Humidity-Induced Phase Transitions of Surfactants Embedded in Latex Coatings Can Drastically Alter Their Water Barrier and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Latex Coatings
2.2. Pendulum Hardness
2.3. Quartz Crystal Microbalance with Dissipation (QCM-D)
Data Analysis
2.4. Differential Scanning Calorimetry (DSC)
2.5. Atomic Force Microscopy (AFM)
3. Results
3.1. Pendulum Hardness
3.2. Quartz Crystal Microbalance with Dissipation (QCM-D)
3.2.1. Latex Film Formation
3.2.2. Water Sorption Isotherms of Latex Coatings
3.2.3. Water Sorption Isotherms of Rhodacal® DSB Films
3.3. Differential Scanning Calorimetry (DSC)
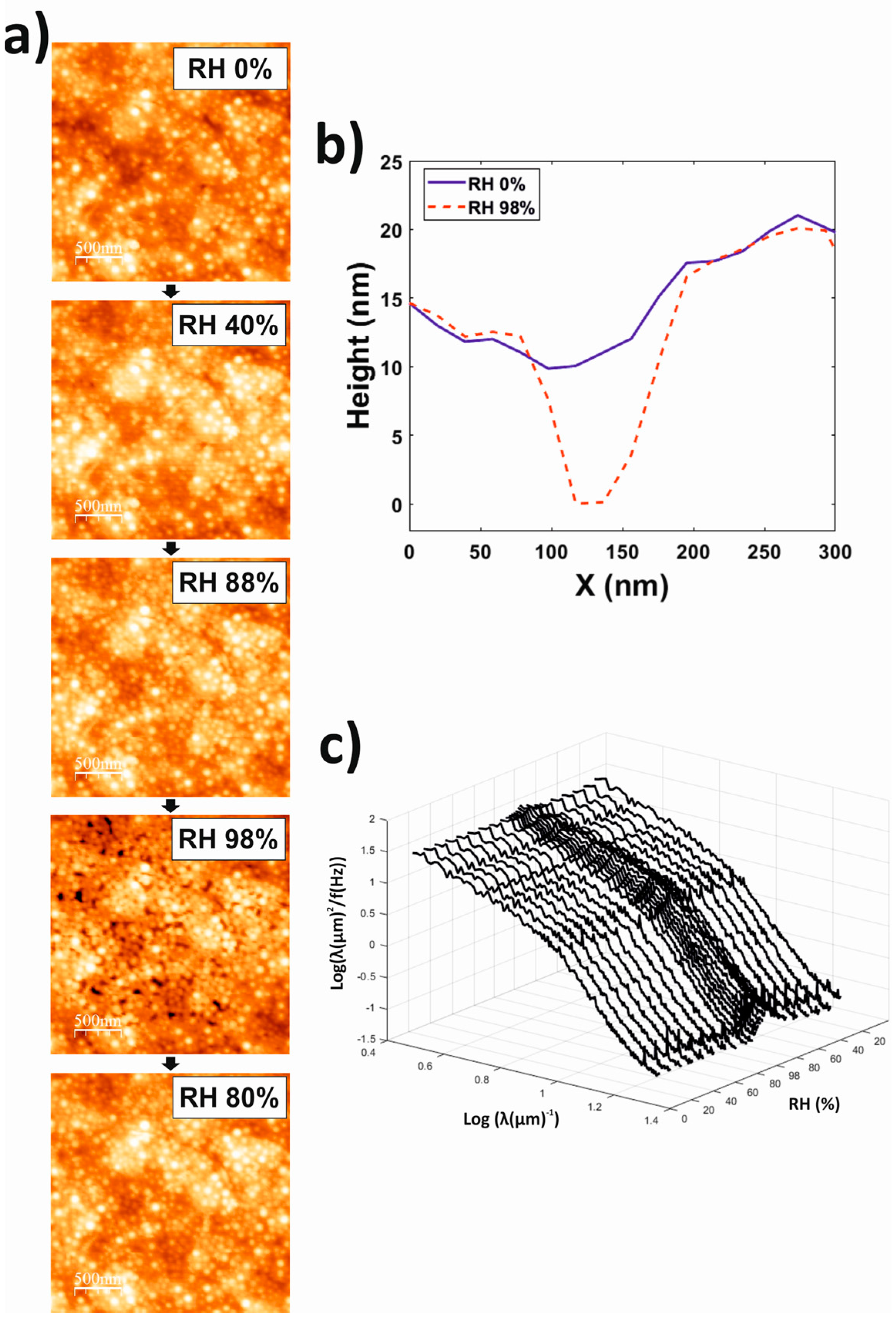
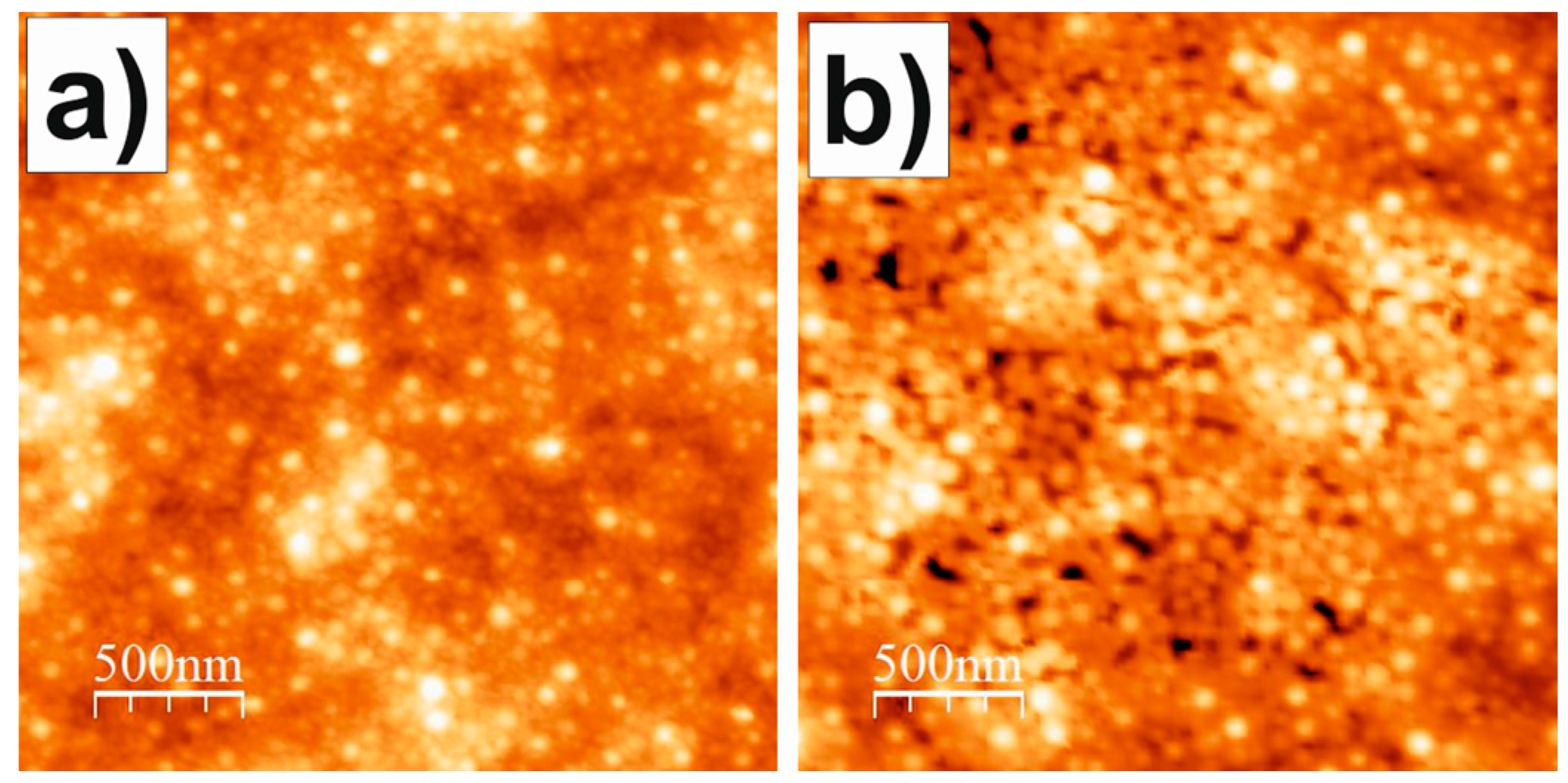
3.4. Atomic Force Microscopy (AFM)
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keddie, J.L.; Routh, A.F. Fundamentals of Latex Film Formation: Processes and Properties; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Hellgren, A.-C.; Weissenborn, P.; Holmberg, K. Surfactants in water-borne paints. Prog. Org. Coat. 1999, 35, 79–87. [Google Scholar] [CrossRef]
