Influence of Chitosan Ascorbate Chirality on the Gelation Kinetics and Properties of Silicon-Chitosan-Containing Glycerohydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Preparation
2.2.1. Solution Preparation
2.2.2. Precipitation
2.2.3. Synthesis of Organically-Modified Silicon
2.2.4. Sol–Gel Synthesis of Glycerohydrogels
2.3. Methods of Examination
2.3.1. Spectroscopic Methods
2.3.2. Elemental Analysis
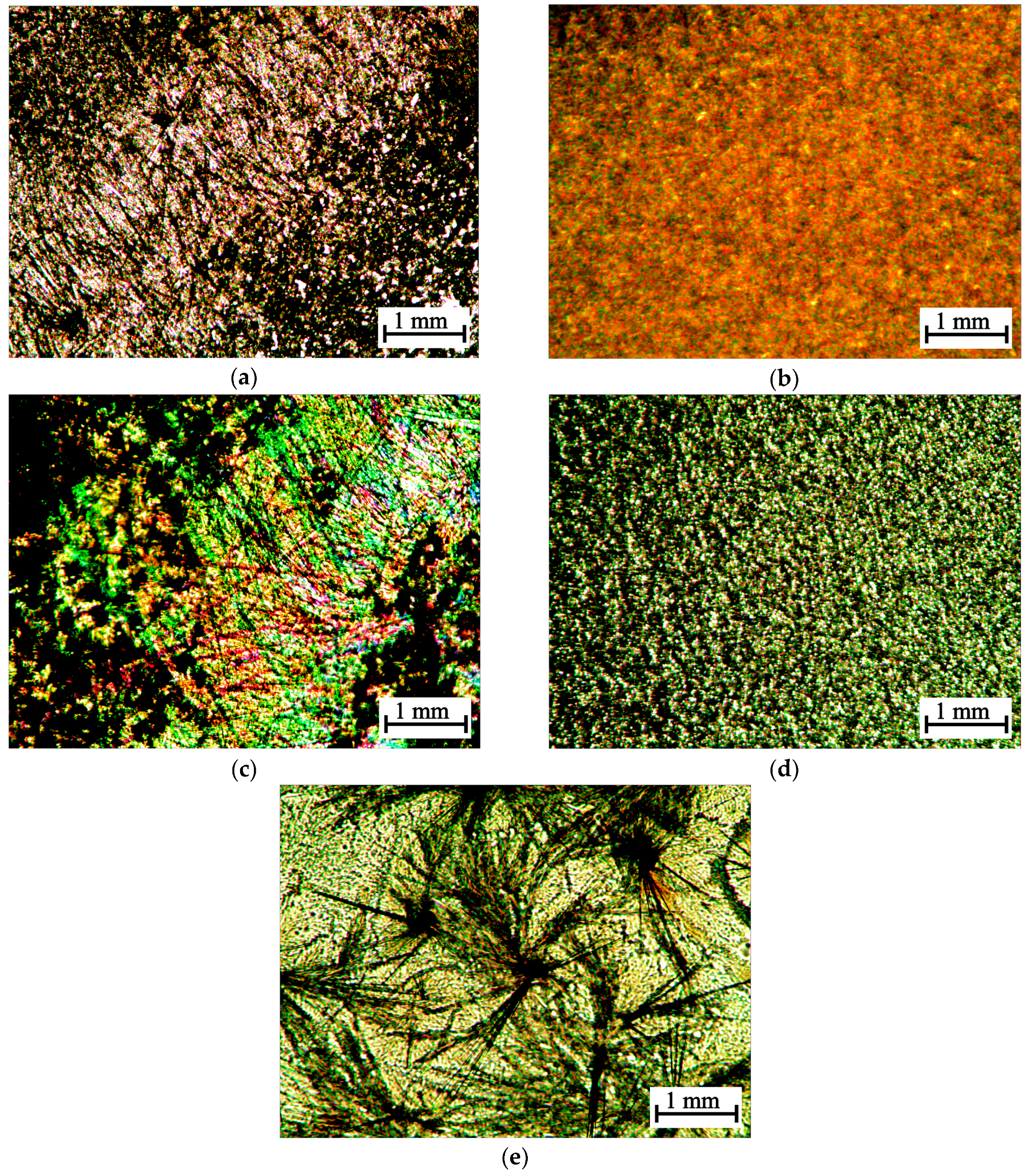
2.3.3. Polarization Microscopy
2.3.4. Mechanical Properties
2.4. Evaluation of Antibacterial Activity
2.5. Viability Test
2.5.1. Cell Preparation
2.5.2. Cell Viability
2.6. Statistical Analysis
3. Results and Discussion
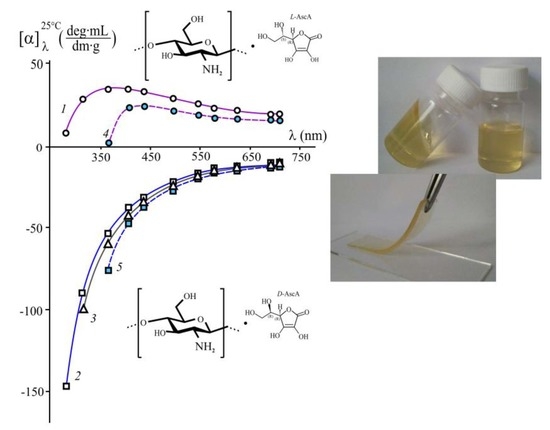
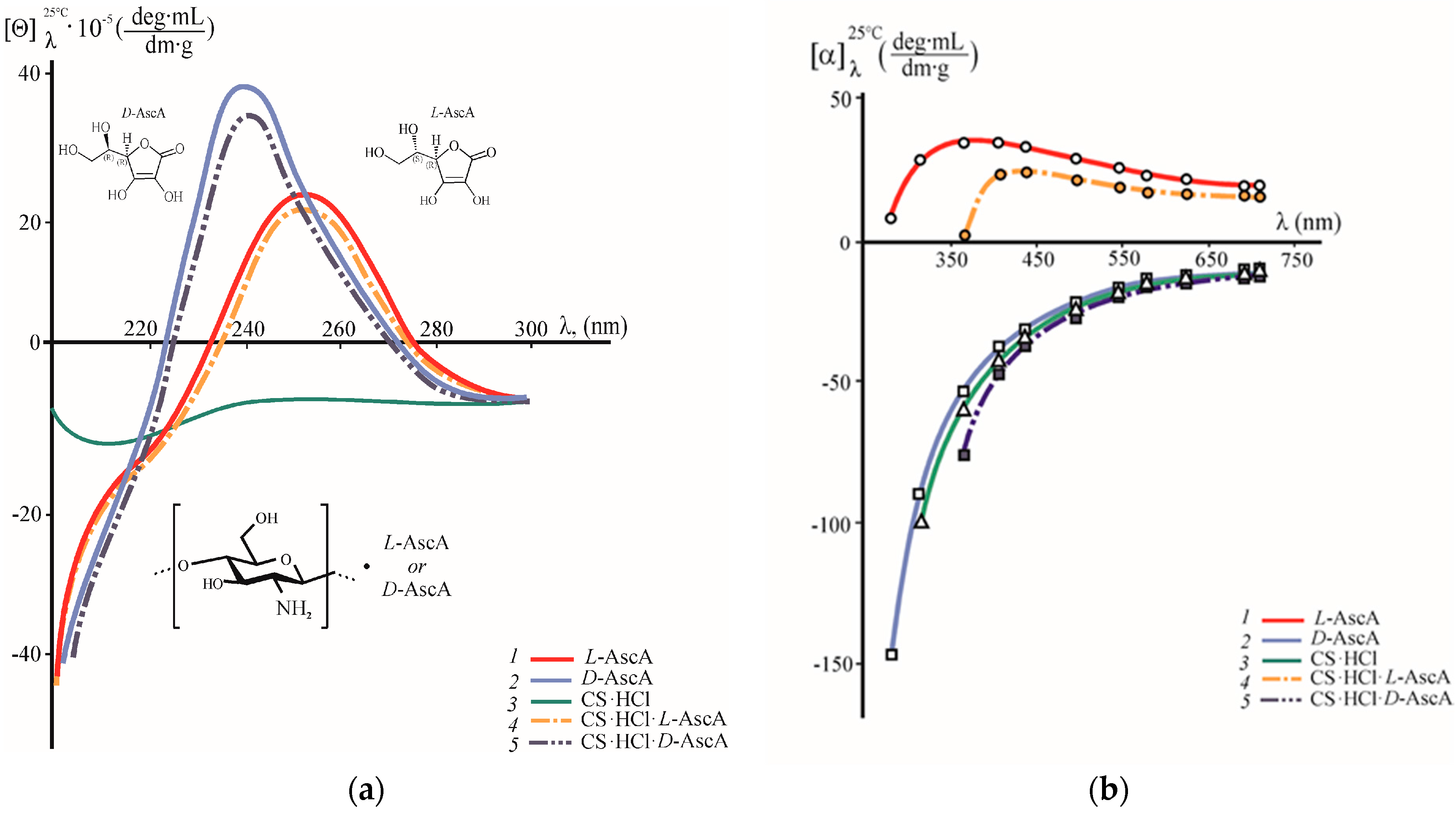
3.1. A Study of the Chirality of Diastereomerically-Enriched Chitosan Salts
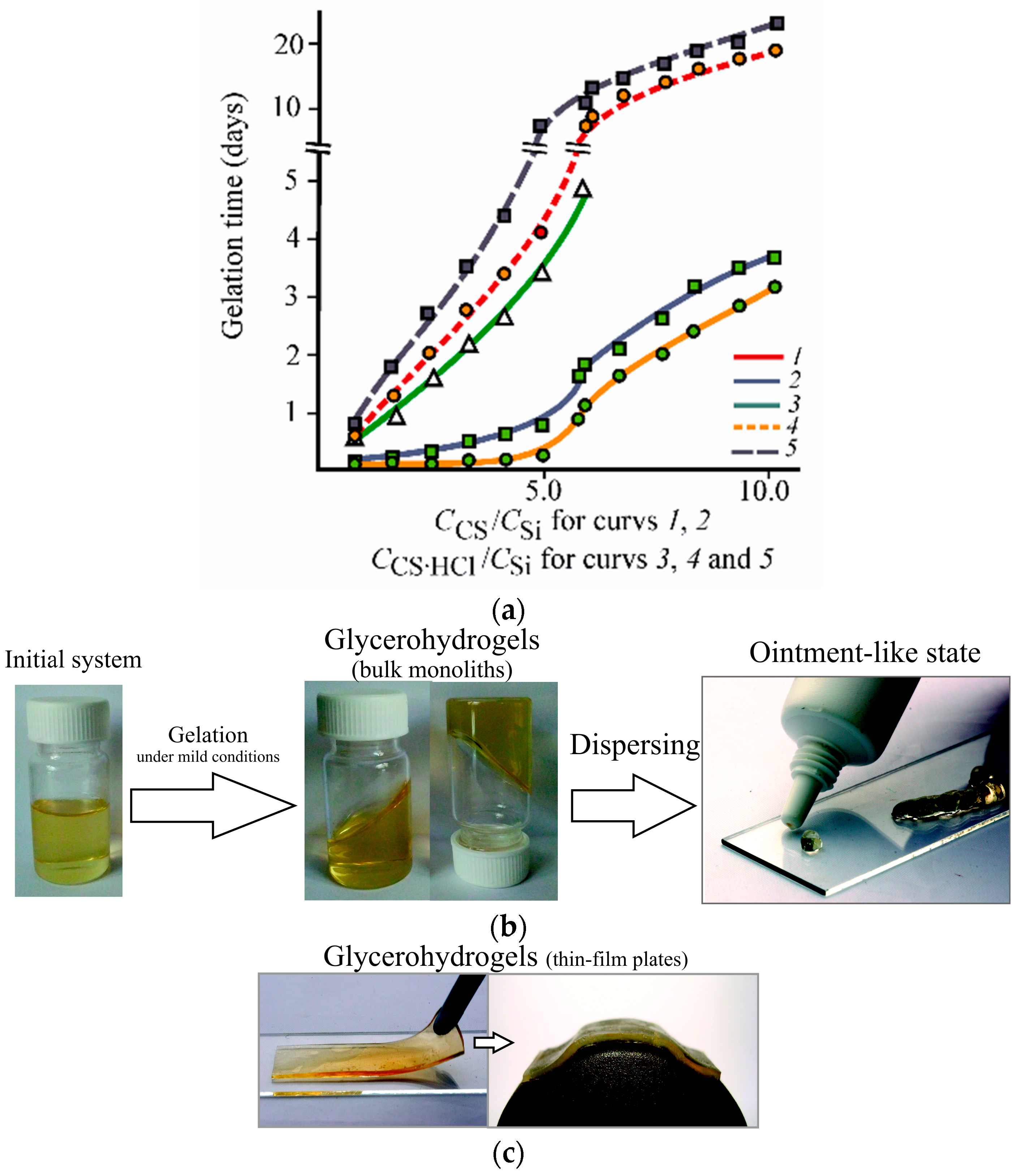
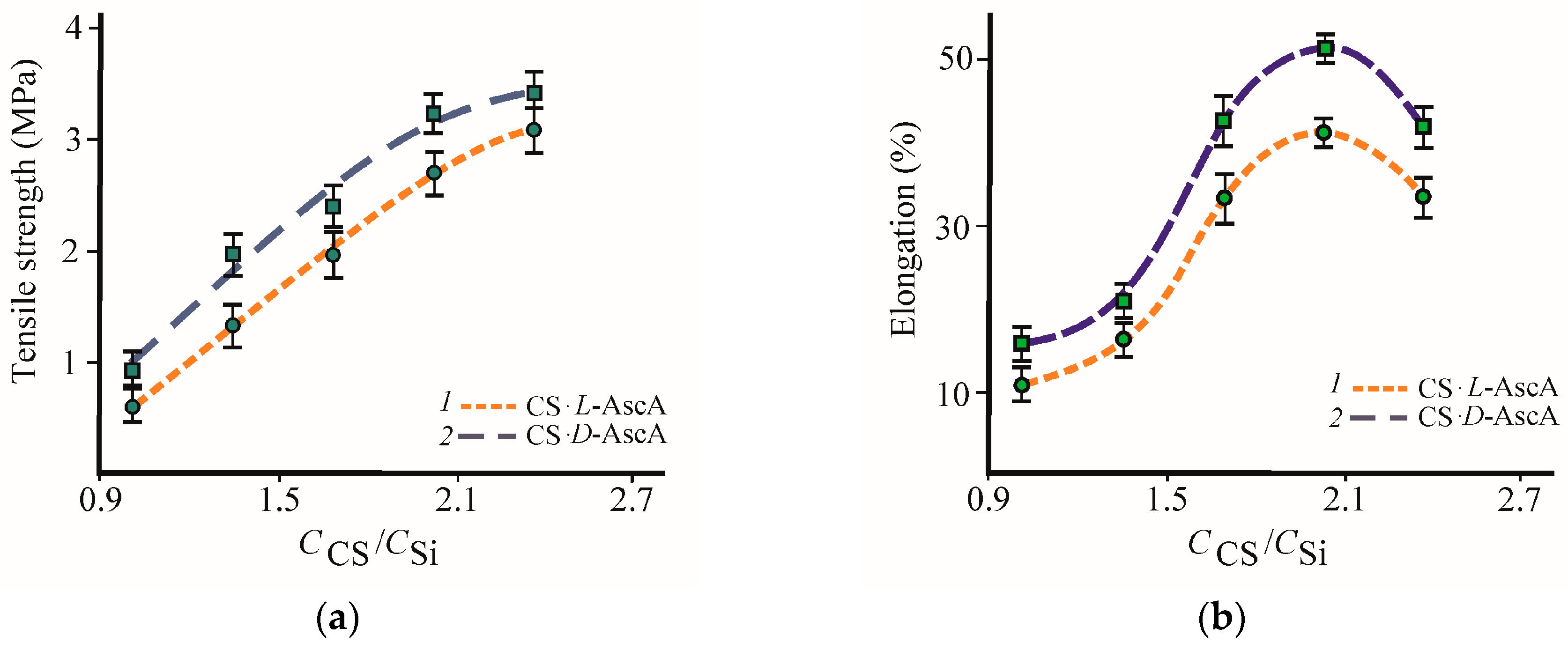
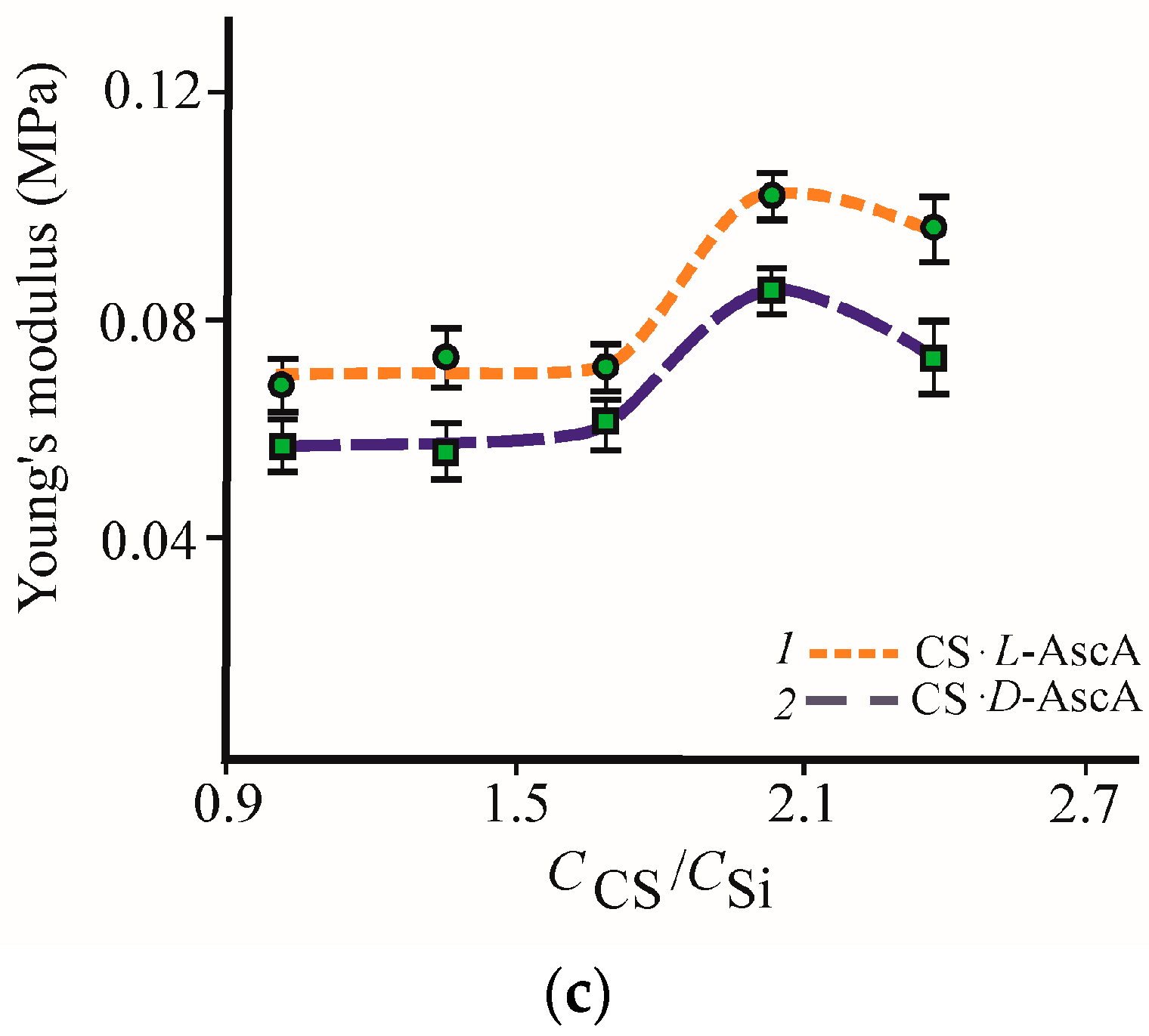
3.2. Formation and Properties of Glycerohydrogels Based on Diastereomerically-Enriched Chitosan Salts
3.3. Antibacterial Activity of l- and d-Diastereomerically-Enriched Chitosan Salts
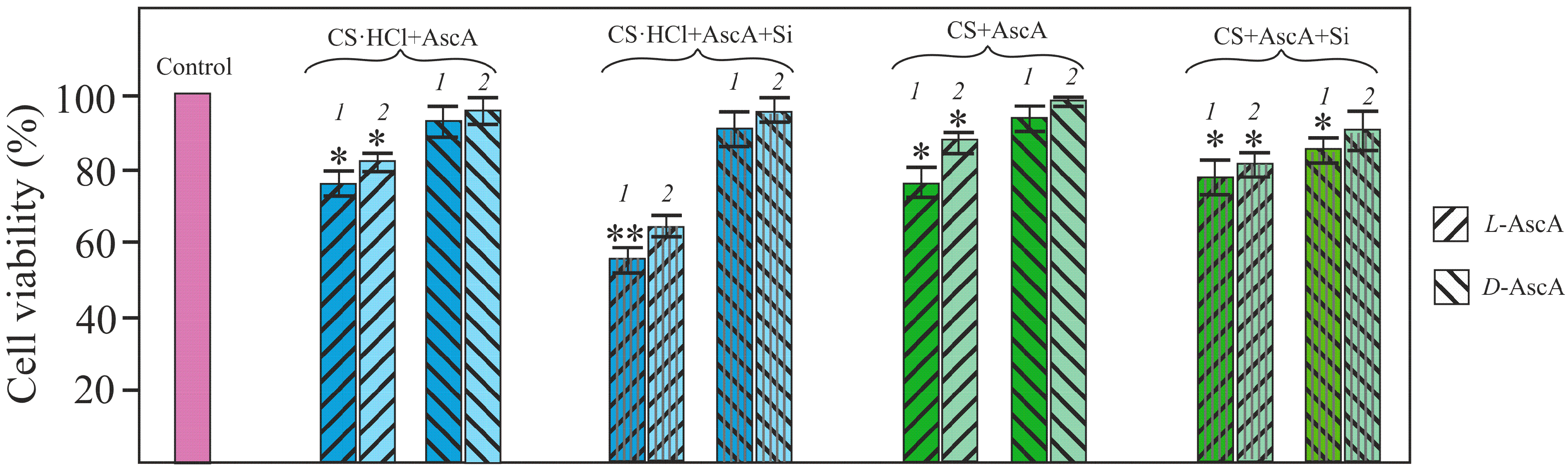
3.4. Evaluation of the Viability of NHDF Cells in the Presence of l- and d-Diastereomerically-Enriched Chitosan Salts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for cartilage regeneration, from polysaccharides to hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef]
