Extraction Optimization, Purification, Antioxidant Activity, and Preliminary Structural Characterization of Crude Polysaccharide from an Arctic Chlorella sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Algae Growth Conditions
2.3. Polysaccharide Extraction
2.4. RSM Experimental Design
2.5. Purification of Crude Chlorella-Arc Polysaccharide
2.6. DPPH Radical Scavenging Assay
2.7. Hydroxyl Radical Scavenging Assay
2.8. Superoxide Radical Scavenging Assay
2.9. Ultraviolet-Visible (UV-Vis) and Fourier-Transform Infrared (FT-IR) Analyses
2.10. Monosaccharide Compositional Analysis
2.11. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.12. Analysis of Sulfate-Group Content
2.13. Statistical Analysis
3. Results and Discussion
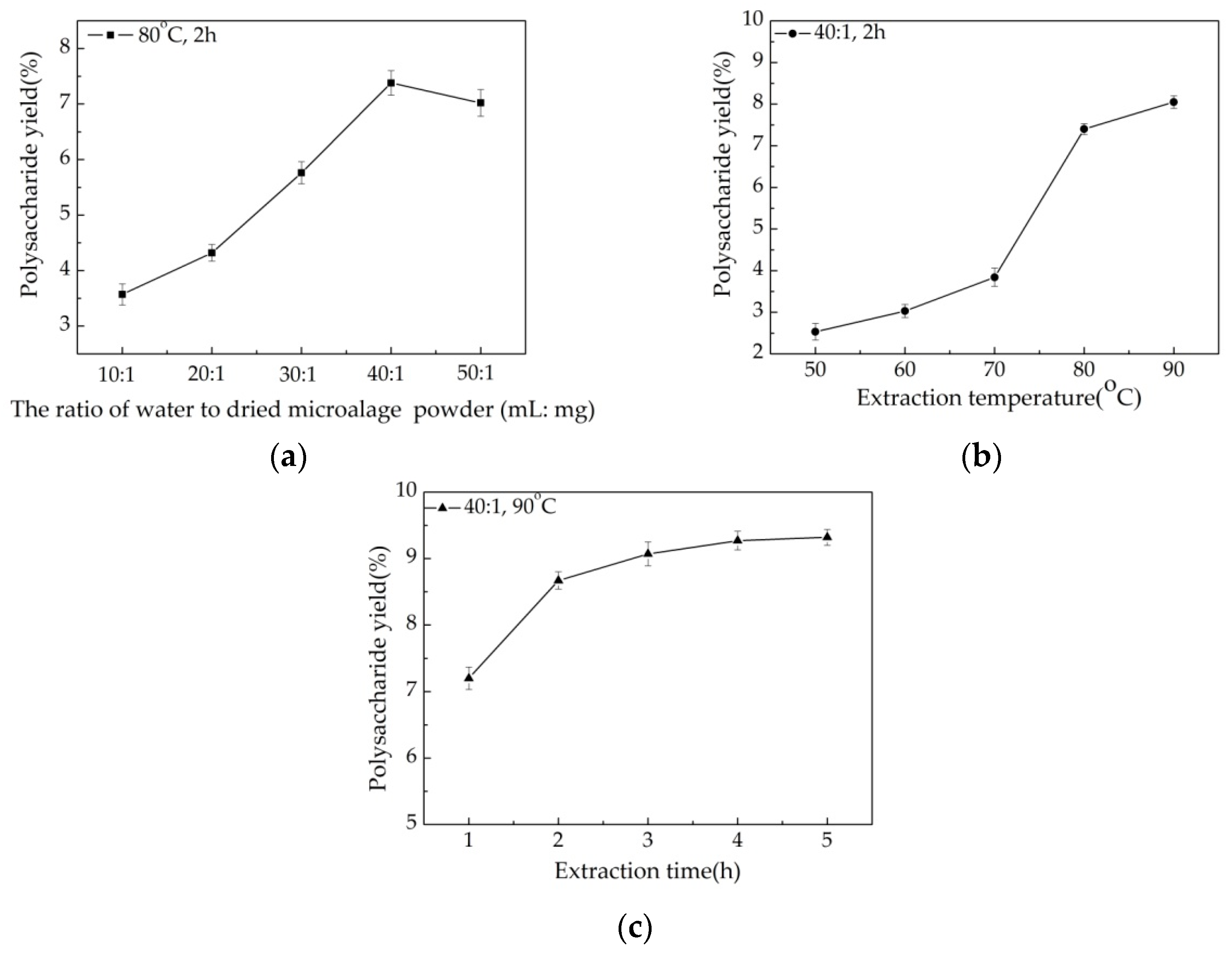
3.1. Selection of Extraction Optimization Factors
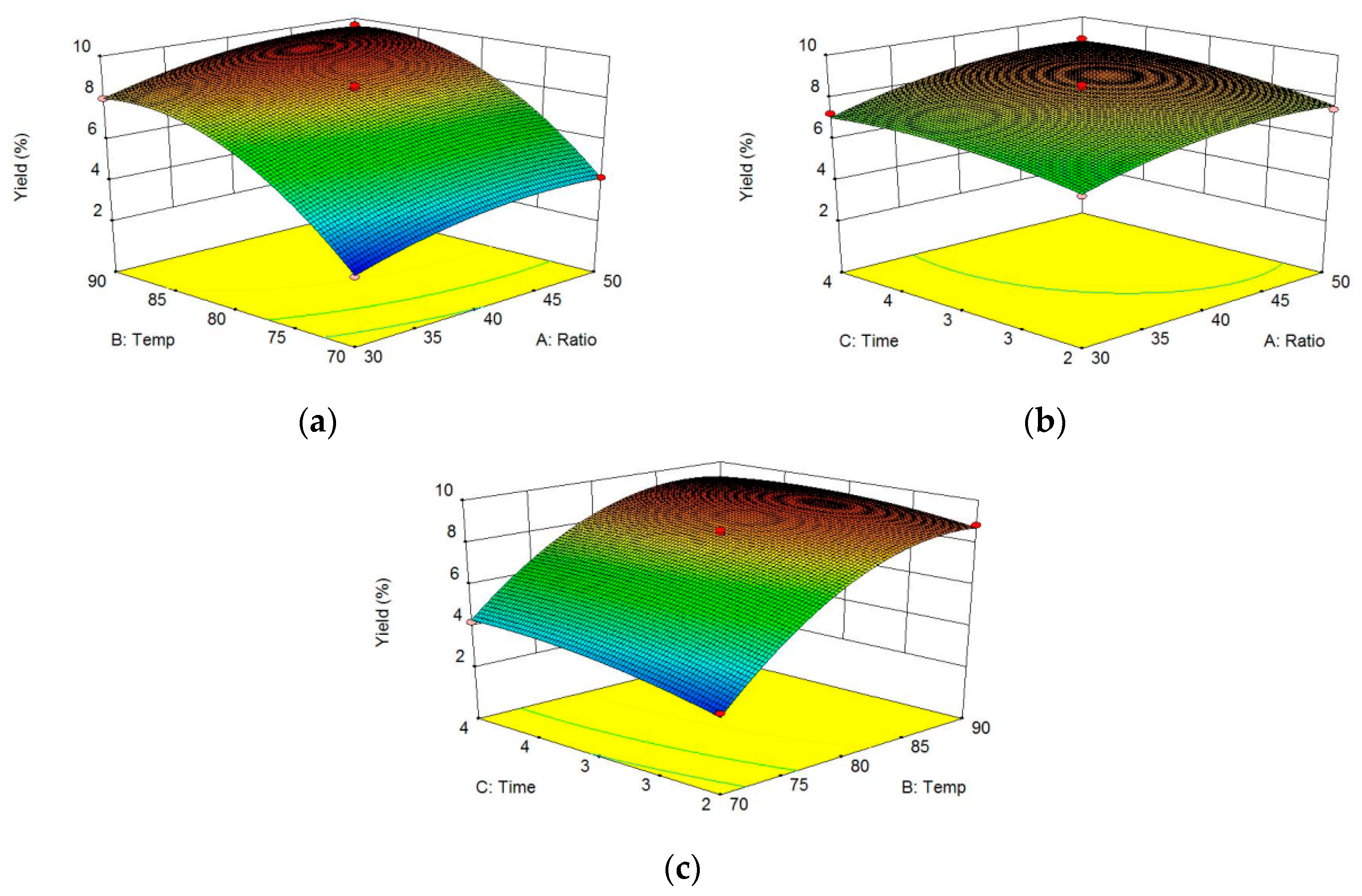
3.2. Box-Behnken Design (BBD) to Optimize Polysaccharide Extraction
3.3. Analysis of the Response Surface
3.4. Purification of Crude Chorella-Arc Polysaccharide
3.5. Antioxidant Properties
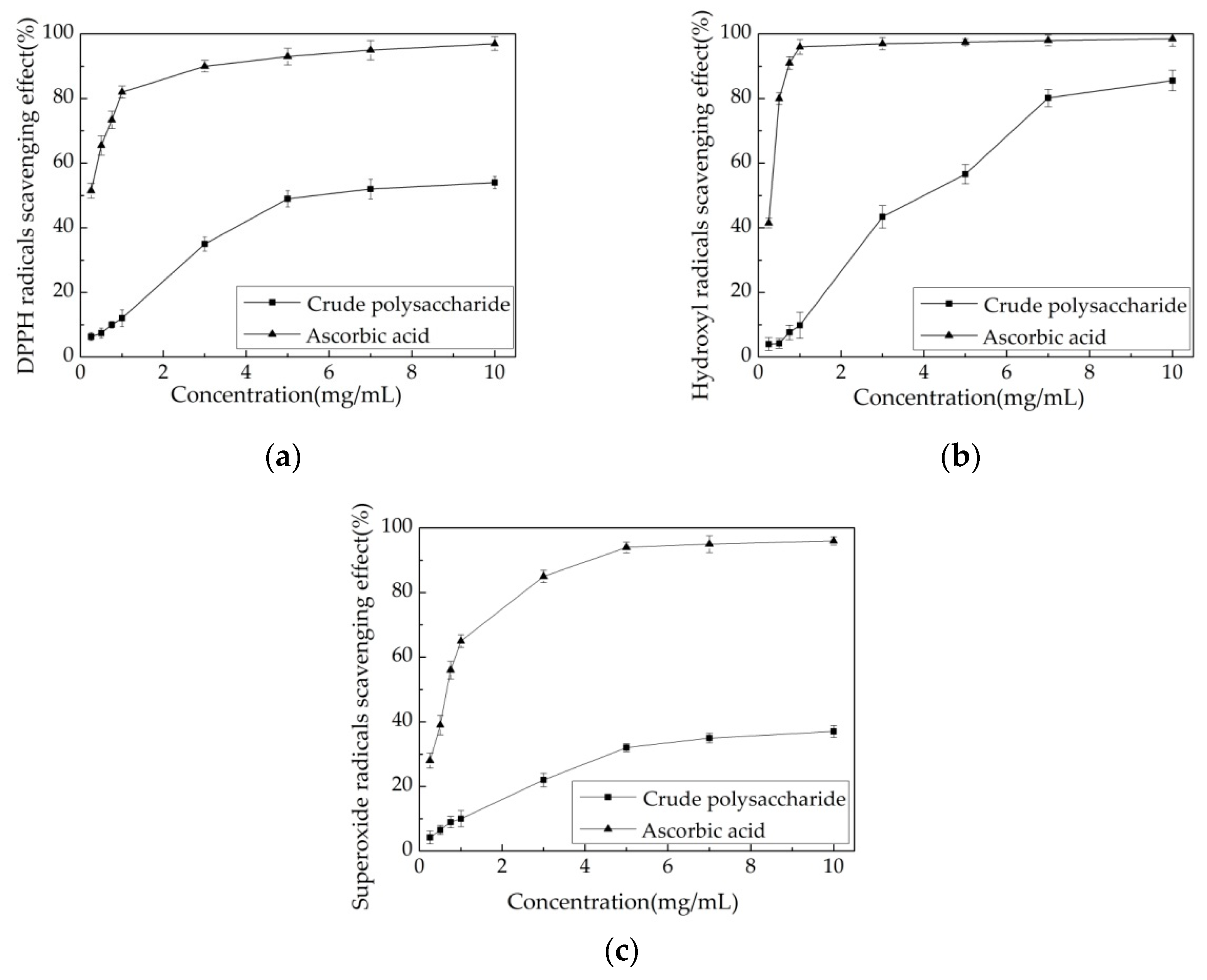
3.5.1. Scavenging Effects of Polysaccharide on DPPH Radical
3.5.2. Scavenging Effects of Polysaccharide on Hydroxyl Radical
3.5.3. Scavenging Effects of the Polysaccharide Extract on Superoxide Radical
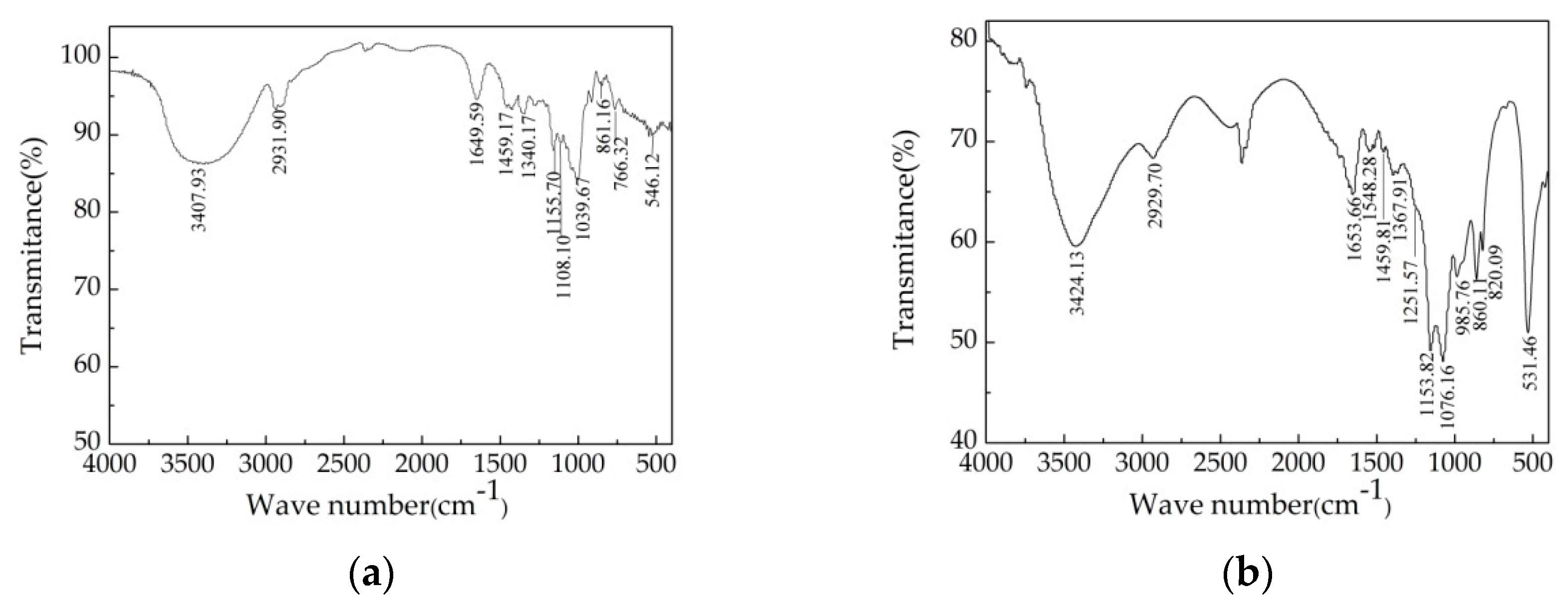
3.6. FTIR Spectroscopic Characterization
3.7. Monosaccharide Composition and Sulfate-Group Content Analysis
3.8. NMR Spectra Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moskovitz, J.; Yim, M.B.; Chock, P.B. Free radicals and disease. Arch. Biochem. Biophys. 2002, 397, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J. Appl. Phycol. 2007, 19, 449–458. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Pan, B.; Xu, J.; Sheng, J.; Shi, Y. Effects of supercritical carbon dioxide extraction conditions on yields and antioxidant activity of Chlorella pyrenoidosa extracts. J. Food Eng. 2007, 80, 997–1001. [Google Scholar] [CrossRef]
