Multi-Responsive Behaviors of Copolymers Bearing N-Isopropylacrylamide with or without Phenylboronic Acid in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Copolymer Synthesis and Identification
2.3. Smart Responsive Behaviors of Copolymers
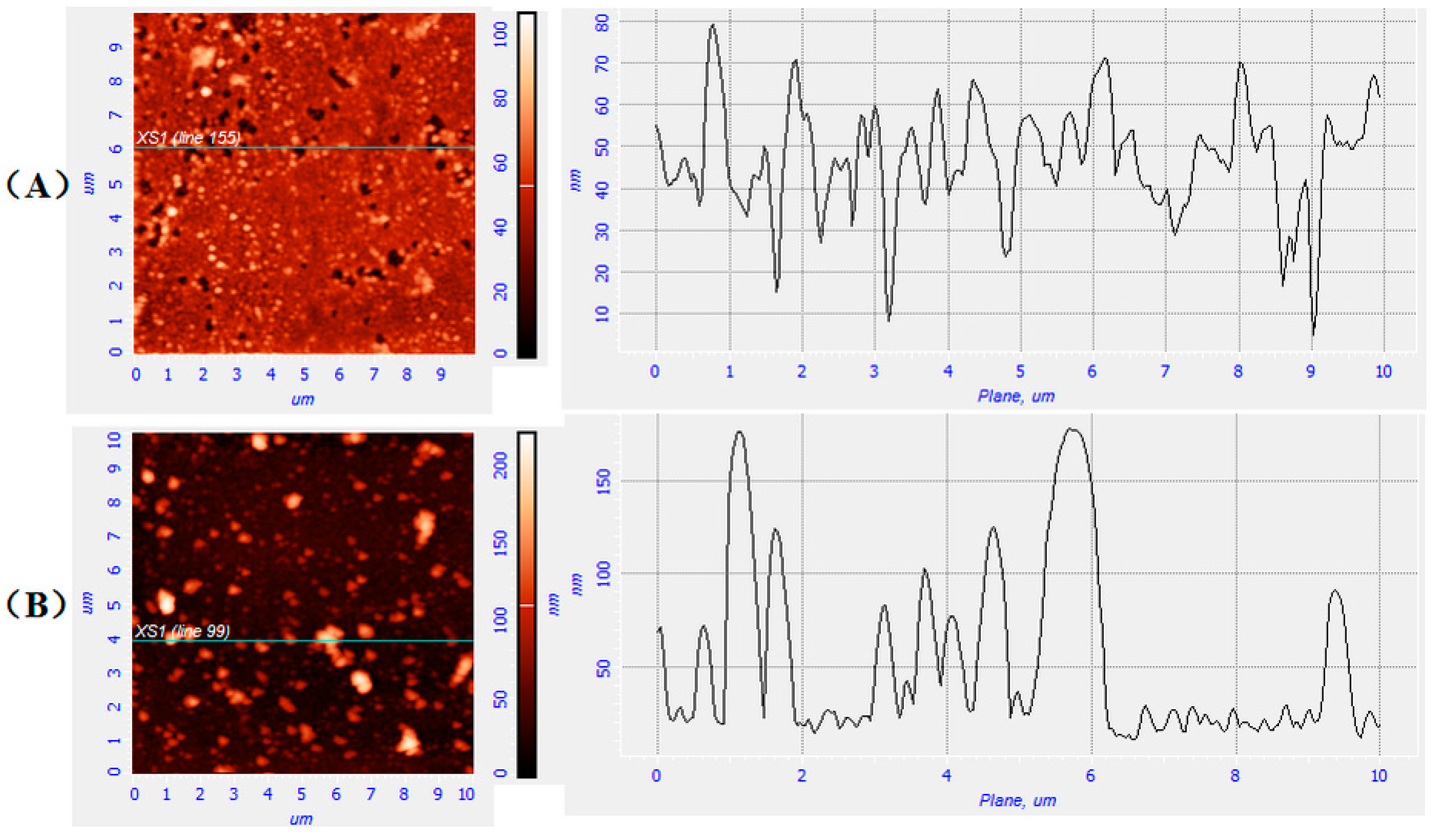
2.4. Morphology Analysis of Glass Surfaces with Copolymer Deposition
3. Results and Discussion
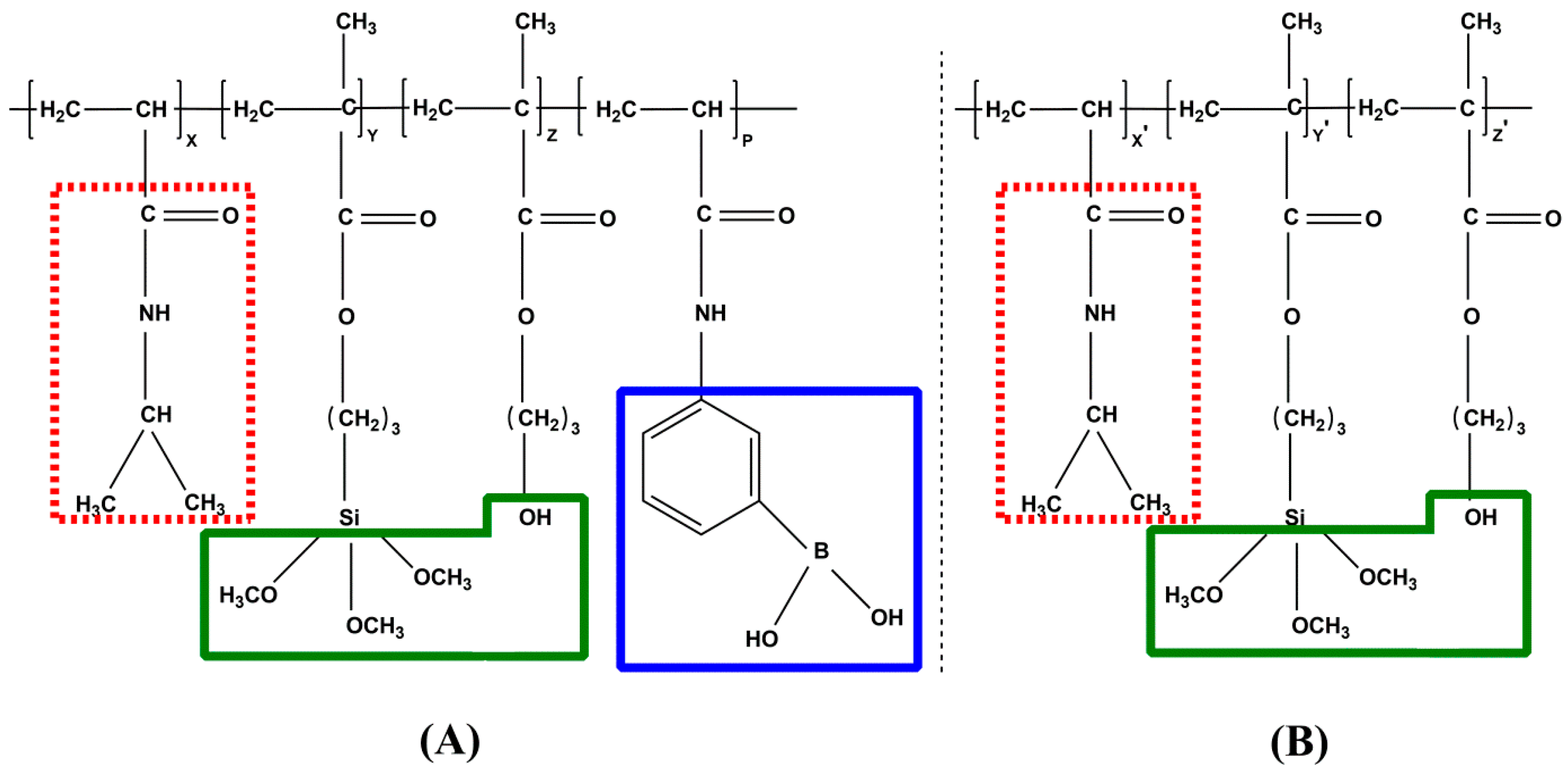
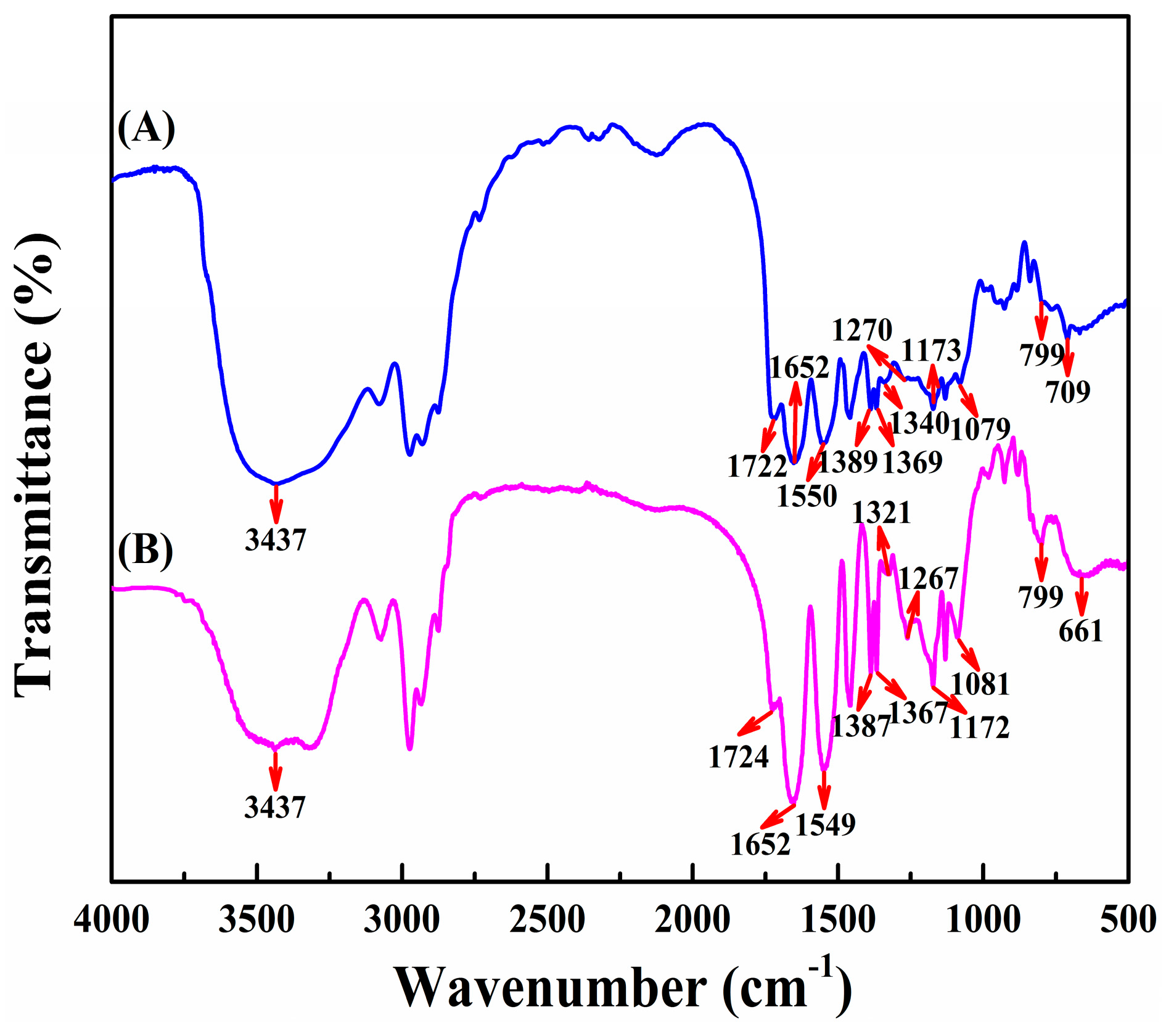
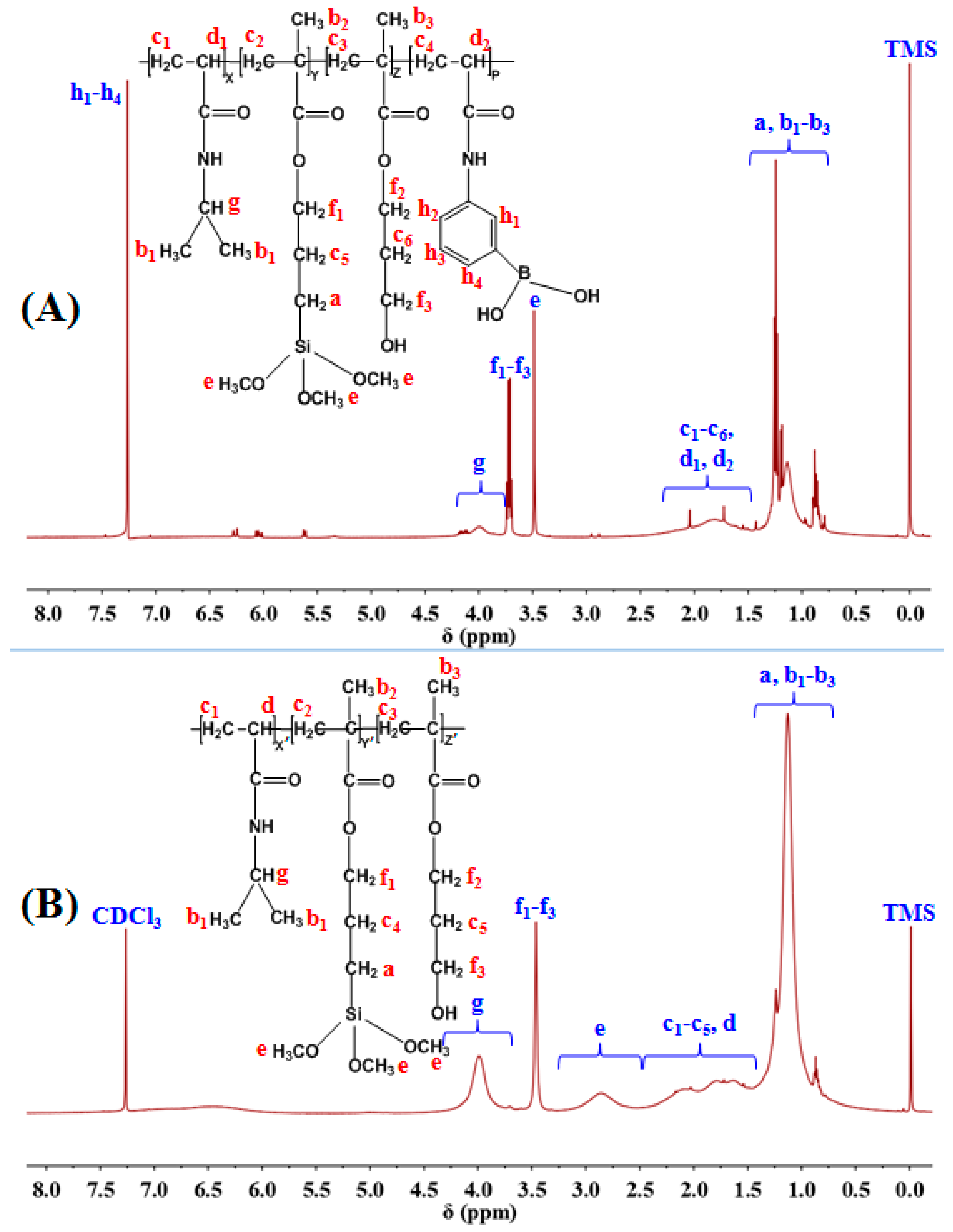
3.1. Copolymer Synthesis and Identification
3.1.1. Synthesis of PNAHT and PNHT Copolymers
3.1.2. Identification of PNAHT and PNHT Copolymers
3.2. Smart Responsive Behaviors of Copolymers
3.2.1. Copolymers in Absolute Ethanol
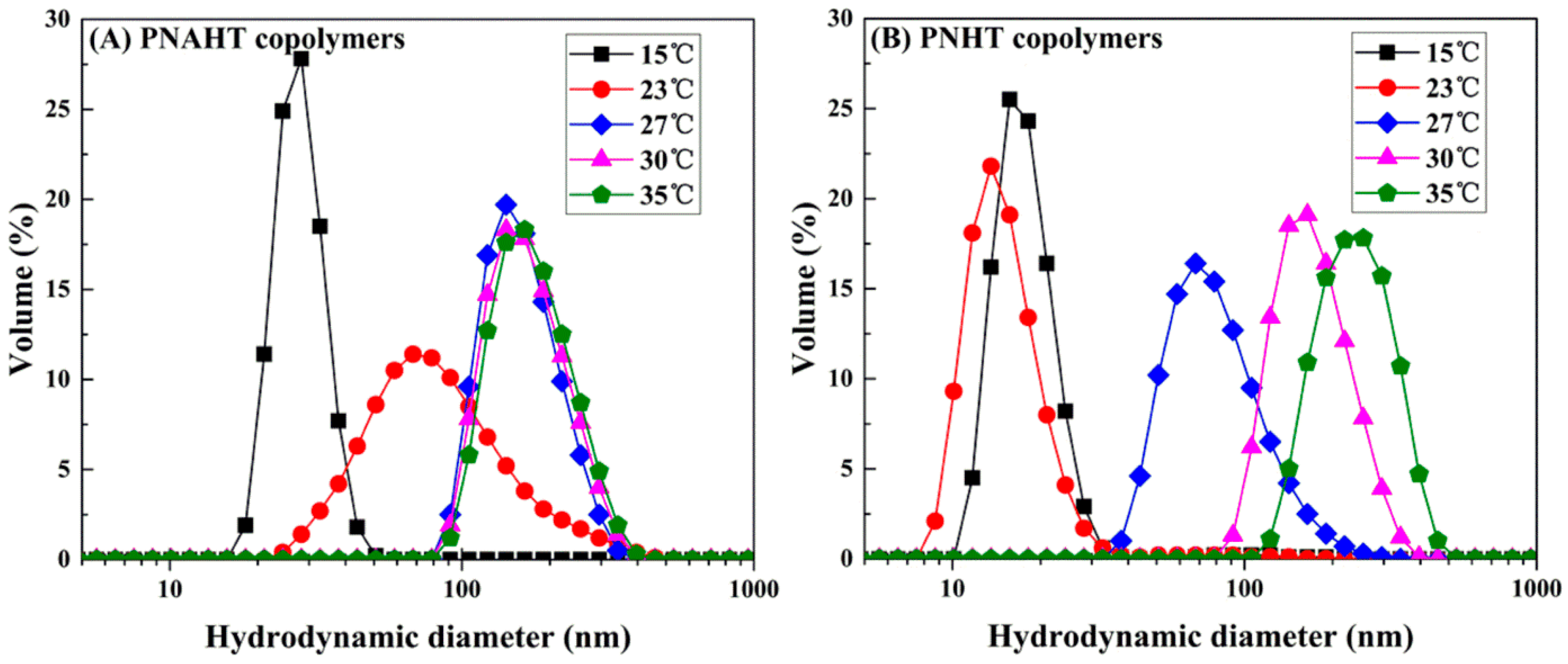
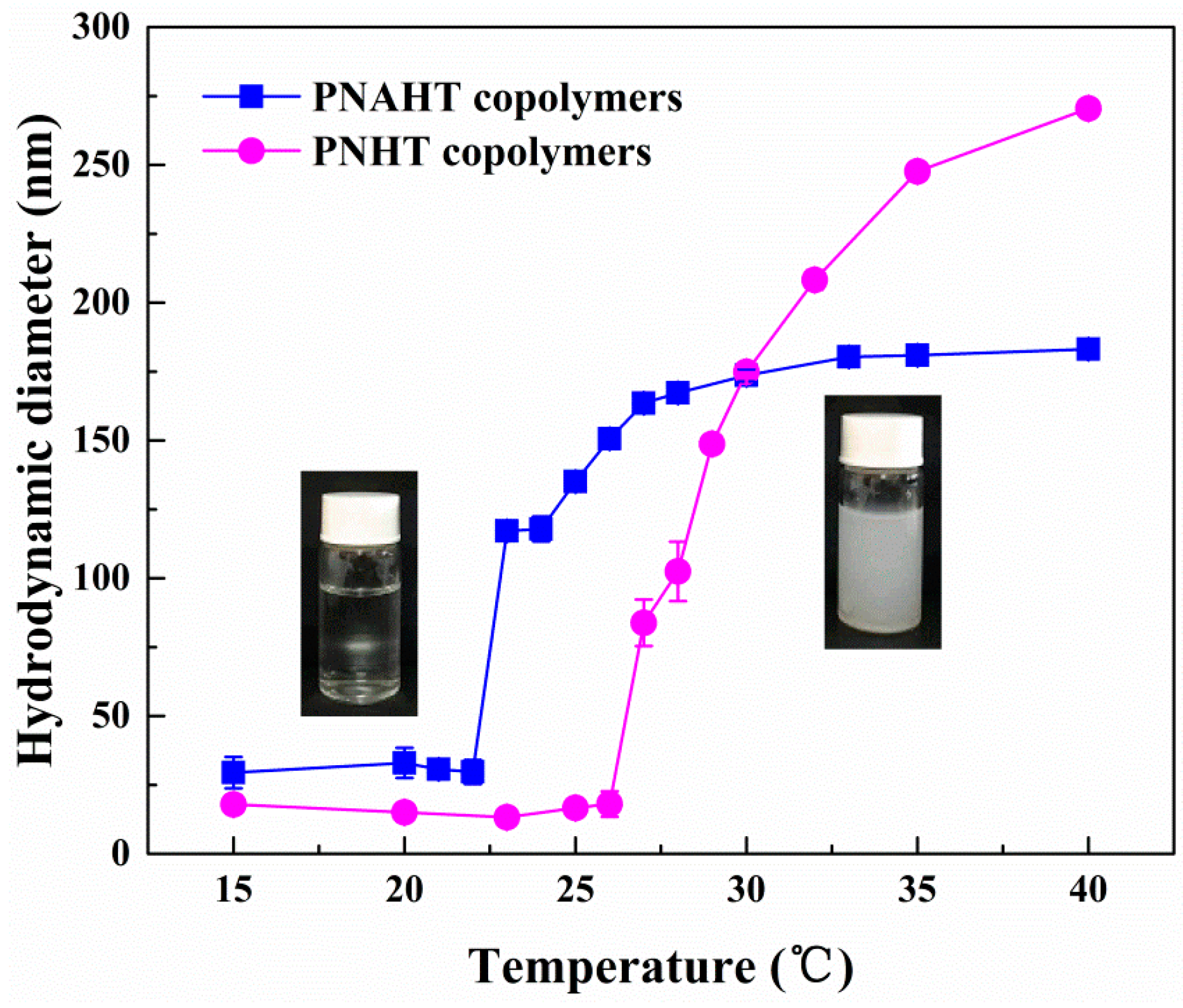
3.2.2. Copolymers in UHQ Water
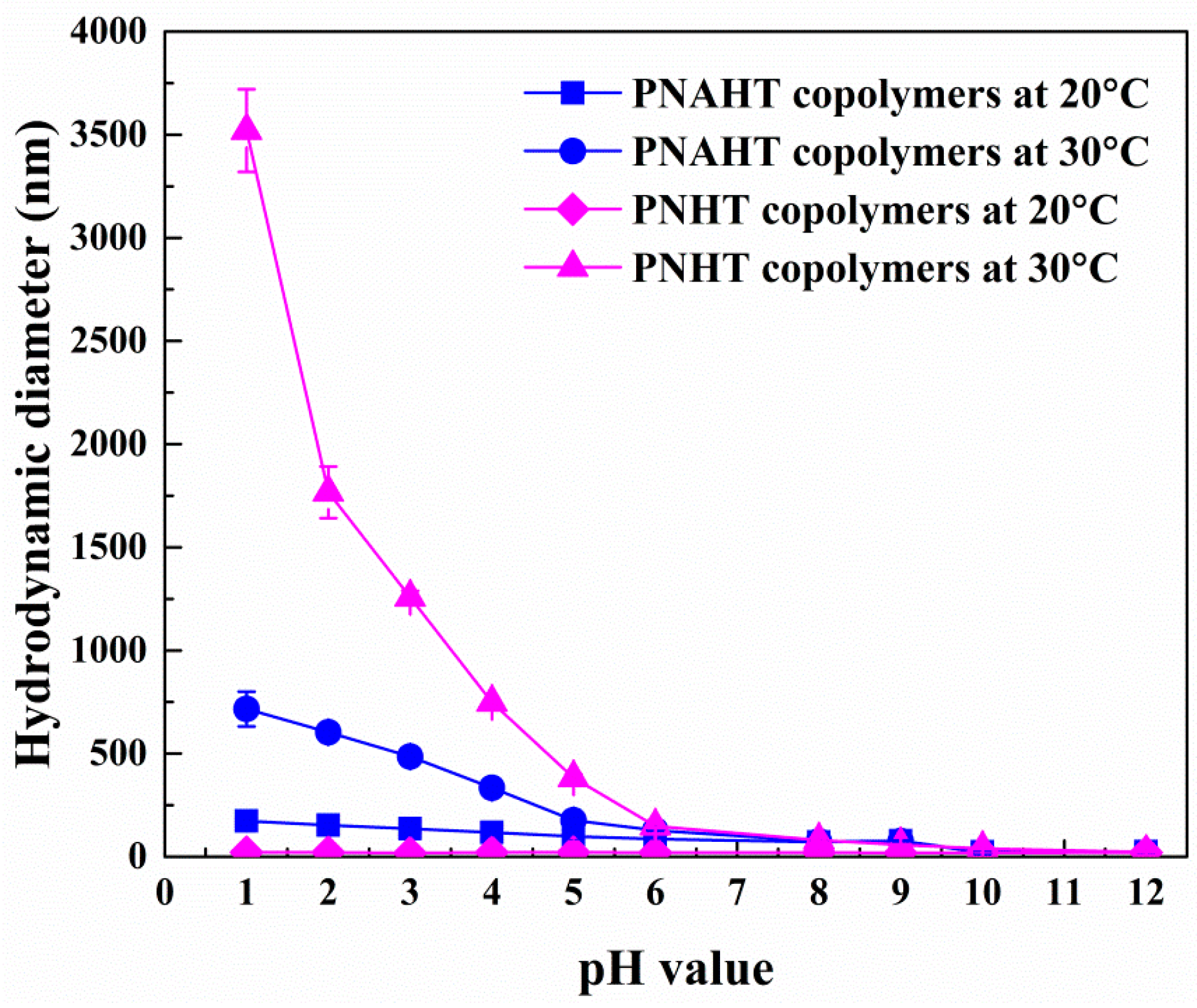
3.2.3. Copolymers in Acidic or Alkaline Solution
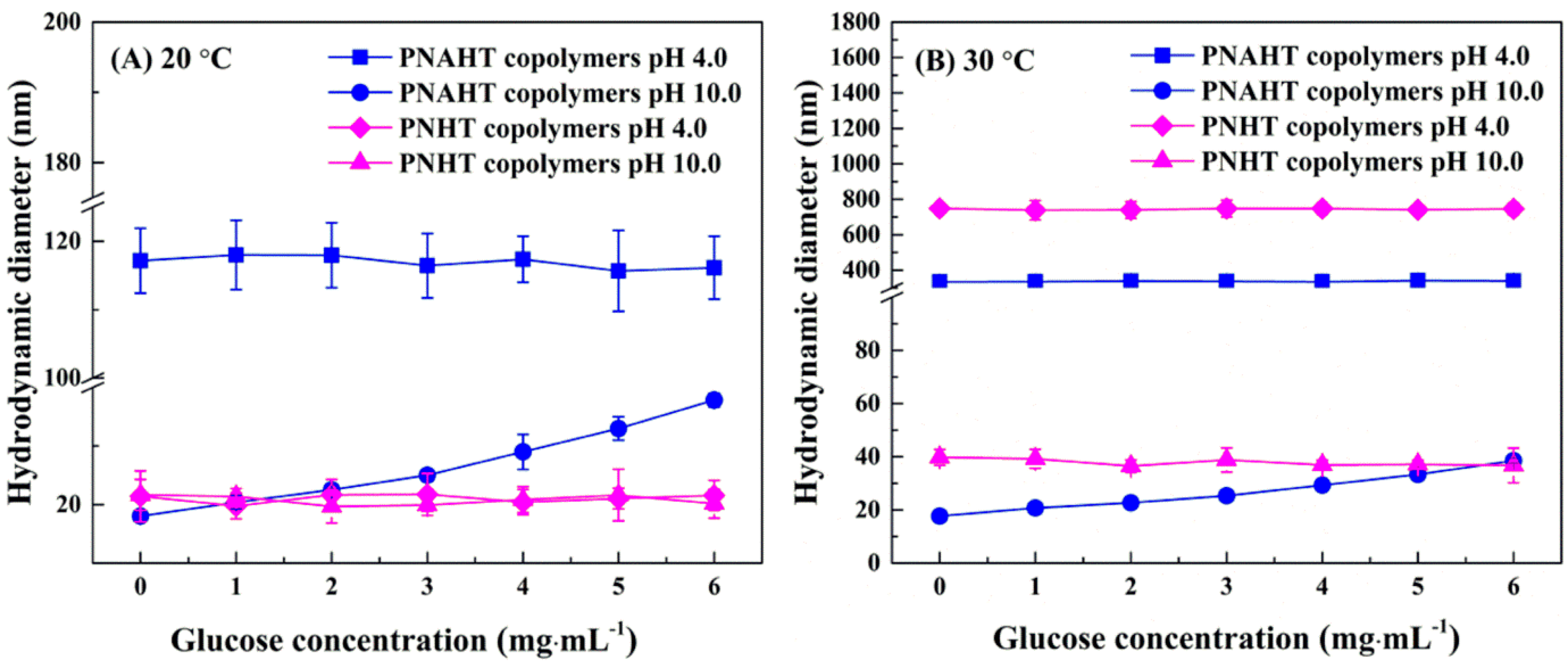
3.2.4. Copolymers in Glucose Solution
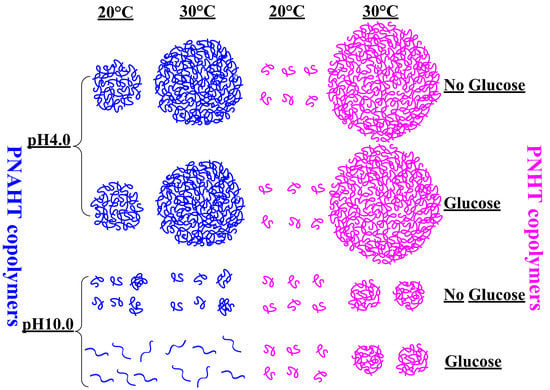
3.2.5. Smart Responsive Behaviors of Copolymers