- Pohontu, C.; Popa, M.; Desbrieres, J.; Verestiuc, L. Acrylates and methylcellulose based hydrogels. Synthesis, swelling properties and applications to inclusion and controlled release of bioactive matters. Cellul. Chem. Technol. 2016, 50, 609–620. [Google Scholar]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Kijkowska, R.; Sobczak-Kupiec, A. Preparation and cytotoxicity of chitosan-based hydrogels modified with silver nanoparticles. Colloid Surfaces B 2017, 160, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Franzén, H.M.; Draget, K.I.; Langebäck, J.; Nilsen-Nygaard, J. Characterization and properties of hydrogels made from neutral soluble chitosans. Polymers 2015, 7, 373–389. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, P.K. Spectroscopic and conformational study of chitosan acid salts. J. Polym. Res. 2009, 16, 231–238. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, P.K. Preparation, circular dichroism induced helical conformation and optical property of chitosan acid salt complexes for biomedical applications. Int. J. Biol. Macromol. 2009, 45, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Domard, A. pH and C.D. measurements on a fully deacetylated, chitosan: Application to CuII-polymer interactions. Inter. J. Biol. Macromol. 1987, 9, 98–104. [Google Scholar] [CrossRef]
- Koralewski, M.; Bodek, K.H.; Marczewska, K. Optical properties of chitosan in aqueous solution. Pol. Chitin Soc. 2006, 9, 29–39. [Google Scholar]
- Kumar, S.; Koh, J. Physiochemical, circular dichroism-induced helical conformation and optical property of chitosan azo-based amino methanesulfonate complex. J. Appl. Polym. Sci. 2012, 124, 4897–4903. [Google Scholar] [CrossRef]
- Shipovskaya, A.B.; Fomina, V.I.; Kazmicheva, O.F.; Rudenko, D.A.; Malinkina, O.N. Optical activity of films based on chitosan of various molecular masses and modifications. Polym. Sci. Ser. A 2017, 59, 330–341. [Google Scholar] [CrossRef]
- Shipovskaya, A.B.; Fomina, V.I.; Rudenko, D.A.; Shchyogolev, S.Y. Influence of physical and chemical modification on the optical rotatory dispersion and biological activity of chitosan films. Int. J. Polym. Sci. 2013, 2013, 825296. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, Y.; Chen, H.; Xu, D.; Lin, K.; Liu, W. Effect of chitosan on the enantioselective bioavailability of the herbicide dichlorprop to Chlorella pyrenoidosa. Environ. Sci. Technol. 2010, 44, 4981–4987. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.K.; Ferreira, J.; Luo, T.J.M.; Geng, H.; Lin, F.C.; Ko, C.C. Direct scaffolding of biomimetic hydroxyapatite-gelatin nanocomposites using aminosilane cross-linker for bone regeneration. J. Mater. Sci. Mater. Med. 2012, 23, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Spinde, K.; Kammer, M.; Freyer, K.; Ehrlich, H.; Vournakis, J.N.; Brunner, E. Biomimetic silicification of fibrous chitin from diatoms. Chem. Mater. 2011, 23, 2973–2978. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Karpenko, T.Y.; Krekoten, A.V.; Postnova, I.V. Gelling of otherwise nongelable polysaccharides. J. Colloid Interface Sci. 2005, 287, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Chae, T.; Yang, H.; Leung, V.; Ko, F.; Troczynski, T. Novel biomimetic hydroxyapatite/alginate nanocomposite fibrous scaffolds for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Li, D.; Lian, Q.; Li, X.; Zhang, W.; Wang, K.; Jin, Z. Fabrication of a bio-inspired beta-Tricalcium phosphate/collagen scaffold based on ceramic stereolithography and gel casting for osteochondral tissue engineering. Rapid Prototyp. J. 2012, 18, 68–80. [Google Scholar] [CrossRef]
- Bogdanova, E.A.; Sabirzyanov, N.A.; Khonina, T.G. Hydroxyapathite gel as a basis for pharmaceutic composites. Glass Phys. Chem. 2011, 37, 533. [Google Scholar] [CrossRef]