- Arnold, C.; Klein, G.; Maaloum, M.; Ernstsson, M.; Larsson, A.; Marie, P.; Holl, Y. Surfactant distribution in waterborne acrylic films: 2. Surface investigation. Colloids Surf. A 2011, 374, 58–68. [Google Scholar] [CrossRef]
- Arnold, C.; Thalmann, F.; Marques, C.; Marie, P.; Holl, Y. Surfactant Distribution in Waterborne Acrylic Films. 1. Bulk Investigation. J. Phys. Chem. B 2010, 114, 9135–9147. [Google Scholar] [CrossRef] [PubMed]
- Gromer, A.; Thalmann, F.; Hébraud, P.; Holl, Y. Simulation of Vertical Surfactant Distributions in Drying Latex Films. Langmuir 2017, 33, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Dong, J.; Severtson, S.J.; Houtman, C.J.; Gwin, L.E. Modifications of Surfactant Distributions and Surface Morphologies in Latex Films Due to Moisture Exposure. J. Phys. Chem. B 2009, 113, 10189–10195. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.N.; Fellows, C.M.; Gilbert, R.G. Effect of surfactants used for binder synthesis on the properties of latex paints. Prog. Org. Coat. 2005, 53, 112–118. [Google Scholar] [CrossRef]
- Asua, J.M.; Schoonbrood, H.A.S. Reactive surfactants in heterophase polymerisation. Acta Polym. 1998, 49, 671–686. [Google Scholar] [CrossRef]
- Jablonski, E.; Hayes, J.; Learner, T.; Golden, M. Conservation concerns for acrylic emulsion paints. Rev. Conserv. 2003, 4, 3–12. [Google Scholar] [CrossRef]
- Liu, Y.; Gajewicz, A.M.; Rodin, V.; Soer, W.-J.; Scheerder, J.; Satgurunathan, G.; Mcdonald, P.J.; Keddie, J.L. Explanations for water whitening in secondary dispersion and emulsion polymer films. J. Polym. Sci. B 2016, 54, 1658–1674. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kats, A.; Juhue, D.; Winnik, M.A.; Shivers, R.R.; Dinsdale, C.J. Freeze-fracture studies of latex films formed in the absence and presence of surfactant. Langmuir 1992, 8, 1435–1442. [Google Scholar] [CrossRef]
- Zhao, C.-L.; Holl, Y.; Pith, T.; Lambla, M. Surface analysis and adhesion properties of coalesced latex films. Br. Polym. J. 1989, 21, 155–160. [Google Scholar] [CrossRef]
- Butler, L.N.; Fellows, C.M.; Gilbert, R.G. Effect of surfactant systems on the water sensitivity of latex films. J. Appl. Polym. Sci. 2004, 92, 1813–1823. [Google Scholar] [CrossRef]
