Structure and Properties Study of PA6 Nanocomposites Flame Retarded by Aluminium Salt of Diisobutylphosphinic Acid and Different Organic Montmorillonites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
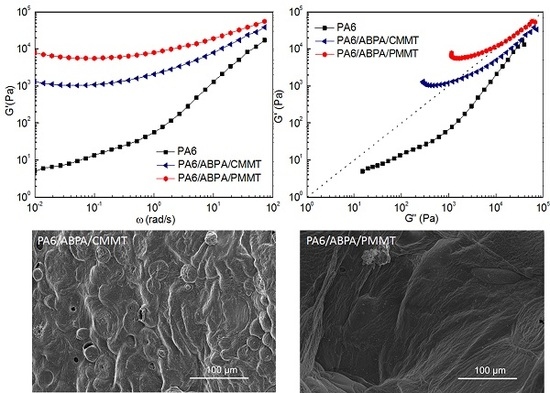
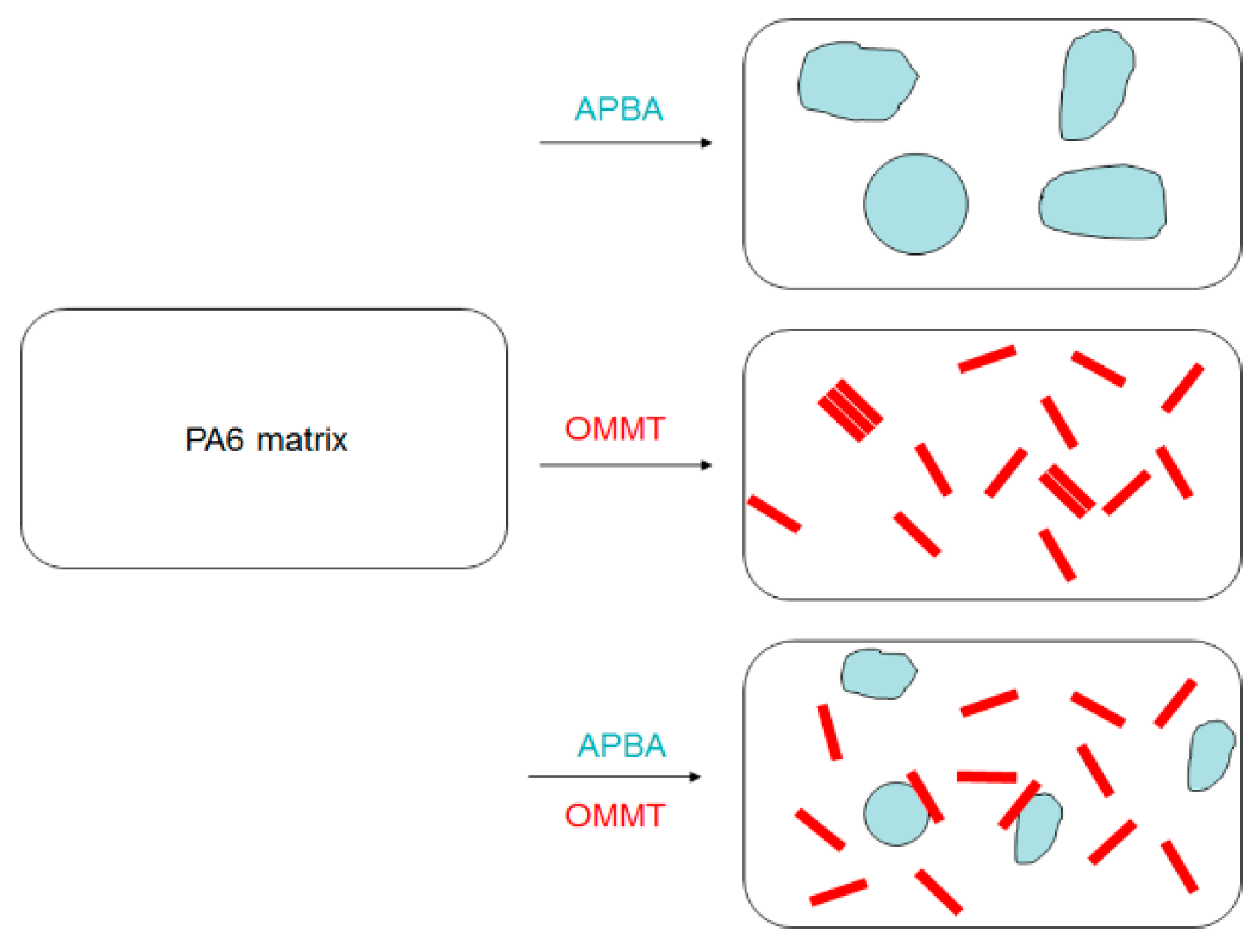
3.1. Microstructure Characterization of PA6 Composites
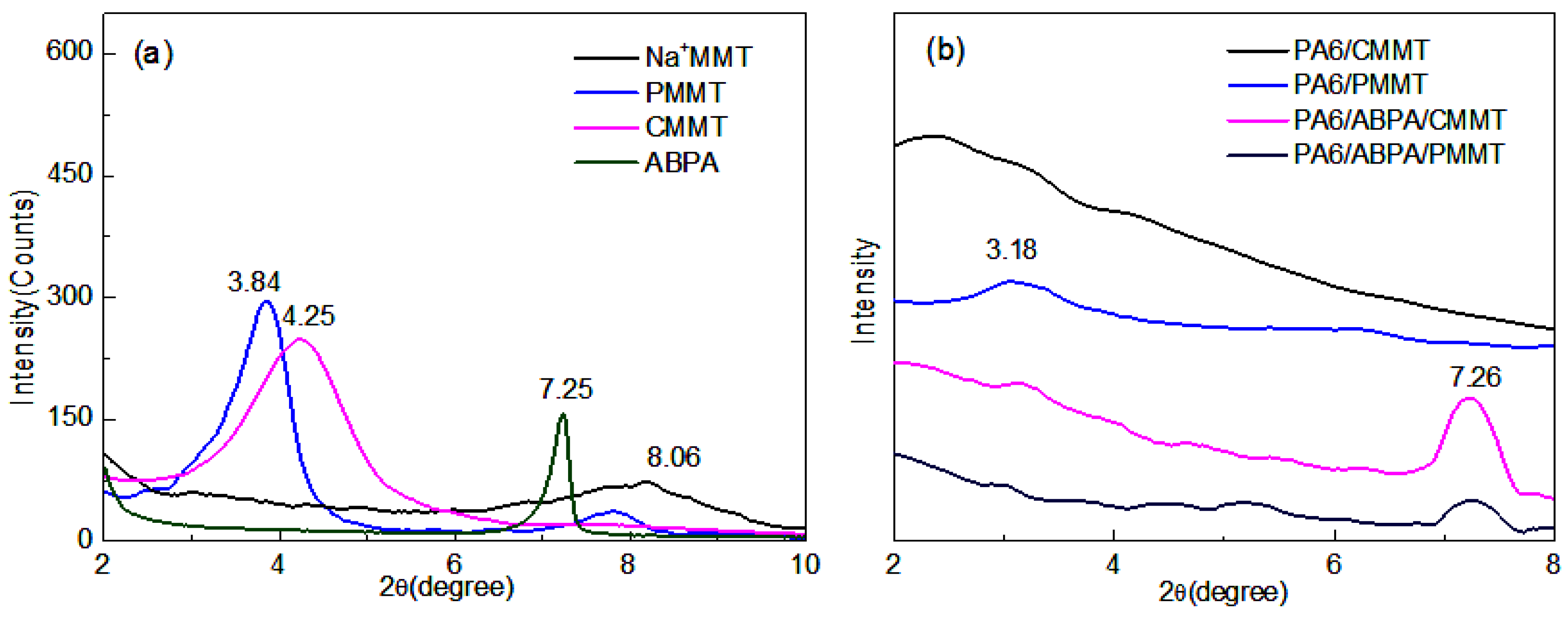
3.1.1. XRD Analysis
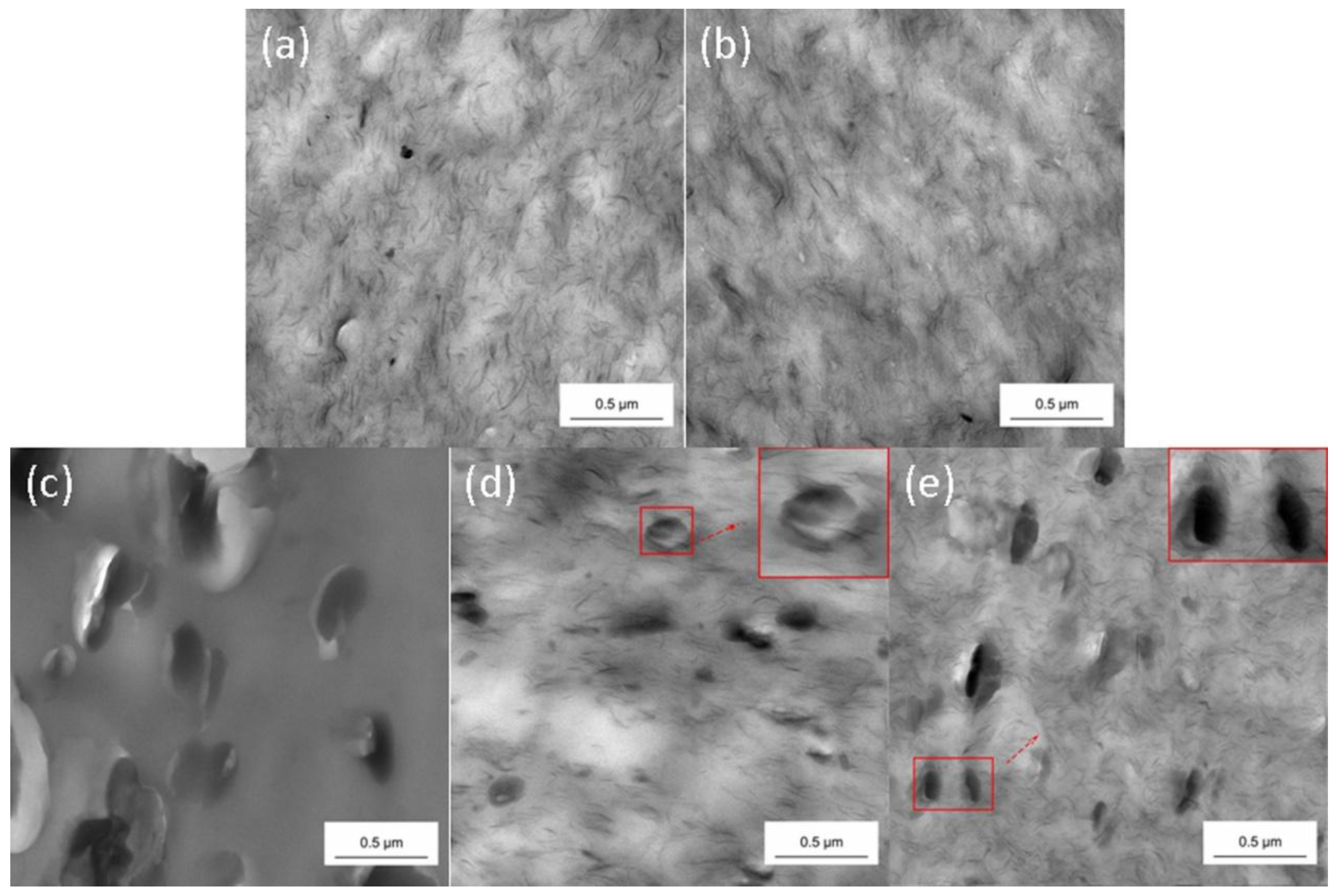
3.1.2. TEM Analysis
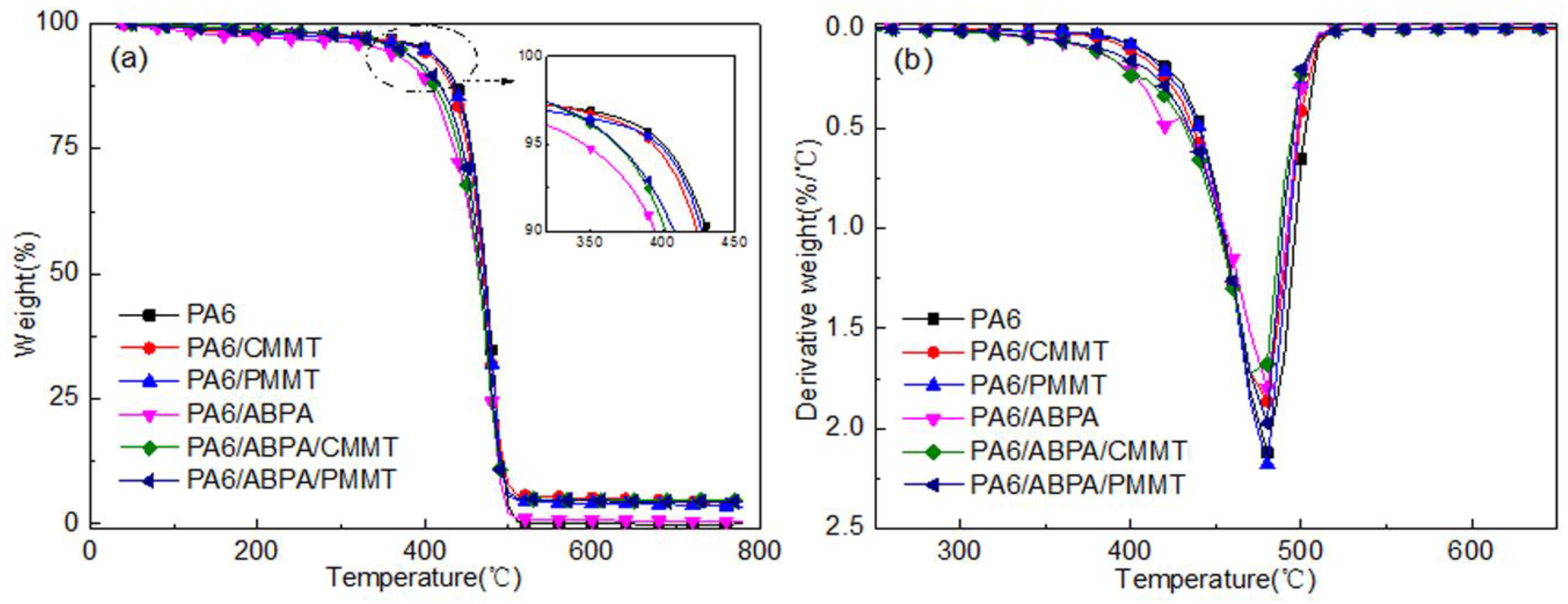
3.2. TG Analysis
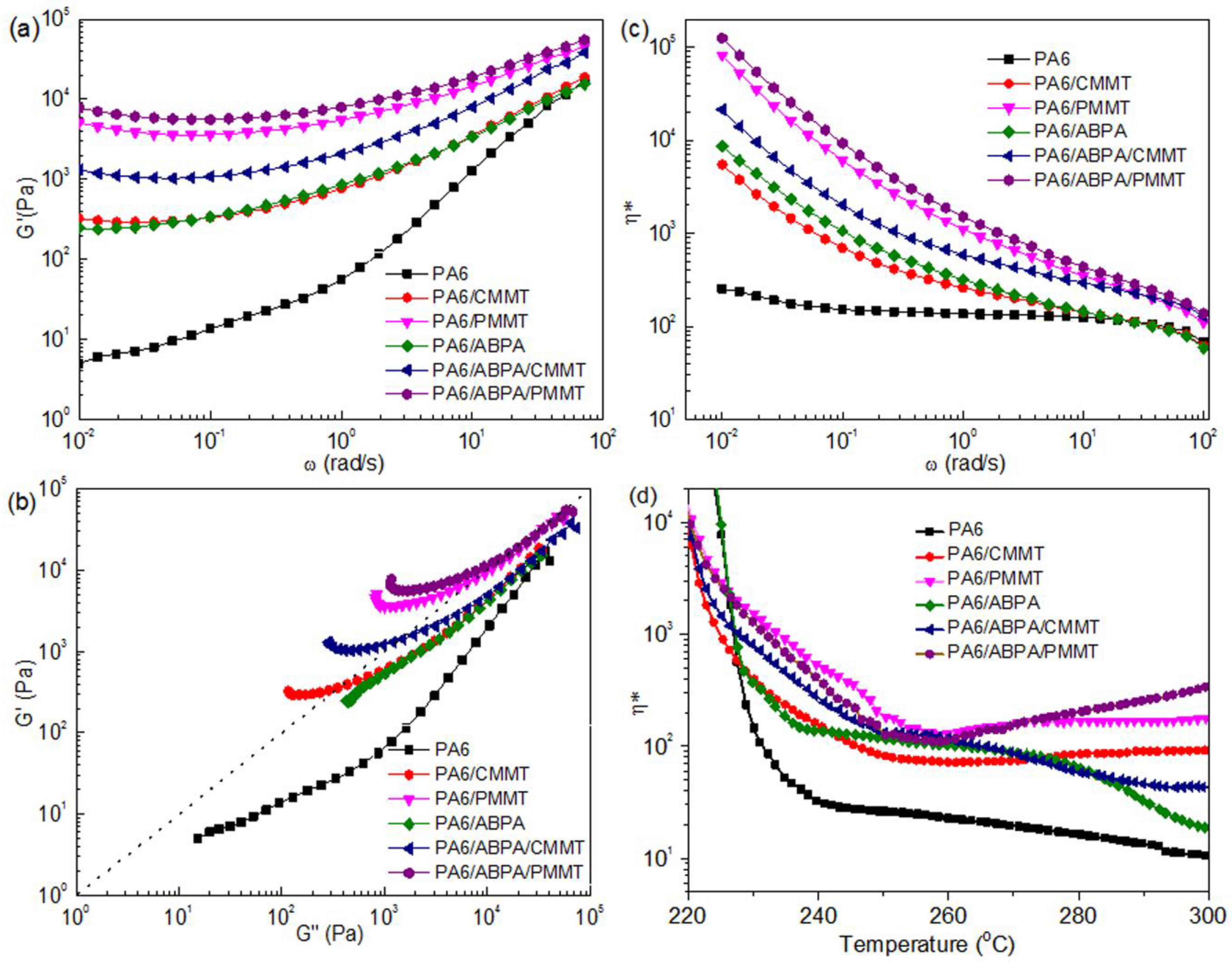
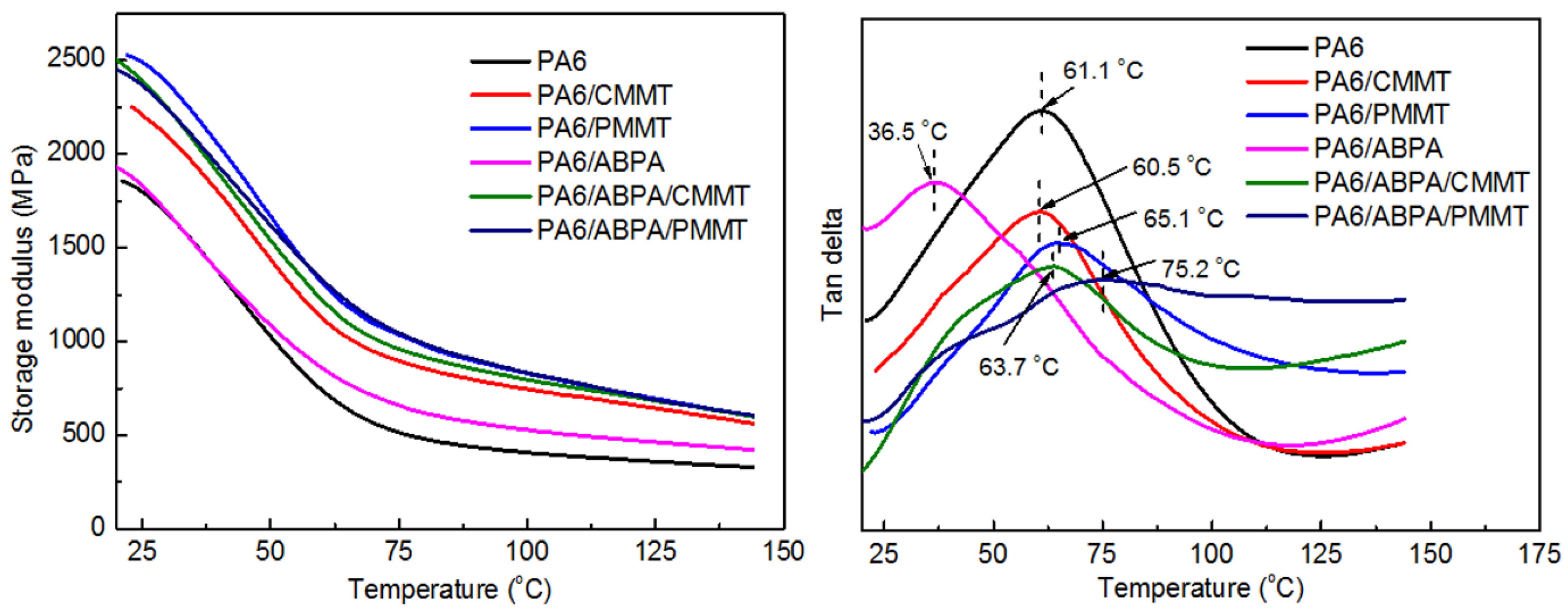
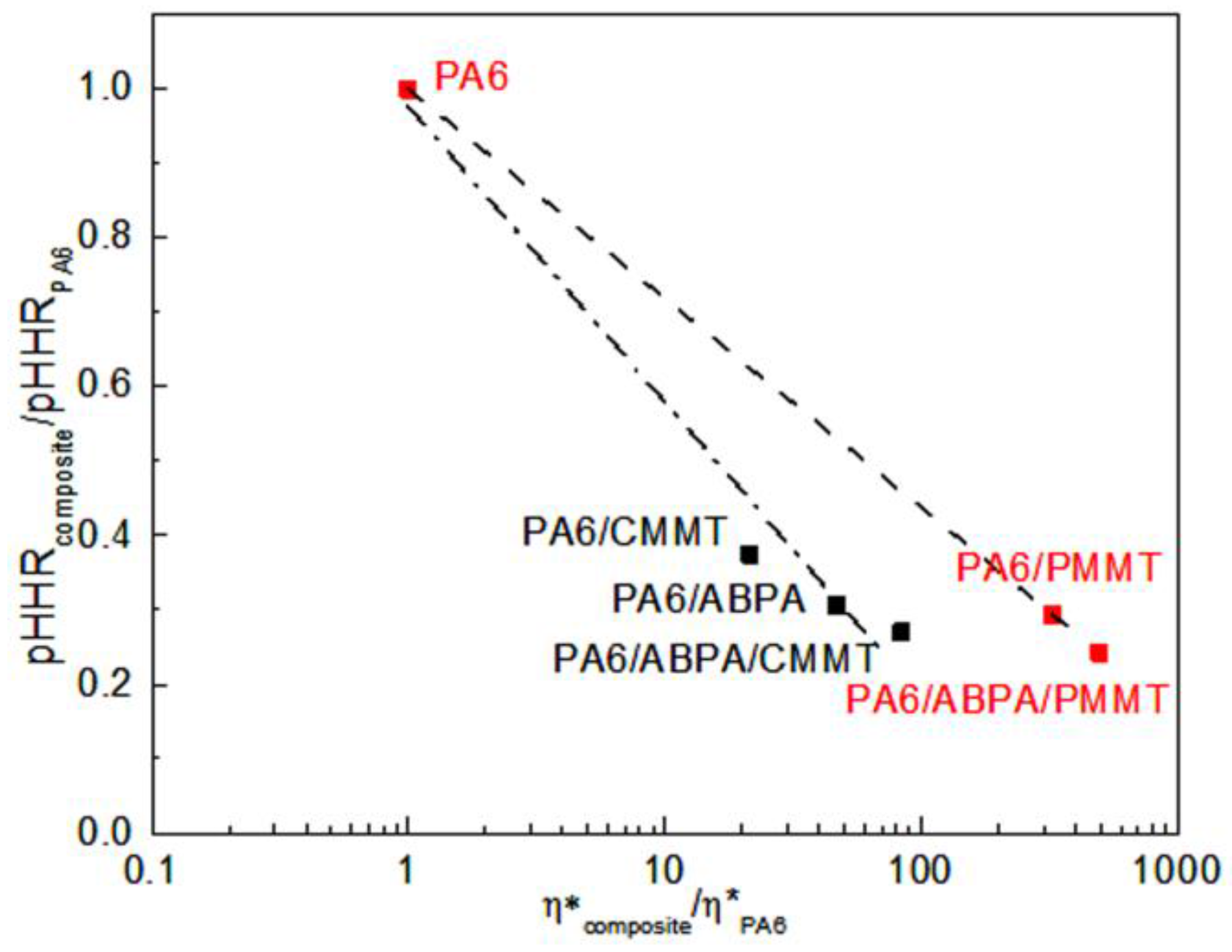
3.3. Viscoelastic Measurements
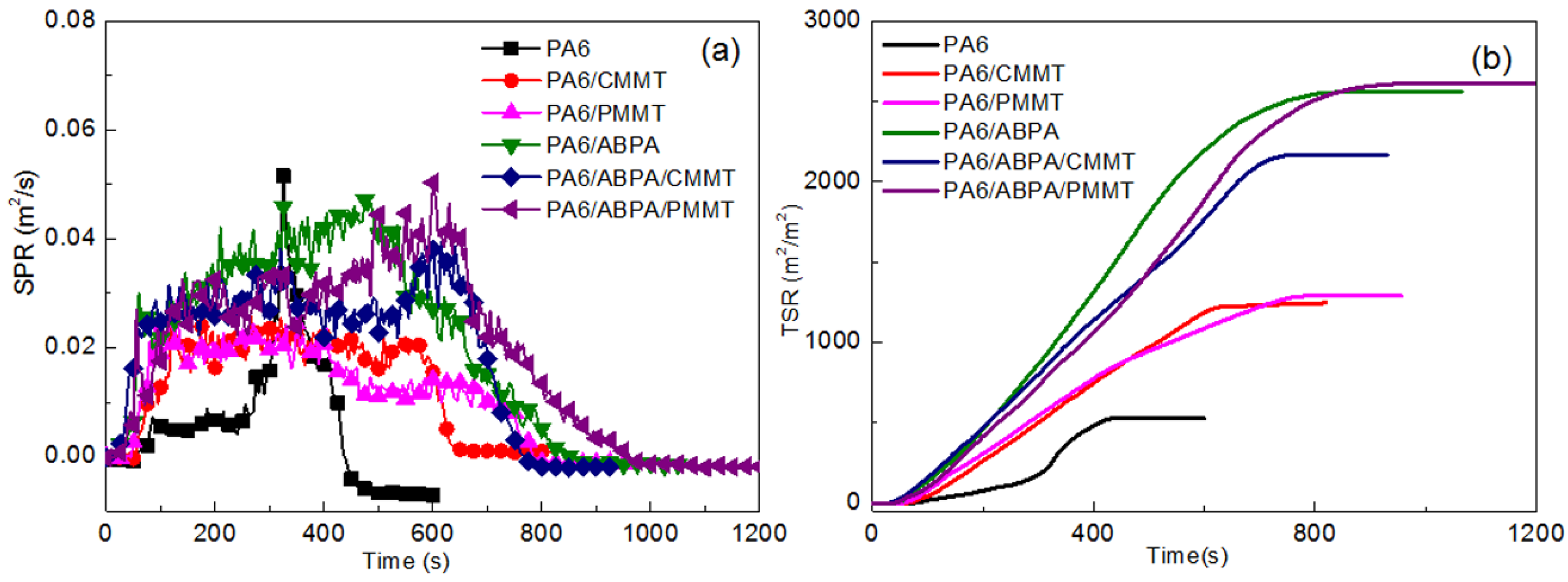
3.4. Flame Retardancy Performance
3.4.1. UL-94, LOI and Cone Calorimetric Results
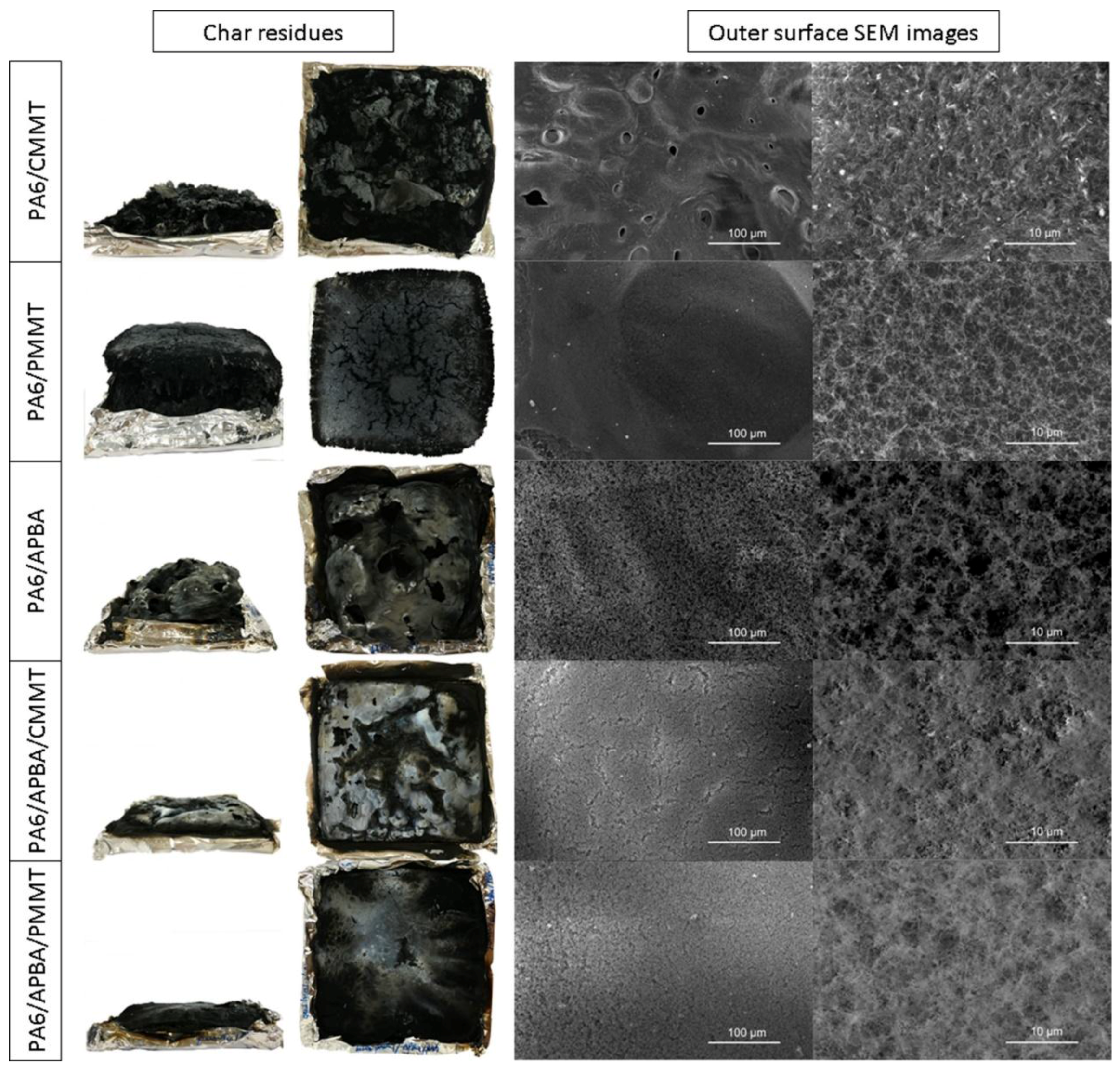
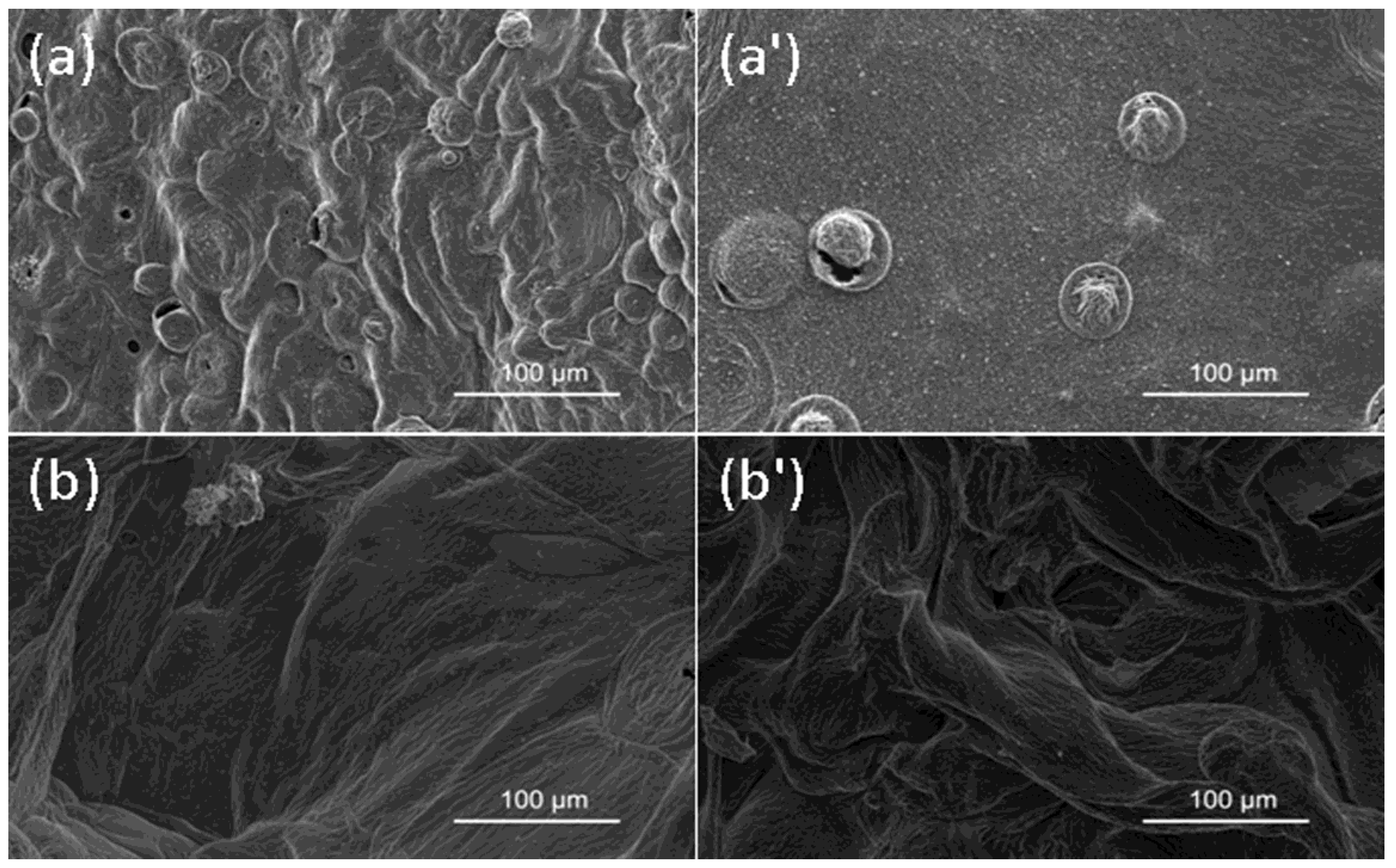
3.4.2. Char Residue Analysis
3.4.3. EDS Analysis
3.4.4. Flame-Retardant Mechanism Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dasari, A.; Yu, Z.Z.; Mai, Y.W.; Liu, S. Flame retardancy of highly filled polyamide 6/clay nanocomposites. Nanotechnology 2007, 18, 445602. [Google Scholar] [CrossRef]
- Barbos, R.; Araújo, E.M.; Melo, T.J.A.; Ito, E.N. Comparison of flammability behavior of polyethylene/Brazilian clay nanocomposites and polyethylene/flame retardants. Mater. Lett. 2007, 61, 2575–2578. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Ling, Z.; Zhuang, Y.; Chen, Z.; Fan, W. Preparation and combustion properties of flame retardant nylon 6/montmorillonite nanocomposite. Macromol. Mater. Eng. 2003, 288, 272–276. [Google Scholar] [CrossRef]
- Isitman, N.A.; Gunduz, H.O.; Kaynak, C. Nanoclay synergy in flame retarded/glass fibre reinforced polyamide 6. Polym. Degrad. Stab. 2009, 94, 2241–2250. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, Z.J.; Yao, J.G.; Zhang, Y.H. Organic nano-montmorillonite for simultaneously improving the flame retardancy, thermal stability, and mechanical properties of intumescent flame-retardant silicone rubber composites. J. Macromol. Sci. B 2015, 54, 1282–1296. [Google Scholar] [CrossRef]