- Shadrina, E.V.; Malinkina, O.N.; Khonina, T.G.; Shipovskaya, A.B.; Fomina, V.I.; Larchenko, E.Y.; Popova, N.A.; Zyryanova, I.G.; Larionov, L.P. Formation and pharmacological activity of silicon-chitosan-containing glycerohydrogels obtained by biomimetic mineralization. Rus. Chem. B 2015, 64, 1633–1639. [Google Scholar] [CrossRef]
- Chumlea, W.M.C. Silica, a mineral of unknown but emerging health importance. J. Nutr. Health Aging 2007, 11, 93. [Google Scholar] [PubMed]
- Shirosaki, Y.; Okayama, T.; Tsuru, K.; Hayakawa, S.; Osaka, A. In vitro bioactivity and MG63 cytocompatibility of chitosan-silicate hybrids. Int. J. Mater. Chem. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Roosen, J.; Spooren, J.; Binnemans, K. Adsorption performance of functionalized chitosan–silica hybrid materials toward rare earths. J. Mater. Chem. A 2014, 2, 19415–19426. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Pylypchuk, I.V.; Tertykh, V.A.; Yanovska, E.S.; Kolodynska, D. Synthesis and adsorption properties of chitosan-silica nanocomposite prepared by sol-gel method. Nanoscale Res. Let. 2015, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Romer, F.; Connell, L.S.; Walter, C.; Saiz, E.; Yue, S.; Jones, J.R. Highly flexible silica/chitosan hybrid scaffolds with oriented pores for tissue regeneration. J. Mater. Chem. B 2015, 3, 7560–7576. [Google Scholar] [CrossRef]
