A Simple, Green Method to Fabricate Composite Membranes for Effective Oil-in-Water Emulsion Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
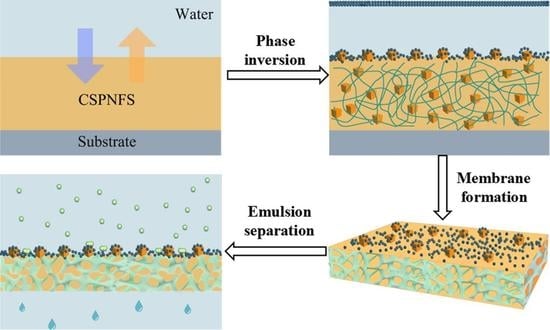
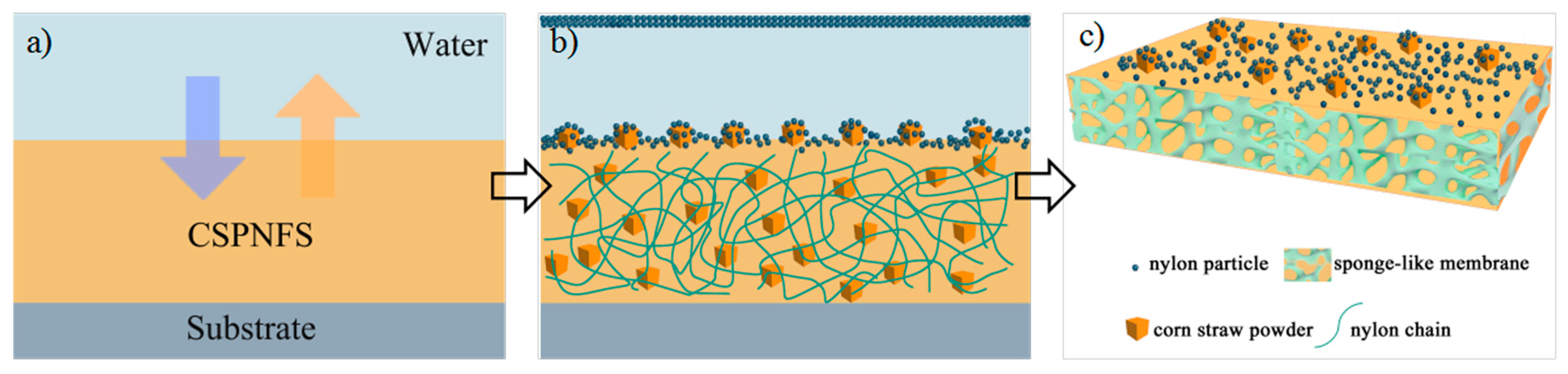
2.2. Fabrication of Superhydrophilic and Underwater Superoleophobic CSPNMs and Pristine Nylon 6,6 Membranes
2.3. Preparation of the Oil-in-Water Emulsion
2.4. Emulsion Separation Experiment
2.5. Continuous Separation
2.6. Cycle Experiment
2.7. Environmental Durability Experiments
2.8. Characterizations
3. Results
3.1. Membrane Characterization
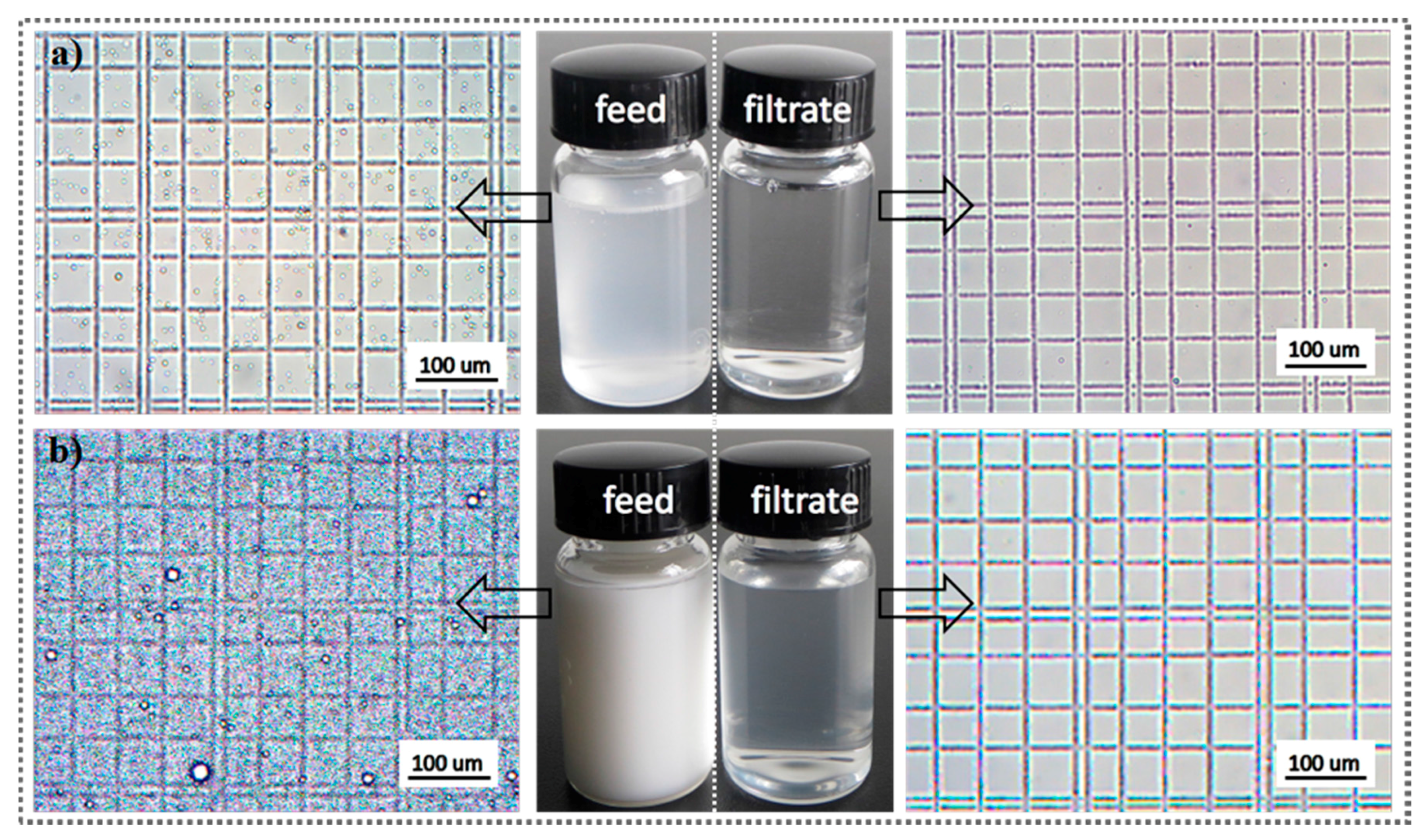
3.2. Filtration
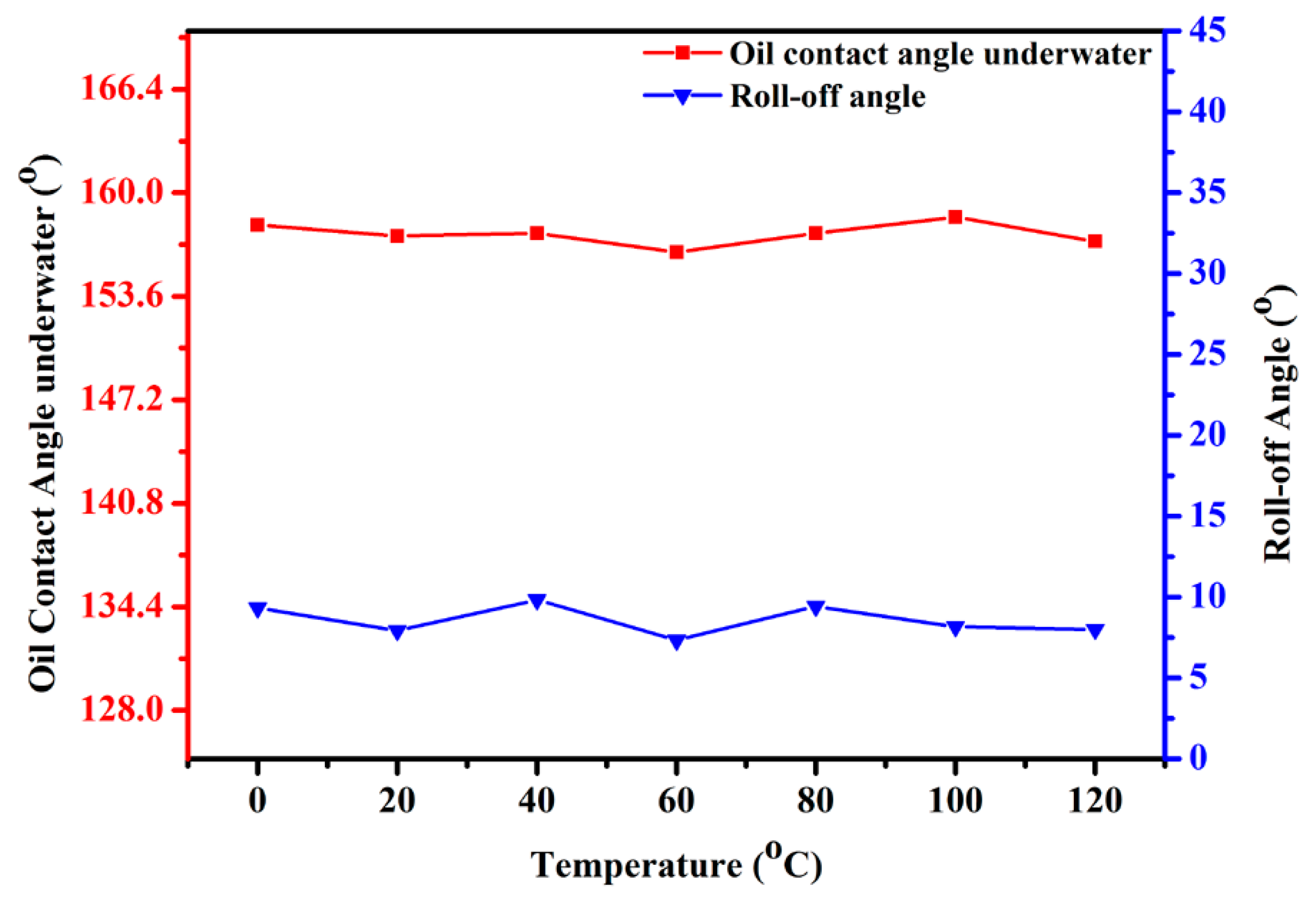
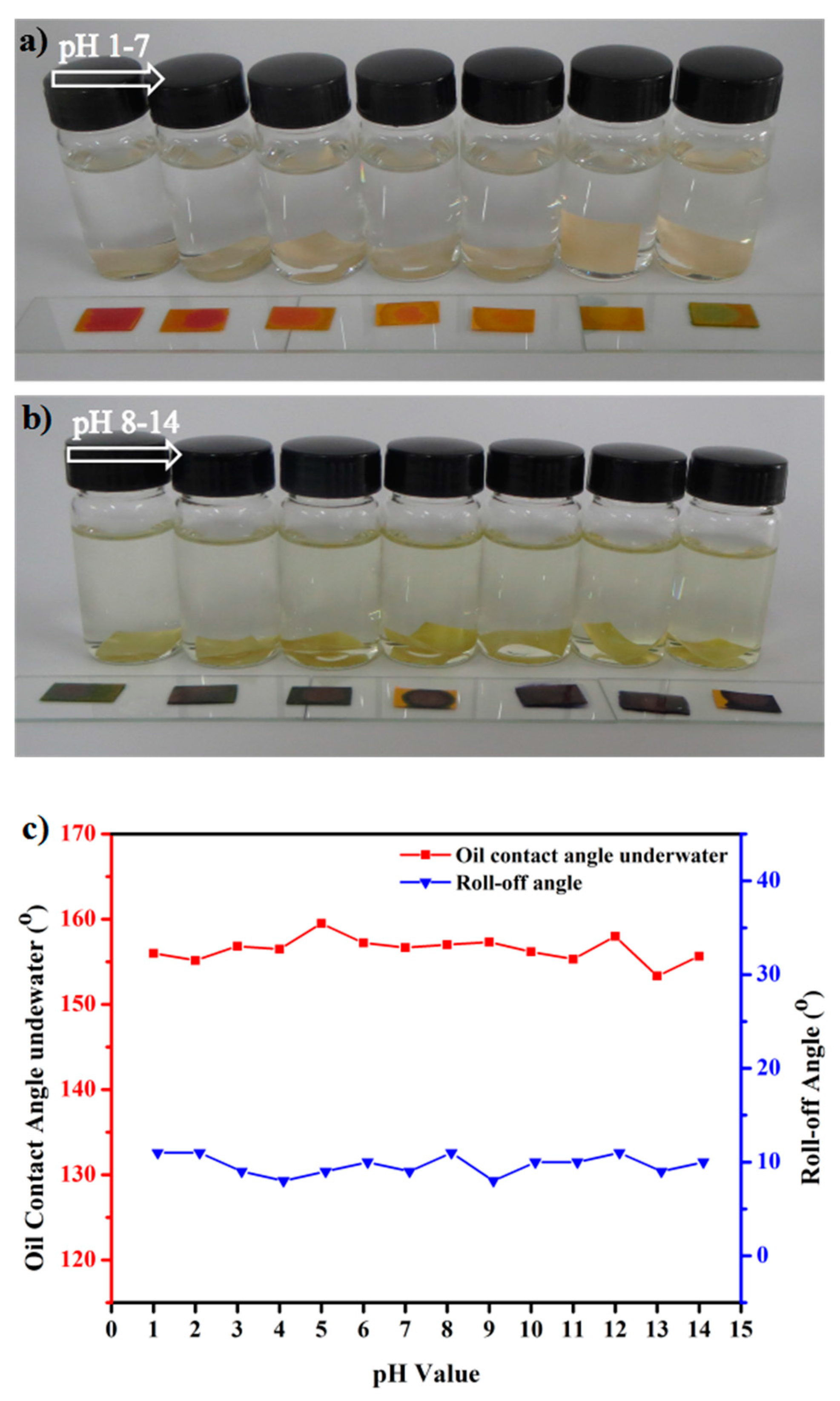
3.3. Recyclability and Chemical Durability Test Experiments
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Thebo, A.L.; Drechsel, P.; Lambin, E.F.; Nelson, K.L. A global, spatially-explicit assessment of irrigated croplands influenced by urban wastewater flows. Environ. Res. Lett. 2017, 12, 074008. [Google Scholar] [CrossRef]
- Barbu, M.; Vilanova, R.; Meneses, M.; Santin, I. On the evaluation of the global impact of control strategies applied to wastewater treatment plants. J. Clean. Prod. 2017, 149, 396–405. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Bringas, E.; Tan, N.R.; Ortiz, I.; Ghahramani, M.; Alaei Shahmirzadi, M.A. Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review. J. Water Process Eng. 2016, 9, 78–110. [Google Scholar] [CrossRef]