3.3. Morphology and Size of Copolymer Particles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, H.W.; Lee, A.W.; Huang, C.H.; Chen, J.K. Characterization of poly(N-isopropylacrylamide)-nucleobase supramolecular complexes featuring bio-multiple hydrogen bonds. Soft Matter 2014, 10, 8330–8340. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.D.; Guerrero, S.; Benito, M.; Fernández, A.; Teijón, C.; Olmo, R.; Katime, I.; Teijón, J.M. In vitro and in vivo evaluation of a folate-targeted copolymeric submicrohydrogel based on N-isopropylacrylamide as 5-Fluorouracil delivery system. Polymers 2011, 3, 1107–1125. [Google Scholar] [CrossRef]
- Pandiyarajan, C.K.; Genzer, J. Effect of network density in surface-anchored poly(N-isopropylacrylamide) hydrogels on adsorption of fibrinogen. Langmuir 2017, 33, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, C.A. Poly(N-isopropylacrylamide) modified polydopamine as a temperature-responsive surface for cultivation and harvest of mesenchymal stem cells. Biomater. Sci. 2017, 5, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.L.; Genever, P.G.; Rimmer, S.; Coles, M.C. Collagen-poly(N-isopropylacrylamide) hydrogels with tunable properties. Biomacromolecules 2016, 17, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.J.; Yang, H.; Li, Z.; Liu, G.; Sun, X.C.; Liu, X.J.; An, Y.L.; Shi, L.Q. Phenylboronic acid-based complex micelles with enhanced glucose-responsiveness at physiological pH by complexation with glycopolymer. Biomacromolecules 2012, 13, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, B.; Cheng, X.; Wang, J.; Tang, R. Phenylboronic acid-decorated gelatin nanoparticles for enhanced tumor targeting and penetration. Nanotechnology 2016, 27, 385101. [Google Scholar] [CrossRef] [PubMed]
- Bajgrowicz-Cieslak, M.; Alqurashi, Y.; Elshereif, M.I.; Yetisen, A.K.; Hassan, M.U.; Butt, H. Optical glucose sensors based on hexagonally-packed 2.5-dimensional photonic concavities imprinted in phenylboronic acid functionalized hydrogel films. RSC Adv. 2017, 7, 53916–53924. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.Y.; Meng, C.; Liu, Z.; Sun, L.; Zhang, X.S.; Zhang, M.K.; Sun, M.R.; Ma, L.W.; Liu, M.Z.; Wei, H. Synthesis and phase transition of poly(N-isopropylacrylamide)-based thermo-sensitive cyclic brush polymer. Polymers 2017, 9, 301. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Courier Dover Publications: New York, NY, USA, 2000; ISBN 0-486-41155-9. [Google Scholar]