- Horrocks, R.; Sitpalan, A.; Chen, Z.; Kandola, B.K. Flame retardant polyamide fibres: The challenge of minimising flame retardant additive contents with added nanoclays. Polymers 2016, 8, 288. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Hu, Y.; Song, L. Nanocomposites with halogen and nonintumescent phosphorus flame retardant additives. In Flame Retardant Polymer Nanocomposites; Morgan, A.B., Wilkie, C.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 191–234. [Google Scholar]
- Wilkie, C.A.; Morgan, A.B. Nanocomposites I: Current developments in nanocomposites as novel flame retardants. In Advances in Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Sawston, UK, 2008; pp. 95–123. [Google Scholar]
- Samyn, F.; Bourbigot, S.; Jama, C.; Bellayer, S.; Nazare, S.; Hull, R. Characterisation of the dispersion in polymer flame retarded nanocomposites. Eur. Polym. J. 2008, 44, 1631–1641. [Google Scholar] [CrossRef]
- Isitman, N.A.; Kaynak, C. Nanostructure of montmorillonite barrier layers: A new insight into the mechanism of flammability reduction in polymer nanocomposites. Polym. Degrad. Stab. 2011, 96, 2284–2289. [Google Scholar] [CrossRef]
- Song, P.; Wang, C.; Chen, L.; Zheng, Y.; Liu, L.; Wu, Q.; Huang, G.; Yu, Y.; Wang, H. Thermally stable, conductive and flame-retardant nylon 612 composites created by adding two-dimensional alumina platelets. Compos. Part A Appl. Sci. Manuf. 2017, 97, 100–110. [Google Scholar] [CrossRef]
- Song, P.; Yu, Y.; Zhang, T.; Fu, S.; Fang, Z.; Wu, Q. Permeability, viscoelasticity, and flammability performances and their relationship to polymer nanocomposites. Ind. Eng. Chem. Res. 2012, 51, 7255–7263. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.; Douglas, J.F.; Winey, K.I.; Harris, H.R., Jr.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, T.; Du, F.; Winey, K.I.; Groth, K.M.; Shields, J.R.; Bellayer, S.P.; Kim, H.; Douglas, J.F. Flammability properties of polymer nanocomposites with single-walled carbon nanotubes: Effects of nanotube dispersion and concentration. Polymer 2005, 46, 471–481. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Mu, M.; Winey, K.; Cipriano, B.; Raghavan, S.R.; Pack, S.; Rafailovich, M.; Yang, Y.; Grulke, E.; Shields, J.; et al. Relation between the viscoelastic and flammability properties of polymer nanocomposites. Polymer 2008, 49, 4358–4368. [Google Scholar] [CrossRef]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z. Clay network in ABS-graft-MAH nanocomposites: Rheology and flammability. Polym. Degrad. Stab. 2007, 92, 1439–1445. [Google Scholar] [CrossRef]
- He, W.T.; Zhu, H.; Xiang, Y.S.; Long, L.J.; Qin, S.H.; Yu, J. Enhancement of flame retardancy and mechanical properties of polyamide 6 by incorporating an aluminium salt of diisobutylphosphinic combined with organoclay. Polym. Degrad. Stab. 2017, 144, 442–453. [Google Scholar] [CrossRef]
- Yao, Q.; Levchik, S.; Alessio, G. Phosphorus-Containing Flame Retardant for Thermoplastic Polymers. WO Patent 2,006,009,983, 5 October 2010. [Google Scholar]
- Qin, S.H.; Yao, Y.; He, W.T.; Yu, J.; He, M.; Xu, C.; Xu, G.M.; Zhang, Q. Effect of intercalation method and intercalating agent type on the structure of silane-grafted montmorillonite. Clays Clay Miner. 2013, 61, 580–589. [Google Scholar] [CrossRef]
- He, W.T.; Yao, Y.; Qin, S.H.; Yu, J.; He, M.; Xu, C.; Xu, G.M. Effect of morphology and composition of organic montmorillonite on the structure and properties of poly(butylene terephthalate) nanocomposites. J. Macromol. Sci. B 2016, 55, 445–456. [Google Scholar] [CrossRef]
- Lu, C.; Liu, L.; Chen, N.; Wang, X.; Yang, D.; Huang, X.H.; Yao, D.H. Influence of clay dispersion on flame retardancy of ABS/PA6/APP blends. Polym. Degrad. Stab. 2015, 114, 16–29. [Google Scholar] [CrossRef]