- Cornish, M.L.; Garbary, D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae 2010, 25, 155–171. [Google Scholar] [CrossRef]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakrishnan, G.; Vasanthi, H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (marine brown alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Huang, X.; Cheong, K. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.W.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Suárez, E.R.; Kralovec, J.A.; Noseda, M.D.; Ewart, H.S.; Barrow, C.J.; Lumsden, M.D.; Grindley, T.B. Isolation, characterization and structural determination of a unique type of arabinogalactan from an immunostimulatory extract of Chlorella pyrenoidosa. Carbohydr. Res. 2005, 340, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, Y.; Sheng, J.; Hu, Q. In vivo immunomodulatory activity of polysaccharides derived from Chlorella pyrenoidosa. Eur. Food Res. Technol. 2006, 224, 225–228. [Google Scholar] [CrossRef]
- Tabarsa, M.; Shin, I.-S.; Lee, J.H.; Surayot, U.; Park, W.; You, S. An immune-enhancing water-soluble α-glucan from Chlorella vulgaris and structural characteristics. Food Sci. Biotechnol. 2015, 24, 1933–1941. [Google Scholar] [CrossRef]
- Qi, J.; Kim, S.M. Characterization and immunomodulatory activities of polysaccharides extracted from green alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, F.; Shi, M.; Zhang, X.; Zhou, B.; Zhang, Y.; Chen, X. Characterization and biotechnological potential analysis of a new exopolysaccharide from the arctic marine bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef] [PubMed]
- Krembsa, C.; Eickenb, H.; Jungea, K.; Deming, J.W. High concentrations of exopolymeric substances in arctic winter sea ice: Implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep-Sea Res. I 2002, 49, 2163–2181. [Google Scholar] [CrossRef]