- Qiu, X.P.; Kwan, C.M.S.; Wu, C. Laser light scattering study of the formation and structure of poly(N-isopropylacrylamide-co-acrylic acid) nanoparticles. Macromolecules 1997, 30, 6090–6094. [Google Scholar] [CrossRef]
- Özyürek, Z.; Voit, B.; Krahl, F.; Arndt, K.-F. Thermoresponsive aggregation behavior of NiPAAm/glyco monomer block copolymers studied by dynamic light scattering. e-Polymers 2010, 10, 482–494. [Google Scholar] [CrossRef]
- Śliwa, T.; Jarzębski, M. Dynamic light scattering investigation of Pnipam-Co-Maa microgel solution. Curr. Top. Biophys. 2015, 37, 29–33. [Google Scholar] [CrossRef]
- Wang, D.; Liu, T.; Yin, J.; Liu, S.Y. Stimuli-responsive fluorescent poly(N-isopropylacrylamide) microgels labeled with phenylboronic acid moieties as multifunctional ratiometric probes for glucose and temperatures. Macromolecules 2011, 44, 2282–2290. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Wu, W.T.; Zhou, S.Q.; Siddiq, M. Engineering of phenylboronic acid based glucose-sensitive microgels with 4-Vinylpyridine for working at physiological pH and temperature. Macromol. Chem. Phys. 2011, 212, 1510–1514. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Khan, A.; Siddiq, M. Temperature-induced volume change and glucose sensitivity of poly[(N-isopropylacry-lamide)-co-acrylamide-co-(phenylboronic acid)] microgels. Polym. Int. 2011, 60, 1481–1486. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, L.; Yu, H.J.; Chen, Z.F. The synthesis and responsive properties of novel glucose-responsive microgels. Polym. Sci. Ser. A 2012, 54, 209–213. [Google Scholar] [CrossRef]
- Tang, Y.C.; Wu, J.H.; Duan, J.X. The micellization and dissociation transitions of thermo-, pH- and sugar-sensitive block copolymer investigated by laser light scattering. eXPRESS Polym. Lett. 2012, 6, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, T.Q.; Song, K.D.; Wu, S.; Fan, X. Effect of intermolecular and intramolecular forces on hydrodynamic diameters of poly(N-isopropylacrylamide) copolymers in aqueous solutions. J. Appl. Polym. Sci. 2013, 127, 4280–4287. [Google Scholar] [CrossRef]
- Wedel, B.; Hertle, Y.; Wrede, O.; Bookhold, J.; Hellweg, T. Smart homopolymer microgels: Influence of the monomer structure on the particle properties. Polymers 2016, 8, 162. [Google Scholar] [CrossRef]