- Larchenko, E.Y.; Shadrina, E.V.; Khonina, T.G.; Chupakhin, O.N. New hybrid chitosan–silicone-containing glycerohydrogels. Mendeleev Commun. 2014, 24, 201–202. [Google Scholar] [CrossRef]
- Shipovskaya, A.B.; Malinkina, O.N.; Zhuravleva, Y.Y.; Rogacheva, S.M. Synthesis of silicon-containing chitosan hydrogels in a glycolic acid medium. Adv. Mater. Sci. Eng. 2016, 2016, 3951703. [Google Scholar] [CrossRef]
- Malinkina, O.N.; Sobolev, A.M.; Shipovskaya, A.B. Hybrid nanogels based on hydrochloride–ascorbate chitosan derived from a sol-gel biomimetic synthesis. BioNanoScience 2016, 6, 157–161. [Google Scholar] [CrossRef]
- Ogawa, K.; Nakata, K.; Yamamoto, A.; Nitta, Y.; Yui, T. X-ray study of chitosan l-and d-ascorbates. Chem. Mater. 1996, 8, 2349–2351. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds; John Wiley & Sons: New York, NY, USA, 2014; ISBN 978-0-470-61637-6. [Google Scholar]
- Back, S.A.; Khan, R.; Gan, X.; Rosenberg, P.A.; Volpe, J.J. A new alamar blue viability assay to rapidly quantify oligodendrocyte death. J. Neurosci. Methods 1999, 91, 47–54. [Google Scholar] [CrossRef]
- Wittine, K.; Gazivoda, T.; Markus, M.; Mrvos-Sermek, D.; Hergold-Brundic, A.; Cetina, M.; Ziher, D.; Gabelica, V.; Mintas, M.; Raic-Malic, S. Crystal structures, circular dichroism spectra and absolute configurations of some l-ascorbic acid derivatives. J. Mol. Struct. 2004, 687, 101–106. [Google Scholar] [CrossRef]