- Ahn, J.W.; Hwangbo, K.; Lee, S.Y.; Choi, H.G.; Park, Y.I.; Liu, J.R.; Jeong, W.J. A new arctic Chlorella species for biodiesel production. Bioresour. Technol. 2012, 125, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; He, M.; Yang, W.; Chen, B.; Luo, W.; Zou, S.; Wang, C. The eurythermal adaptivity and temperature tolerance of a newly isolated psychrotolerant arctic Chlorella sp. J. Appl. Phycol. 2016, 28, 877–888. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Pizzoferrato, L.; Liquori, A.M. A physico-chemical study on the polysaccharide ulvan from hot water extraction of the macroalga Ulva. Int. J. Biol. Macromol. 1999, 25, 309–315. [Google Scholar] [CrossRef]
- Balavigneswaran, C.K.; Sujin Jeba Kumar, T.; Moses Packiaraj, R.; Veeraraj, A.; Prakash, S. Anti-oxidant activity of polysaccharides extracted from Isocrysis galbana using RSM optimized conditions. Int. J. Biol. Macromol. 2013, 60, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, J.; Zhang, H.; Zhang, Q.; Lu, J.; Wang, J. Structure elucidation of a polysaccharide from Umbilicaria esculenta and its immunostimulatory activity. PLoS ONE 2016, 11, e0168472. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mao, W.; Chen, Y.; Guo, S.; Li, H.; Qi, X.; Chen, Y.; Xu, J. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydr. Polym. 2009, 78, 117–124. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Xiao, Z.; Huang, Y.; Liu, B. Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Geun Goo, B.; Baek, G.; Jin Choi, D.; Il Park, Y.; Synytsya, A.; Bleha, R.; Ho Seong, D.; Lee, C.G.; Kweon Park, J. Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta. Bioresour. Technol. 2013, 129, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liang, M.; Xu, L.; Li, H.; Zhang, X.; Kang, J.; Zhao, Q.; Zhao, H. Cloning and functional characterization of two abiotic stress-responsive Jerusalem artichoke (Helianthus tuberosus) fructan 1-exohydrolases (1-FEHs). Plant Mol. Biol. 2014, 87, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Pan, R.; Deng, X.; Chen, Y.; Zhao, G.; Wang, C. Separation, purification, anticoagulant