- Al-Shamrani, A.A.; James, A.; Xiao, H. Separation of oil from water by dissolved air flotation. Colloids Surf. A Physicochem. Eng. Asp. 2002, 209, 15–26. [Google Scholar] [CrossRef]
- Sokolovic, S.; Secerov-Sokolovic, R.; Sevic, S. Two-Stage Coalescer for Oil/Water Separation. Water Sci. Technol. 1992, 26, 2073–2076. [Google Scholar]
- Paul, U.C.; Fragouli, D.; Bayer, I.S.; Athanassiou, A. Functionalized cellulose networks for efficient oil removal from oil-water emulsions. Polymers 2016, 8, 52. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Pan, Y.; Zhao, X. A Facile and Effective Method to Fabricate Superhydrophobic/Superoeophilic Surface for the Separation of Both Water/Oil Mixtures and Water-in-Oil Emulsions. Polymers 2017, 9, 563. [Google Scholar] [CrossRef]
- Chen, F.; Song, J.; Liu, Z.; Liu, J.; Zheng, H.; Huang, S.; Sun, J.; Xu, W.; Liu, X. Atmospheric Pressure Plasma Functionalized Polymer Mesh: An Environmentally Friendly and Efficient Tool for Oil/Water Separation. ACS Sustain. Chem. Eng. 2016, 4, 6828–6837. [Google Scholar] [CrossRef]
- Thiam, A.R.; Bremond, N.; Bibette, J. Breaking of an Emulsion under an ac Electric Field. Phys. Rev. Lett. 2009, 102, 188304. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.P.; Vadodariya, N.; Nataraj, S.K.; Meena, R. Chitosan-Based Aerogel Membrane for Robust Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 24957–24962. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Li, J.; Zhou, W.; Zhang, R.; Ma, G.; Su, Z. Improved stability of emulsions in preparation of uniform small-sized konjac glucomanna (KGM) microspheres with epoxy-based polymer membrane by premix membrane emulsification. Polymers 2016, 8, 53. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Siekierka, A.; Bryjak, M. Preparation of Fouling-Resistant Nanofibrous Composite Membranes for Separation of Oily Wastewater. Polymers 2017, 9, 679. [Google Scholar] [CrossRef]
- Maartens, A.; Jacobs, E.P.; Swart, P. UF of pulp and paper effluent: Membrane fouling-prevention and cleaning. J. Membr. Sci. 2002, 209, 81–92. [Google Scholar] [CrossRef]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Chen, Z.; Chen, G.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y. Robust translucent superhydrophobic PDMS/PMMA film by facile one-step spray for self-cleaning and efficient emulsion separation. Chem. Eng. J. 2017, 330, 26–35. [Google Scholar] [CrossRef]
- Wang, B.; Chen, W.; Zhang, L.; Li, Z.; Liu, C.; Chen, J.; Shen, C. Hydrophobic polycarbonate monolith with mesoporous nest-like structure: An effective oil sorbent. Mater. Lett. 2017, 188, 201–204. [Google Scholar] [CrossRef]
- Yang, H.C.; Liao, K.J.; Huang, H.; Wu, Q.Y.; Wan, L.S.; Xu, Z.K. Mussel-inspired modification of a polymer membrane for ultra-high water permeability and oil-in-water emulsion separation. J. Mater. Chem. A 2014, 2, 10225–10230. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Yuan, H.; Liu, H.; Liu, C.; Li, T. Non-covalently functionalized graphene strengthened poly(vinyl alcohol). Mater. Des. 2018, 139, 372–379. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Rana, D.; Doraiswamy, M. Separation of oil/water emulsions using nano MgO anchored hybrid ultrafiltration membranes for environmental abatement. J. Appl. Polym. Sci. 2016, 133, 42848. [Google Scholar] [CrossRef]