- Cao, X.J.; Lu, K.; Li, Y.J. Isolated protective char layers by nanoclay network: Significantly improved flame retardancy and mechanical performance of TPV/MH composites by small amount of nanoclay. Ind. Eng. Chem. Res. 2015, 54, 6912–6921. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.; Fang, Z. Relationship between the distribution of organo-montmorillonite and the flammability of flame retardant polypropylene. Polym. Eng. Sci. 2012, 52, 390–398. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Z.; Yang, C.; Wang, Y.; Guo, Z.; Zhang, Y. Effect of clay dispersion on the synergism between clay and intumescent flame retardants in polystyrene. J. Appl. Polym. Sci. 2010, 115, 777–783. [Google Scholar] [CrossRef]
- Buczko, A.; Stelzig, T.; Bommer, L.; Rentsch, D.; Heneczkowski, M.; Gaan, S. Bridged DOPO derivatives as flame retardants for PA6. Polym. Degrad. Stab. 2014, 107, 158–165. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Harris, R.H., Jr.; Xin, Z.; Briber, R.M.; Cipriano, B.H.; Raghavan, S.R.; Awad, W.H.; Shields, J.R. Flame retardant mechanism of polyamide 6-clay nanocomposites. Polymer 2004, 45, 881–891. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.W.; Jian, R.K.; Wang, Y.Z. Synergistic effect between aluminium hypophosphite and alkyl-substituted phosphinate in flameretarded polyamide 6. Ind. Eng. Chem. Res. 2013, 52, 17162–17170. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.W.; Chen, H.B.; Wang, Y.Z. Aluminium hypophosphite versus alkyl-substituted phosphinate in polyamide 6: Flame retardance, thermal degradation, and pyrolysis behavior. Ind. Eng. Chem. Res. 2013, 52, 2875–2886. [Google Scholar] [CrossRef]
- Ye, L.; Ren, J.; Cai, S.Y.; Wang, Z.G.; Li, J.B. Poly(lactic acid) nanocomposites with improved flame retardancy and impact strength by combining of phosphinates and organoclay. Chin. J. Polym. Sci. 2016, 34, 785–796. [Google Scholar] [CrossRef]
- Ren, J.X.; Silva, A.S.; Krishnamoorti, R. Linear viscoelasticity of disordered polystyrene−polyisoprene block copolymer based layered-silicate nanocomposites. Macromolecules 2000, 33, 3739–3746. [Google Scholar] [CrossRef]
- Pluta, M.; Jeszka, J.K.; Boiteux, G. Polylactide/montmorillonite nanocomposites: Structure, dielectric, viscoelastic and thermal properties. Eur. Polym. J. 2007, 43, 2819–2835. [Google Scholar] [CrossRef]