activity and preliminary structural characterization of two sulfated polysaccharides from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2014, 49, 1352–1361. [Google Scholar] [CrossRef]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [PubMed]
- Bhadja, P.; Tan, C.-Y.; Ouyang, J.-M.; Yu, K. Repair effect of seaweed polysaccharides with different contents of sulfate group and molecular weights on damaged HK-2 cells. Polymers 2016, 8, 188. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Y.; Yang, H.; Mao, L. Extraction of Angelica sinensis polysaccharides using ultrasound-assisted way and its bioactivity. Int. J. Biol. Macromol. 2016, 88, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, N.; Wang, Y.; Sun, Y.; Lu, K.; Guan, W. Extraction optimization of Bruguiera gymnorrhiza polysaccharides with radical scavenging activities. Carbohydr. Polym. 2013, 96, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sheng, J.C.; Yang, F.M.; Hu, Q.H. Purification and identification of polysaccharide derived from Chlorella pyrenoidosa. Food Chem. 2007, 103, 101–105. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Shu, X.; Du, H.; Li, N.; Wang, J. Extraction, characterization and immunological activity of polysaccharides from Rhizoma gastrodiae. Int. J. Mol. Sci. 2016, 17, 1011. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, B.; Wang, X.; Zhang, J. Preparation and characterization of sulfated galactomannan from guar gum: Optimization of reaction conditions by BBD and molecule conformational studies. J. Taiwan Inst. Chem. Eng. 2012, 43, 889–896. [Google Scholar] [CrossRef]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ben Hafsa, M.; Ben Ismail, M.; Garrab, M.; Aly, R.; Gagnon, J.; Naghmouchi, K. Antimicrobial, antioxidant, cytotoxic and anticholinesterase activities of water-soluble polysaccharides extracted from microalgae Isochrysis galbana and Nannochloropsis oculata. J. Serb. Chem. Soc. 2017, 82, 509–522. [Google Scholar]