- Lin, X.; Heo, J.; Jeong, H.; Choi, M.; Chang, M.; Hong, J. Robust superhydrophobic carbon nanofiber network inlay-gated mesh for water-in-oil emulsion separation with high flux. J. Mater. Chem. A 2016, 4, 17970–17980. [Google Scholar] [CrossRef]
- Yang, H.C.; Pi, J.K.; Liao, K.J.; Huang, H.; Wu, Q.Y.; Huang, X.J.; Xu, Z.K. Silica-Decorated Polypropylene Microfiltration Membranes with a Mussel-Inspired Intermediate Layer for Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2014, 6, 12566–12572. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, W.; Zhang, F.; Xing, T.; Jin, J. Superhydrophilic In-Situ-Cross-Linked Zwitterionic Polyelectrolyte/PVDF-Blend Membrane for Highly Efficient Oil/Water Emulsion Separation. ACS Appl. Mater. Interfaces 2017, 9, 9603–9613. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, Y.; Zeng, G.; Chen, X.; Shi, H.; Qing, D.; Li, F.; Chen, Q. Bio-inspired method for preparation of multiwall carbon nanotubes decorated superhydrophilic poly(vinylidene fluoride) membrane for oil/water emulsion separation. Chem. Eng. J. 2017, 321, 245–256. [Google Scholar] [CrossRef]
- Liu, X.; Lian, M.; Pan, Y.; Wang, X.; Zheng, G.; Liu, C. An Alternating Skin–Core Structure in Melt Multi-Injection-Molded Polyethylene. Macromol. Mater. Eng. 2018, 303, 1700465. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, L.; Dong, Y.; Li, L.; Liu, J. Waste-to-Resource Strategy to Fabricate Highly Porous Whisker-Structured Mullite Ceramic Membrane for Simulated Oil-in-Water Emulsion Wastewater Treatment. ACS Sustain. Chem. Eng. 2016, 4, 2098–2106. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Yang, Y.; Li, J.; Zha, F.; Lei, Z. A prewetting induced underwater superoleophobic or underoil (super) hydrophobic waste potato residue-coated mesh for selective efficient oil/water separation. Green Chem. 2016, 18, 541–549. [Google Scholar] [CrossRef]
- Zang, D.; Zhang, M.; Liu, F.; Wang, C. Superhydrophobic/superoleophilic corn straw fibers as effective oil sorbents for the recovery of spilled oil. J. Chem. Technol. Biotechnol. 2016, 91, 2449–2456. [Google Scholar] [CrossRef]
- Hong, J.; Ren, L.; Hong, J.; Xu, C. Environmental impact assessment of corn straw utilization in China. J. Clean. Prod. 2016, 112, 1700–1708. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Qi, Y.; Li, C.; Chen, J.; Wang, X.; He, J.; Wang, S.; Hao, J.; Zhang, L.; et al. Emission characterization, environmental impact, and control measure of PM2.5 emitted from agricultural crop residue burning in China. J. Clean. Prod. 2017, 149, 629–635. [Google Scholar] [CrossRef]
- Lin, D.J.; Chang, C.L.; Lee, C.K.; Cheng, L.P. Fine structure and crystallinity of porous Nylon 66 membranes prepared by phase inversion in the water/formic acid/Nylon 66 system. Eur. Polym. J. 2006, 42, 356–367. [Google Scholar] [CrossRef]