- Cai, J.; Wirasaputra, A.; Zhu, Y.; Liu, S.; Zhou, Y.; Zhang, C.; Zhao, J. The flame retardancy and rheological properties of PA6/MCA modified by DOPO-based chain extender. RSC Adv. 2017, 7, 19593–19603. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Zhang, M.; Zhao, Y. Viscoelasticity and thermal stability of polylactide composites with various functionalized carbon nanotubes. Polym. Degrad. Stab. 2008, 93, 1577–1584. [Google Scholar] [CrossRef]
- Liu, T.; Tjiu, W.C.; He, C.; Na, S.S.; Chung, T.S. A processing-induced clay dispersion and its effect on the structure and properties of polyamide 6. Polym. Int. 2010, 53, 392–399. [Google Scholar] [CrossRef]
- Gomari, S.; Ghasemi, I.; Karrabi, M.; Azizi, H. Organoclay localization in polyamide 6/ethylene-butene copolymer grafted maleic anhydride blends: The effect of different types of organoclay. J. Polym. Res. 2012, 19, 1–11. [Google Scholar] [CrossRef]
- Ansari, D.M.; Price, G.J. Chromatographic estimation of filler surface energies and correlation with photodegradation of kaolin filled polyethylene. Polymer 2004, 45, 1823–1831. [Google Scholar] [CrossRef] [Green Version]
- Lewin, M.; Mey-Marom, A.; Frank, R. Surface free energies of polymeric materials, additives and minerals. Polym. Adv. Technol. 2005, 16, 429–441. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Khayyam, H.; Naebe, M. Fish DNA-modified clays: Towards highly flame retardant polymer nanocomposite with improved interfacial and mechanical performance. Sci. Rep. 2016, 6, 38194–38210. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, R.; Mohan, T.P. Room temperature processing of epoxy-clay nanocomposites. J. Mater. Sci. 2004, 39, 7333–7339. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Ou, Y.C.; Liu, L. Mechanical, morphological and rheological properties of polyamide 6/organo-montmorillonite nanocomposites. Express Polym. Lett. 2007, 1, 77–83. [Google Scholar]