- Steward, P.A.; Hearn, J.; Wilkinson, M.C. Studies on permeation through polymer latex films, I. Films containing no or only low levels of additives. Polym. Int. 1995, 38, 1–12. [Google Scholar] [CrossRef]
- Eckersley, S.T.; Rudin, A. The effect of plasticization and pH on film formation of acrylic latexes. J. Appl. Polym. Sci. 1993, 48, 1369–1381. [Google Scholar] [CrossRef]
- Jiang, B.; Tsavalas, J.; Sundberg, D. Measuring the Glass Transition of Latex-Based Polymers in the Hydroplasticized State via Differential Scanning Calorimetry. Langmuir 2010, 26, 9408–9415. [Google Scholar] [CrossRef] [PubMed]
- Tsavalas, J.G.; Sundberg, D.C. Hydroplasticization of Polymers: Model Predictions and Application to Emulsion Polymers. Langmuir 2010, 26, 6960–6966. [Google Scholar] [CrossRef] [PubMed]
- Rodahl, M.; Hook, F.; Fredriksson, C.; Keller, C.A.; Krozer, A.; Brzezinski, P.; Voinova, M.; Kasemo, B. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss. 1997, 107, 229–246. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Krozer, A.; Brzezinski, P.; Kasemo, B. Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum. 1995, 66, 3924–3930. [Google Scholar] [CrossRef]