- Custódio, L.; Soares, F.; Pereira, H.; Rodrigues, M.J.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Botryococcus braunii and Nannochloropsis oculata extracts inhibit cholinesterases and protect human dopaminergic SH-SY5Y cells from H2O2-induced cytotoxicity. J. Appl. Phycol. 2014, 27, 839–848. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Xu, Y.; Song, S.; Wei, Y.; Wang, F.; Zhao, M.; Guo, J.; Zhang, J. Sulfated modification of the polysaccharide from Sphallerocarpus gracilis and its antioxidant activities. Int. J. Biol. Macromol. 2016, 87, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gao, B.; Li, A.; Xiong, J.; Ao, Z.; Zhang, C. Preliminary characterization, antioxidant properties and production of chrysolaminarin from marine diatom Odontella aurita. Mar. Drugs 2014, 12, 4883–4897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xu, J.; Xu, X. Bioactivity of fucoidan extracted from Laminaria japonica using a novel procedure with high yield. Food Chem. 2018, 245, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Galley, H.F.; Webster, N.R. Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 2003, 90, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wei, X.; Xu, J.; Chen, B.; Zhao, D.; Cui, S.; Zhou, T. Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food Chem. 2017, 215, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, Q.; Zhou, X.; Zeng, F.; Lu, X. Extraction of Opuntia dillenii Haw. polysaccharides and their antioxidant activities. Molecules 2016, 21, 1612. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Kanmani, P.; Kumar, R.S.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Production and purification of a novel exopolysaccharide from lactic acid bacterium Streptococcus phocae PI80 and its functional characteristics activity in vitro. Bioresour. Technol. 2011, 102, 4827–4833. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Tang, Z.; Zhang, X.; Zhong, Y.; Yao, S.; Wang, L.; Lin, C.; Luo, X. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cai, F.; Yu, Z.; Zhang, L.; Li, X.; Yang, Y.; Liu, G. Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chem. 2016, 194, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, R.; Zhang, S.; Zhu, W.; Tang, J.; Liu, J.; Chen, P.; Zhang, D.; Ye, W.; Zheng, Y. Polysaccharides from Panax japonicus C.A. Meyer and their antioxidant activities. Carbohydr. Polym. 2014, 101, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Fang, J.M.; Goodwin, A.; Lawther, J.M.; Bolton, A.J. Fractionation and characterization of polysaccharides from abaca fibre. Carbohydr. Polym. 1998, 37, 351–359. [Google Scholar] [CrossRef]
- Fimbres-Olivarria, D.; Carvajal-Millan, E.; Lopez-Elias, J.A.; Martinez-Robinson, K.G.; Miranda-Baeza, A.; Martinez-Cordova, L.R.; Enriquez-Ocaña, F.; Valdez-Holguin, J.E. Chemical characterization and antioxidant activity of sulfated polysaccharides from Navicula sp. Food Hydrocoll. 2018, 75, 229–236. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Wang, A.; Yu, Z.; Xu, Y.; Yang, Y. Purification, characterization and bioactivity determination of a novel polysaccharide from pumpkin (Cucurbita moschata) seeds. Food Hydrocoll. 2017, 66, 357–364. [Google Scholar] [CrossRef]
- Sheng, J.; Yu, F.; Xin, Z.; Zhao, L.; Zhu, X.; Hu, Q. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007, 105, 533–539. [Google Scholar] [CrossRef]
- He, L.; Ji, P.; Cheng, J.; Wang, Y.; Qian, H.; Li, W.; Gong, X.; Wang, Z. Structural characterization and immunostimulatory activity of a novel protein-bound polysaccharide produced by Hirsutella sinensis Liu, Guo, Yu & Zeng. Food Chem. 2013, 141, 946–953. [Google Scholar] [PubMed]