- Feng, J.; Hao, J.; Du, J.; Yang, R. Effects of organoclay modifiers on the flammability, thermal and mechanical properties of polycarbonate nanocomposites filled with a phosphate and organoclays. Polym. Degrad. Stab. 2012, 97, 108–117. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the main fire retardancy mechanisms in polymer nanocomposites. Polym. Adv. Technol. 2006, 17, 772–777. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Wang, Z.; Du, X.; Yu, H.; Jiang, Z.; Liu, J.; Tang, T. Mechanism on flame retardancy of polystyrene/clay composites-the effect of surfactants and aggregate state of organoclay. Polymer 2009, 50, 5794–5802. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, L.; Lin, G.P. Flame retardation of glass-fibre-reinforced polyamide 6 by a novel metal salt of alkylphosphinic acid. Polym. Degrad. Stab. 2011, 96, 1538–1545. [Google Scholar] [CrossRef]
- Lewin, M. Some comments on the modes of action of nanocomposites in the flame retardancy of polymers. Fire Mater. 2003, 27, 1–7. [Google Scholar] [CrossRef]
- Bartholmai, M.; Schartel, B. Layered silicate polymer nanocomposites: New approach or illusion for fire retardancy? Investigations of the potentials and the tasks using a model system. Polym. Adv. Technol. 2004, 15, 335–364. [Google Scholar] [CrossRef]
- Clerc, L.; Ferry, L.; Leroy, E.; Lopez-Cuesta, J.M. Influence of talc physical properties on the fire retarding behaviour of (ethylene–vinyl acetate copolymer/magnesium hydroxide/talc) composites. Polym. Degrad. Stab. 2005, 88, 504–511. [Google Scholar] [CrossRef]
- Batistella, M.; Otazaghine, B.; Sonnier, R.; Caro-Bretelle, A.S.; Petter, C.; Lopez-Cuesta, J.M. Fire retardancy of ethylene vinyl acetate/ultrafine kaolinite composites. Polym. Degrad. Stab. 2014, 100, 54–62. [Google Scholar] [CrossRef]
- Courtat, J.; Melis, F.; Taulemesse, J.-M.; Bounor-Legare, V.; Sonnier, R.; Ferry, L.; Cassagnau, P. Effect of phosphorous-modified silica on the flame retardancy of polypropylene based nanocomposites. Polym. Degrad. Stab. 2015, 119, 260–274. [Google Scholar] [CrossRef]