- Markarian, S.A.; Sargsyan, H.R. Electronic absorption spectra of ascorbic acid in water and water–dialkylsulfoxide mixtures. J. Appl. Spectrosc. 2011, 78, 6–10. [Google Scholar] [CrossRef]
- Shukla, M.K.; Mishra, P.C. Electronic structures and spectra of two antioxidants: Uric acid and ascorbic acid. J. Mol. Struct. THEOCHEM 1996, 337, 247–259. [Google Scholar] [CrossRef]
- Shipovskaya, A.B.; Zudina, I.V.; Fomina, V.I.; Malinkina, O.N. Novel antimicrobial drugs based on complex chitosan salts with chiral organic ligands. Butlerov Commun. 2015, 41, 82–94. [Google Scholar]
- Shipovskaya, A.B.; Fomina, V.I.; Kazmicheva, O.F.; Timofeeva, G.N.; Komarov, B.A. Effect of molecular mass on the optical activity of chitosan. Polym. Sci. Ser. B 2007, 49, 288–291. [Google Scholar] [CrossRef]
- Shipovskaya, A.B.; Malinkina, O.N.; Fomina, V.I.; Rudenko, D.A.; Shchegolev, S.Y. Optical activity of solutions and films of chitosan acetate. Rus. Chem. B 2015, 64, 1172–1177. [Google Scholar] [CrossRef]
- Nova, A.; Keten, S.; Pugno, N.M.; Redaelli, A.; Buehler, M.J. Molecular and nanostructural mechanisms of deformation, strength and toughness of spider silk fibrils. Nano Lett. 2010, 10, 2626–2634. [Google Scholar] [CrossRef] [PubMed]