- Yu, Z.; Liu, L.; Xu, Y.; Wang, L.; Teng, X.; Li, X.; Dai, J. Characterization and biological activities of a novel polysaccharide isolated from raspberry (Rubus idaeus L.) fruits. Carbohydr. Polym. 2015, 132, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, H.; Lv, X.; Wang, Y.; Gao, H.; Wang, M. Protection of chronic renal failure by a polysaccharide from Cordyceps sinensis. Fitoterapia 2010, 81, 397–402. [Google Scholar] [CrossRef] [PubMed]
 ) and Coomassie brilliant blue (
) and Coomassie brilliant blue ( ). There were three major fractions in the crude Chlorella-Arc polysaccharide (P-I, P-II, and P-III). (b) Sephadex G-100 chromatogram elution profile of P-II; (c) Sephadex G-100 chromatogram elution profile of P-IIa; (d) UV-Vis spectrum of P-IIa
). There were three major fractions in the crude Chlorella-Arc polysaccharide (P-I, P-II, and P-III). (b) Sephadex G-100 chromatogram elution profile of P-II; (c) Sephadex G-100 chromatogram elution profile of P-IIa; (d) UV-Vis spectrum of P-IIa
 ) and Coomassie brilliant blue (
) and Coomassie brilliant blue ( ). There were three major fractions in the crude Chlorella-Arc polysaccharide (P-I, P-II, and P-III). (b) Sephadex G-100 chromatogram elution profile of P-II; (c) Sephadex G-100 chromatogram elution profile of P-IIa; (d) UV-Vis spectrum of P-IIa
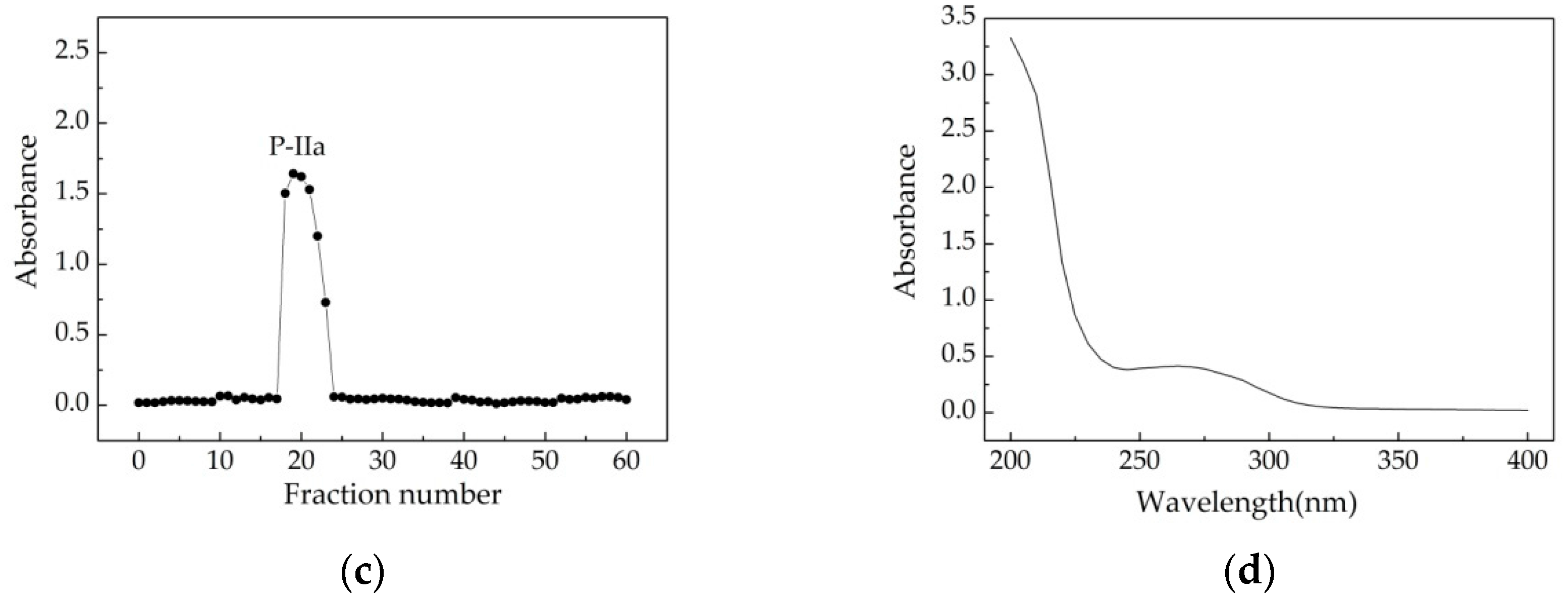
). There were three major fractions in the crude Chlorella-Arc polysaccharide (P-I, P-II, and P-III). (b) Sephadex G-100 chromatogram elution profile of P-II; (c) Sephadex G-100 chromatogram elution profile of P-IIa; (d) UV-Vis spectrum of P-IIa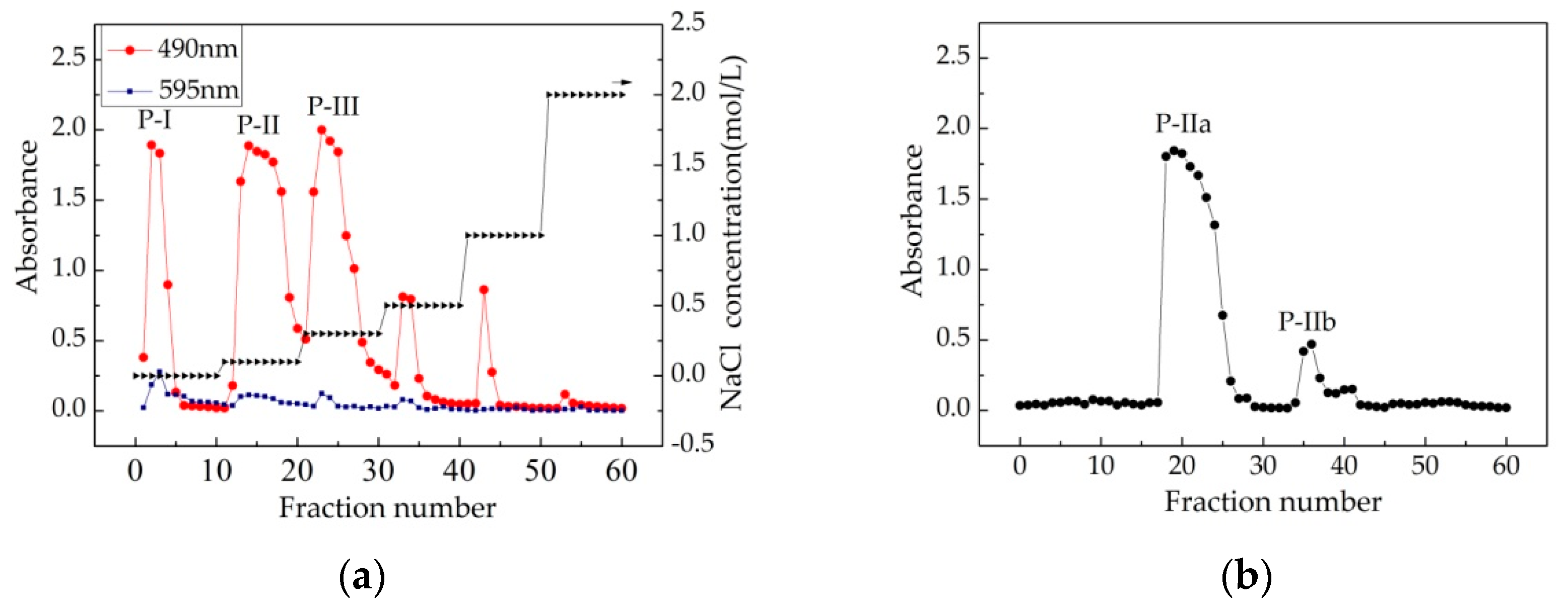


| Factors | Code | Actual | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Ratio of water to dried microalgae powder (mL/g) | X1 | x1 | 30 | 40 | 50 |
| Extraction temperature (°C) | X2 | x2 | 70 | 80 | 90 |
| Extraction time (h) | X3 | x3 | 2 | 3 | 4 |
| Run | X1 (mL:g) | X2 (°C) | X3 (h) | Crude Polysaccharides Yield(%) | |
|---|---|---|---|---|---|
| Actual | Predicted | ||||
| 1 | 40 | 80 | 3 | 8.35 | 8.42 |
| 2 | 30 | 80 | 4 | 7.25 | 7.07 |
| 3 | 30 | 70 | 3 | 2.59 | 2.66 |
| 4 | 30 | 90 | 3 | 7.99 | 8.01 |