- Björklund, S.; Kocherbitov, V. Humidity scanning quartz crystal microbalance with dissipation monitoring setup for determination of sorption-desorption isotherms and rheological changes. Rev. Sci. Instrum. 2015, 86, 055105. [Google Scholar] [CrossRef] [PubMed]
- Graf, G.; Kocherbitov, V. Determination of Sorption Isotherm and Rheological Properties of Lysozyme Using a High-Resolution Humidity Scanning QCM-D Technique. J. Phys. Chem. B 2013, 117, 10017–10026. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.A. The water activities of lithium chloride solutions up to high concentrations at 25°. Trans. Faraday Soc. 1945, 41, 756–758. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung. Z. Phys. A 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Johannsmann, D. Viscoelastic Analysis of Organic Thin Films on Quartz Resonators. Macromol. Chem. Phys. 1999, 200, 501–516. [Google Scholar] [CrossRef]
- Znamenskaya, Y.; Sotres, J.; Gavryushov, S.; Engblom, J.; Arnebrant, T.; Kocherbitov, V. Water Sorption and Glass Transition of Pig Gastric Mucin Studied by QCM-D. J. Phys. Chem. B 2013, 117, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stand. Sect. A 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Burnett, D.J.; Thielmann, F.; Booth, J. Determining the critical relative humidity for moisture-induced phase transitions. Int. J. Pharm. 2004, 287, 123–133. [Google Scholar] [CrossRef] [PubMed]
- González Martínez, J.F.; Nieto-Carvajal, I.; Abad, J.; Colchero, J. Nanoscale measurement of the power spectral density of surface roughness: How to solve a difficult experimental challenge. Nanoscale Res. Lett. 2012, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.J. Wood Handbook: Wood as an Engineering Material; General Technical Report FPL-GTR-190; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010.
- Immergut, E.H.; Mark, H.F. Principles of Plasticization. In Plasticization and Plasticizer Processes; American Chemical Society: Washington, DC, USA, 1965. [Google Scholar]