- Uğuzdoğan, E.; Denkbaş, E.B.; Tuncel, A. RNA-sensitive N-isopropylacrylamide/vinylphenylboronic acid random copolymer. Macromol. Biosci. 2002, 2, 214–222. [Google Scholar] [CrossRef]
- Dinçer, S.; Köseli, V.; Kesim, H.; Rzaev, Z.M.O.; Pişkin, E. Radical copolymerization of N-isopropylacrylamide with anhydrides of maleic and citraconic acids. Eur. Polym. J. 2002, 38, 2143–2152. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, J.R.; Lewis, A.L.; Vick, T.A.; Stratford, P.W. Swelling of zwitterionic polymer films characterized by spectroscopic ellipsometry. Macromolecules 2001, 34, 8768–8776. [Google Scholar] [CrossRef]
- Hoare, T.; Pelton, R. Engineering glucose swelling responses in poly(N-isopropylacrylamide)-based microgels. Macromolecules 2007, 40, 670–678. [Google Scholar] [CrossRef]
- Yang, L.; Pan, F.; Zhao, X.B.; Yaseen, M.; Padia, F.; Coffey, P.; Freund, A.; Yang, L.Y.; Liu, T.Q.; Ma, X.H.; et al. Thermoresponsive copolymer nanofilms for controlling cell adhesion, growth, and detachment. Langmuir 2010, 26, 17304–17314. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Guan, Y.; Zhang, Y.J. Contraction-type glucose-sensitive microgel functionalized with a 2-substituted phenylboronic acid ligand. Polym. Chem. 2014, 5, 1782–1790. [Google Scholar] [CrossRef]