| 5 | 40 | 80 | 3 | 8.50 | 8.42 |
| 6 | 40 | 80 | 3 | 8.61 | 8.42 |
| 7 | 40 | 80 | 3 | 8.34 | 8.42 |
| 8 | 50 | 90 | 3 | 9.65 | 9.58 |
| 9 | 40 | 70 | 4 | 4.20 | 4.31 |
| 10 | 50 | 80 | 4 | 8.86 | 8.76 |
| 11 | 50 | 70 | 3 | 4.16 | 4.14 |
| 12 | 50 | 80 | 2 | 7.45 | 7.63 |
| 13 | 40 | 70 | 2 | 3.04 | 2.87 |
| 14 | 40 | 80 | 3 | 8.30 | 8.42 |
| 15 | 40 | 90 | 4 | 9.07 | 9.24 |
| 16 | 40 | 90 | 2 | 8.84 | 8.73 |
| 17 | 30 | 80 | 2 | 6.17 | 6.17 |
| Sample | Antioxidant Activity (%) | ||
|---|---|---|---|
| DPPH Radical | Hydroxyl Radical | Superoxide Radical | |
| Crude polysaccharide | 49.10 ± 2.50 c | 56.60 ± 2.50 d | 32.10 ± 1.20 c |
| P-I | 42.30 ± 1.70 b | 45.10 ± 2.10 b | 23.10 ± 1.30 b |
| P-III | 30.80 ± 1.20 a | 34.90 ± 1.20 a | 20.20 ± 1.50 a |
| P-II | 55.40 ± 1.30 d | 68.30 ± 2.40 e | 40.10 ± 1.40 d |
| P-IIa | 60.20 ± 1.20 e | 72.10 ± 1.50 f | 42.20 ± 1.60 d |
| P-IIb | 30.70 ± 1.10 a | 52.20 ± 1.60 c | 31.30 ± 1.20 c |
| Ascorbic acid | 93.00 ± 2.60 f | 97.50 ± 1.00 g | 94.00 ± 1.70 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; He, M.; Gu, C.; Wei, D.; Liang, Y.; Yan, J.; Wang, C. Extraction Optimization, Purification, Antioxidant Activity, and Preliminary Structural Characterization of Crude Polysaccharide from an Arctic Chlorella sp. Polymers 2018, 10, 292. https://doi.org/10.3390/polym10030292
Song H, He M, Gu C, Wei D, Liang Y, Yan J, Wang C. Extraction Optimization, Purification, Antioxidant Activity, and Preliminary Structural Characterization of Crude Polysaccharide from an Arctic Chlorella sp. Polymers. 2018; 10(3):292. https://doi.org/10.3390/polym10030292
Chicago/Turabian StyleSong, Hong, Meilin He, Chuankun Gu, Dong Wei, Yuqi Liang, Junmei Yan, and Changhai Wang. 2018. "Extraction Optimization, Purification, Antioxidant Activity, and Preliminary Structural Characterization of Crude Polysaccharide from an Arctic Chlorella sp." Polymers 10, no. 3: 292. https://doi.org/10.3390/polym10030292