- Denolf, G.C.; Haack, L.; Holubka, J.; Straccia, A.; Blohowiak, K.; Broadbent, C.; Shull, K.R. High Frequency Rheometry of Viscoelastic Coatings with the Quartz Crystal Microbalance. Langmuir 2011, 27, 9873–9879. [Google Scholar] [CrossRef] [PubMed]
- Denolf, G.C.; Sturdy, L.F.; Shull, K.R. High-Frequency Rheological Characterization of Homogeneous Polymer Films with the Quartz Crystal Microbalance. Langmuir 2014, 30, 9731–9740. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, A.C.; Wallin, M.; Weissenborn, P.K.; Mcdonald, P.J.; Glover, P.M.; Keddie, J.L. New techniques for determining the extent of crosslinking in coatings. Prog. Org. Coat. 2001, 43, 85–98. [Google Scholar] [CrossRef]
- Björklund, S.; Kocherbitov, V. Hydration-Induced Phase Transitions in Surfactant and Lipid Films. Langmuir 2016, 32, 5223–5232. [Google Scholar] [CrossRef] [PubMed]
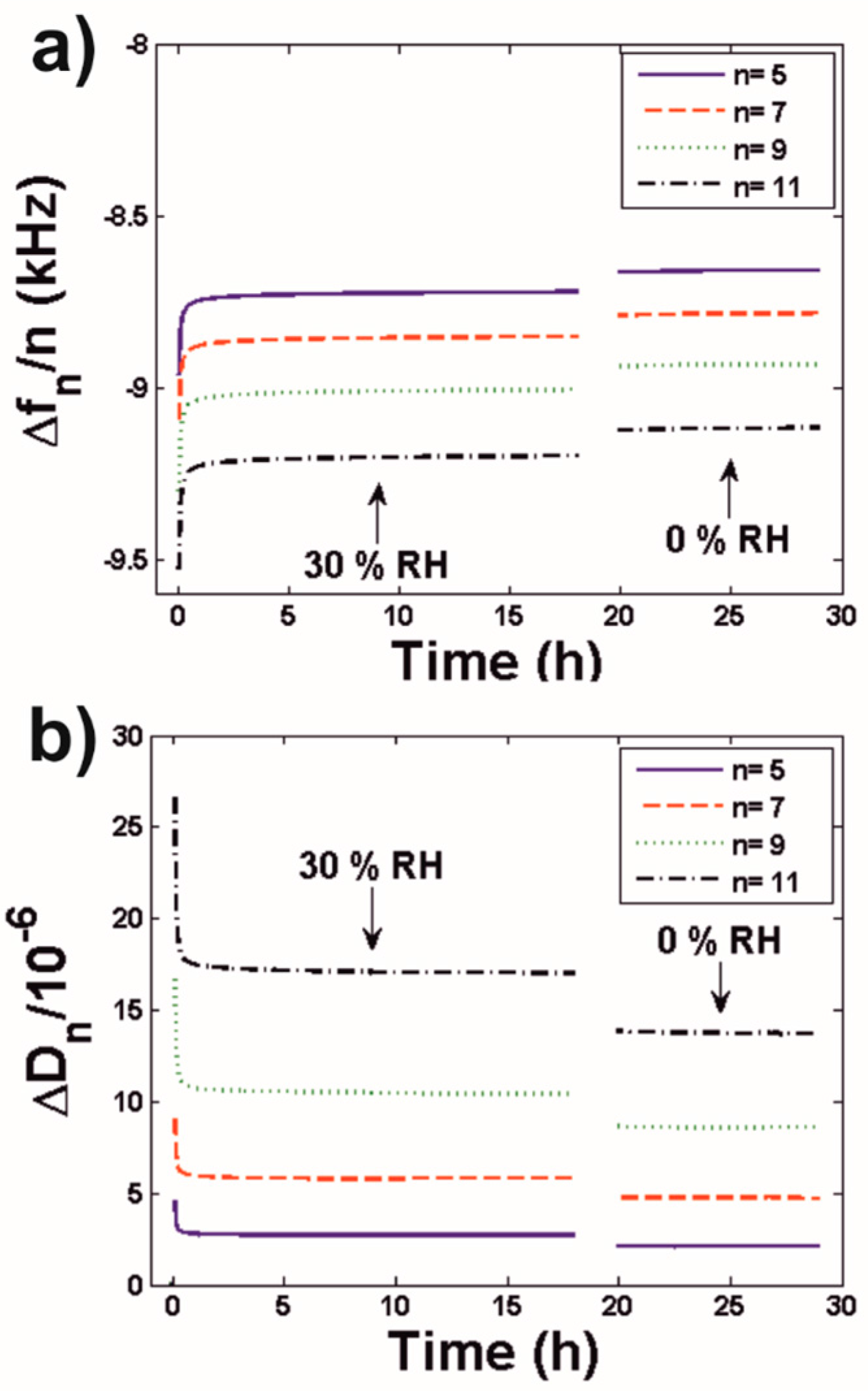

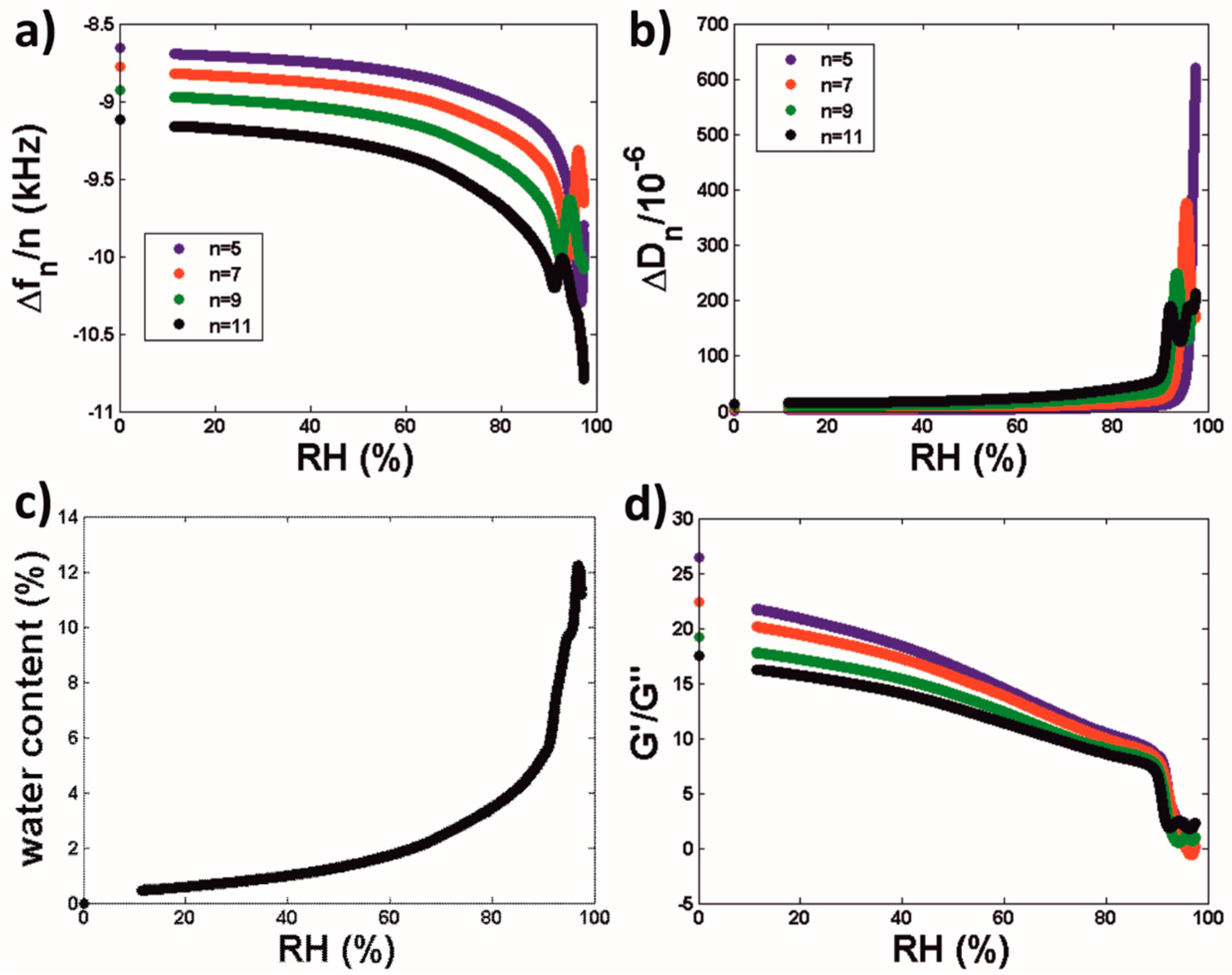
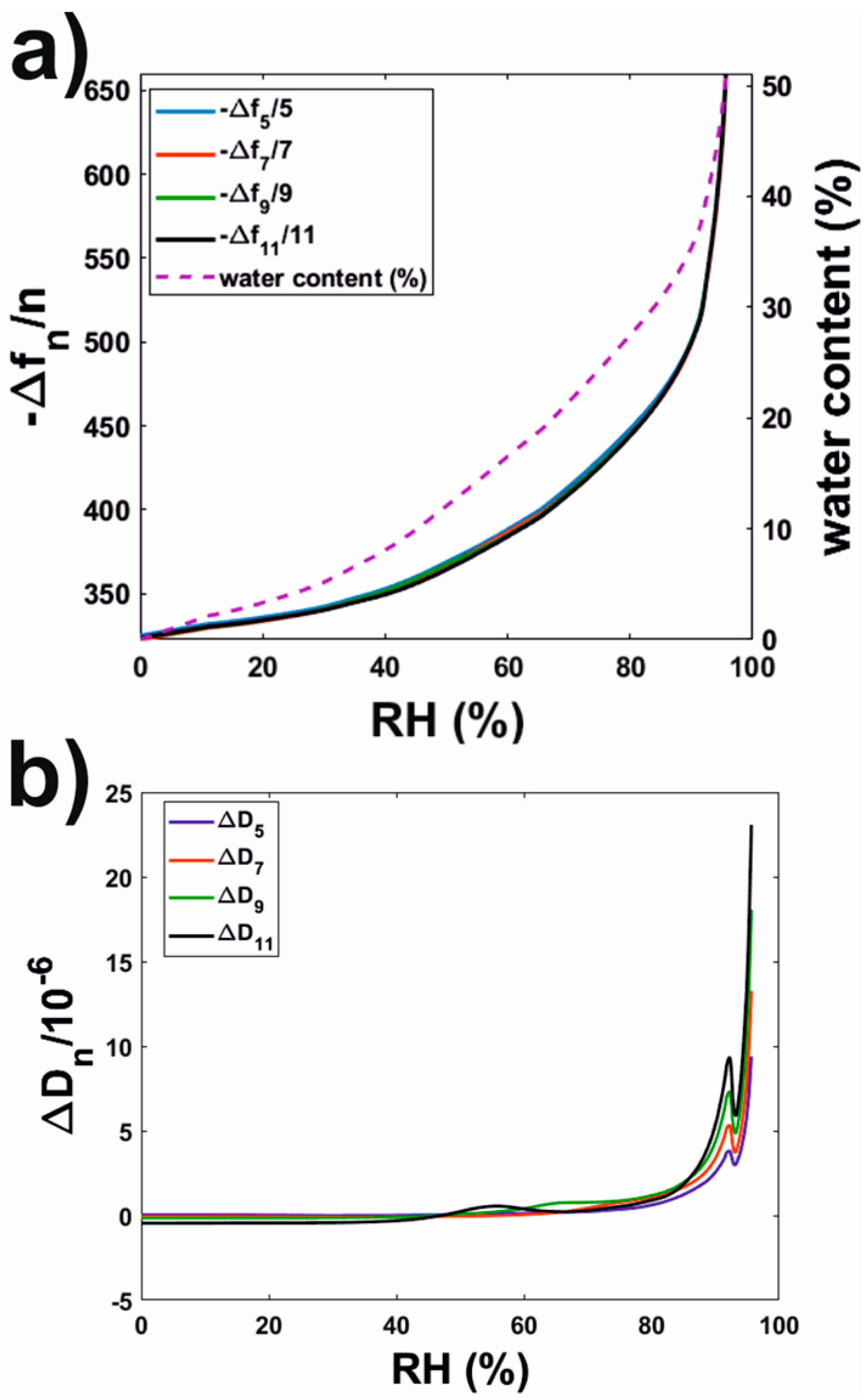
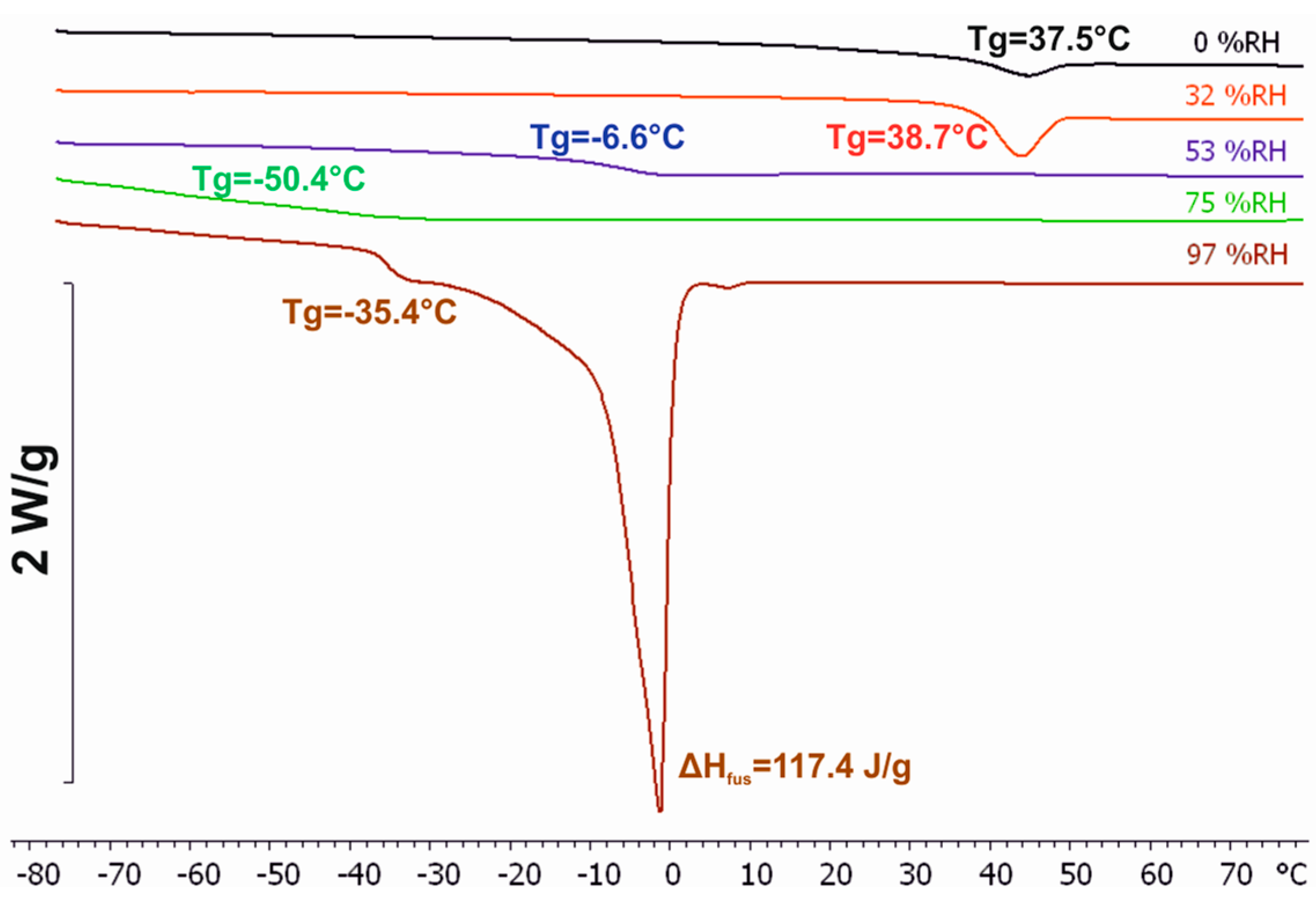
| Equilibration RH | # Oscillations (König Hardness) Equilibration Period 7 days | # Oscillations (König Hardness) Equilibration Period 14 days |