- Brewer, S.H.; Allen, A.M.; Lappi, S.E.; Chasse, T.L.; Briggman, K.A.; Gorman, C.B.; Franzen, S. Infrared detection of a phenylboronic acid terminated alkane thiol monolayer on gold surfaces. Langmuir 2004, 20, 5512–5520. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.P.; Wang, Y.X.; Wei, Y.; Li, G. Preparation of uniform-sized and dual stimuli-responsive microspheres of poly(N-isopropylacrylamide)/poly(acrylic acid) with semi-IPN structure by one-step method. Polymers 2016, 8, 90. [Google Scholar] [CrossRef]
- Khan, A.; Alhoshan, M. Preparation and characterization of pH-responsive and thermoresponsive hybrid microgel particles with gold nanorods. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 39–46. [Google Scholar] [CrossRef]
| Group | Molar Ratio | Mu (g·mol−1) | Mn (g·mol−1) | Mw (g·mol−1) | Mw/Mn | DP | |||
|---|---|---|---|---|---|---|---|---|---|
| NIPAAm | TMSPM | HPM | AAPBA | ||||||
| PNAHT | X | Y | Z | P | 2846.76 | 15182 | 24061 | 1.584 | 6 |
| 20 | 1 | 1 | 1 | ||||||
| PNHT | X′ | Y′ | Z′ | P′ | 2655.77 | 19475 | 29115 | 1.495 | 8 |
| 20 | 1 | 1 | 0 | ||||||
| Item | pH | Glucose | 20 °C | 30 °C |
|---|---|---|---|---|
| PNAHT copolymers | 4.0 | Absence |  |  |
| Presence |  |  | ||
| 10.0 | Absence |  |  | |
| Presence |  |  | ||
| PNHT copolymers | 4.0 | Absence |  |  |
| Presence |  |  | ||
| 10.0 | Absence |  |  | |
| Presence |  |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, L.; Fan, X.; Wang, F.; Zhang, J.; Wang, Z. Multi-Responsive Behaviors of Copolymers Bearing N-Isopropylacrylamide with or without Phenylboronic Acid in Aqueous Solution. Polymers 2018, 10, 293. https://doi.org/10.3390/polym10030293
Li J, Yang L, Fan X, Wang F, Zhang J, Wang Z. Multi-Responsive Behaviors of Copolymers Bearing N-Isopropylacrylamide with or without Phenylboronic Acid in Aqueous Solution. Polymers. 2018; 10(3):293. https://doi.org/10.3390/polym10030293
Chicago/Turabian StyleLi, Jiaxing, Lei Yang, Xiaoguang Fan, Fei Wang, Jing Zhang, and Zhanyong Wang. 2018. "Multi-Responsive Behaviors of Copolymers Bearing N-Isopropylacrylamide with or without Phenylboronic Acid in Aqueous Solution" Polymers 10, no. 3: 293. https://doi.org/10.3390/polym10030293