- Del Mercato, L.L.; Maruccio, G.; Pompa, P.P.; Bochicchi, B.; Tamburro, A.M.; Cingolani, R.; Rinaldi, R. Amyloid-like fibrils in elastin-related polypeptides: Structural characterization and elastic properties. Biomacromolecules 2008, 9, 796–803. [Google Scholar] [CrossRef] [PubMed]

| Solution | Antibacterial activity (Ai), % | |
|---|---|---|
| Staphylococcus aureus 209P | Escherichia coli 113-13 | |
| l-AscA | 36.4 | 45.7 |
| d-AscA | 17.0 | 24.6 |
| CS·HCl·l-AscA | 9.2 | 7.3 |
| CS·HCl·d-AscA | 29.2 | 54.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gegel, N.O.; Zhuravleva, Y.Y.; Shipovskaya, A.B.; Malinkina, O.N.; Zudina, I.V. Influence of Chitosan Ascorbate Chirality on the Gelation Kinetics and Properties of Silicon-Chitosan-Containing Glycerohydrogels. Polymers 2018, 10, 259. https://doi.org/10.3390/polym10030259
Gegel NO, Zhuravleva YY, Shipovskaya AB, Malinkina ON, Zudina IV. Influence of Chitosan Ascorbate Chirality on the Gelation Kinetics and Properties of Silicon-Chitosan-Containing Glycerohydrogels. Polymers. 2018; 10(3):259. https://doi.org/10.3390/polym10030259
Chicago/Turabian StyleGegel, Natalia O., Yulia Yu. Zhuravleva, Anna B. Shipovskaya, Olga N. Malinkina, and Irina V. Zudina. 2018. "Influence of Chitosan Ascorbate Chirality on the Gelation Kinetics and Properties of Silicon-Chitosan-Containing Glycerohydrogels" Polymers 10, no. 3: 259. https://doi.org/10.3390/polym10030259