- Courtat, J.; Melis, F.; Taulemesse, J.M.; Bounor-Legare, V.; Sonnier, R.; Ferry, L.; Cassagnau, P. Effect of phosphorous-modified silica on the flame retardancy of polybutylene terephthalate based nanocomposites. Polym. Degrad. Stab. 2017, 143, 74–84. [Google Scholar] [CrossRef]
- Isitman, N.A.; Kaynak, C. Tailored flame retardancy via nanofiller dispersion state: Synergistic action between a conventional flame-retardant and nanoclay in high-impact polystyrene. Polym. Degrad. Stab. 2010, 95, 1759–1768. [Google Scholar] [CrossRef]
- Samyn, F.; Bourbigot, S.; Jama, C.; Bellayer, S. Fire retardancy of polymer clay nanocomposites: Is there an influence of the nanomorphology? Polym. Degrad. Stab. 2008, 93, 2019–2924. [Google Scholar] [CrossRef]

| Sample | PA6 | PA6/CMMT | PA6/PMMT | PA6/ABPA | PA6/ABPA/CMMT | PA6/ABPA/PMMT |
|---|---|---|---|---|---|---|
| CMMT | - | 6 | - | - | 6 | - |
| PMMT | - | - | 6 | - | - | 6 |
| ABPA | - | - | - | 12 | 6 | 6 |
| Sample | T5% (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|
| PA6 | 400.6 ± 0.1 | 479.8 ± 0.5 | 0.2 ± 0.1 |
| PA6/CMMT | 393.7 ± 0.2 | 477.7 ± 0.1 | 3.7 ± 0.5 |
| PA6/PMMT | 397.4 ± 0.7 | 478.7 ± 0.4 | 3.6 ± 1.2 |
| PA6/ABPA | 345.7 ± 2.1 | 423.7 ± 0.7/478.4 ± 0.2 | 0.7 ± 0.1 |
| PA6/ABPA/CMMT | 367.7 ± 0.5 | 475.5 ± 0.3 | 4.7 ± 0.2 |
| PA6/ABPA/PMMT | 368.6 ± 0.1 | 479.1 ± 0.6 | 4.5 ± 0.1 |
| Sample | Contact Angle (°) | Total Surface Tension at 15 °C (mN/m) | −dγ/dT (mN/(m·°C)) | Surface Tension at 230 °C (mN/m) | Interfacial Tension (mN/m) | |||
|---|---|---|---|---|---|---|---|---|
| H2O | CH2I2 | γ | γ | γd | γp | γij | ||
| PA6 | 73.5 ± 0.6 | 42.4 ± 0.2 | 50.5 | 0.065 a | 36.6 | 28.3 | 8.3 | - |
| Na+MMT | 45.6 ± 0.7 | 28.1 ± 0.6 | 68.9 | 0.43 b | 23.5 | 15.4 | 8.1 | 1.94 |
| CMMT | 78.3 ± 0.8 | 35.6 ± 0.2 | 50.9 | 0.1 c | 29.4 | 24.3 | 5.1 | 0.54 |
| PMMT | 86.0 ± 0.2 | 35.8 ± 0.1 | 47.7 | 0.1 c | 26.2 | 23.1 | 3.1 | 1.49 |
| Sample Composition | LOI (%) | UL 94 (3.2 mm) a | UL 94 (1.6 mm) | ||||
|---|---|---|---|---|---|---|---|
| t1/t2 (s) | Dripping | Rating | t1/t2 (s) | Dripping | Rating | ||
| PA6 | 25 | -- | Yes | NR | -- | Yes | NR |