|---|---|---|
| 50% | 60.5 ± 0.7 | 63.5 ± 0.7 |
| 100% | 43.0 ± 4.2 | 35.0 ± 7.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Martinez, J.F.; Znamenskaya Falk, Y.; Björklund, S.; Erkselius, S.; Rehnberg, N.; Sotres, J. Humidity-Induced Phase Transitions of Surfactants Embedded in Latex Coatings Can Drastically Alter Their Water Barrier and Mechanical Properties. Polymers 2018, 10, 284. https://doi.org/10.3390/polym10030284
Gonzalez-Martinez JF, Znamenskaya Falk Y, Björklund S, Erkselius S, Rehnberg N, Sotres J. Humidity-Induced Phase Transitions of Surfactants Embedded in Latex Coatings Can Drastically Alter Their Water Barrier and Mechanical Properties. Polymers. 2018; 10(3):284. https://doi.org/10.3390/polym10030284
Chicago/Turabian StyleGonzalez-Martinez, Juan F., Yana Znamenskaya Falk, Sebastian Björklund, Stefan Erkselius, Nicola Rehnberg, and Javier Sotres. 2018. "Humidity-Induced Phase Transitions of Surfactants Embedded in Latex Coatings Can Drastically Alter Their Water Barrier and Mechanical Properties" Polymers 10, no. 3: 284. https://doi.org/10.3390/polym10030284