| PA6/CMMT | 21 | -- | Yes | NR | -- | Yes | NR |
| PA6/PMMT | 24.2 | -- | Yes | NR | -- | Yes | NR |
| PA6/APBA | 30.4 | 1.9/3.7 | No | V-0 | 4.2/0.9 | Yes | V-0 |
| PA6/APBA/CMMT | 29.8 | 2.3/7.5 | No | V-0 | 10.3/3.8 | Yes | V-1 |
| PA6/APBA/PMMT | 33.0 | 0.8/3.6 | No | V-0 | 3.6/4.5 | Yes | V-0 |
| Sample | PHRR (kW/m2) | AHRR (kW/m2) | THR (MJ/m2) | av-EHC (MJ/Kg) | TSP (m2/m2) | Residue (wt %) |
|---|---|---|---|---|---|---|
| PA6 | 1074.1 | 357.5 | 200.3 | 27.7 | 528.57 | 2.7 |
| PA6/CMMT | 402.3 | 227.2 | 179.5 | 24.7 | 1245.59 | 5.2 |
| PA6/PMMT | 315.1 | 200.7 | 183.7 | 26.3 | 1287.91 | 7.1 |
| PA6/ABPA | 328.7 | 160.6 | 163.0 | 22.5 | 2547.06 | 3.7 |
| PA6/ABPA/CMMT | 289.4 | 189.3 | 168.5 | 23.6 | 2155.98 | 6.9 |
| PA6/ABPA/PMMT | 261.0 | 150.5 | 174.6 | 24.7 | 2602.36 | 8.2 |
| C (wt %) | O (wt %) | Si (wt %) | Al (wt %) | P (wt %) | |
|---|---|---|---|---|---|
| PA6/CMMT | 17.4 | 59.2 | 12.2 | 5.5 | - |
| PA6/PMMT | 42.0 | 42.9 | 8.4 | 4.1 | 0.6 |
| PA6/ABPA | 20.3 | 49.6 | - | 10.5 | 18.0 |
| PA6/ABPA/CMMT | 15.6 | 55.6 | 5.3 | 11.2 | 12.6 |
| PA6/ABPA/PMMT | 29.7 | 44.9 | 7.9 | 7.8 | 7.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.-T.; Liao, S.-T.; Xiang, Y.-S.; Long, L.-J.; Qin, S.-H.; Yu, J. Structure and Properties Study of PA6 Nanocomposites Flame Retarded by Aluminium Salt of Diisobutylphosphinic Acid and Different Organic Montmorillonites. Polymers 2018, 10, 312. https://doi.org/10.3390/polym10030312
He W-T, Liao S-T, Xiang Y-S, Long L-J, Qin S-H, Yu J. Structure and Properties Study of PA6 Nanocomposites Flame Retarded by Aluminium Salt of Diisobutylphosphinic Acid and Different Organic Montmorillonites. Polymers. 2018; 10(3):312. https://doi.org/10.3390/polym10030312
Chicago/Turabian StyleHe, Wen-Tao, Sheng-Tao Liao, Yu-Shu Xiang, Li-Juan Long, Shu-Hao Qin, and Jie Yu. 2018. "Structure and Properties Study of PA6 Nanocomposites Flame Retarded by Aluminium Salt of Diisobutylphosphinic Acid and Different Organic Montmorillonites" Polymers 10, no. 3: 312. https://doi.org/10.3390/polym10030312