- Zeni, M.; Riveros, R.; de Souza, J.F.; Mello, K.; Meireles, C.; Filho, G.R. Morphologic analysis of porous polyamide 6,6 membranes prepared by phase inversion. Desalination 2008, 221, 294–297. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Dai, Z.; Wu, J.; Zhao, N.; Xu, J. Micro-nano hierarchically structured nylon 6,6 surfaces with unique wettability. J. Colloid Interface Sci. 2010, 345, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Y.; Liu, X.; Wang, D.; Li, J.; Jiang, L.; Jin, J. Salt-Induced Fabrication of Superhydrophilic and Underwater Superoleophobic PAA-g-PVDF Membranes for Effective Separation of Oil-in-Water Emulsions. Angew. Chem. Int. Ed. 2014, 53, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An Intelligent Superwetting PVDF Membrane Showing Switchable Transport Performance for Oil/Water Separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Wang, L.; Qiao, Y.; Tang, C.; Jung, C.; Yu, M. Ultrafiltration Membranes with Structure-Optimized Graphene-Oxide Coatings for Antifouling Oil/Water Separation. Adv. Mater. Interfaces 2015, 2, 1400433. [Google Scholar] [CrossRef]
- Islam, M.S.; McCutcheon, J.R.; Rahaman, M.S. A high flux polyvinyl acetate-coated electrospun nylon 6/SiO2 composite microfiltration membrane for the separation of oil-in-water emulsion with improved antifouling performance. J. Membr. Sci. 2017, 537, 297–309. [Google Scholar] [CrossRef]
- Yang, Y.; Yi, H.; Wang, C. Oil Absorbents Based on Melamine/Lignin by a Dip Adsorbing Method. ACS Sustain. Chem. Eng. 2015, 3, 3012–3018. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Shi, S.; Liu, C.; Dai, K.; Yin, R. Annealing induced mechanical reinforcement of injection molded iPP parts. Macromol. Mater. Eng. 2016, 301, 1468–1472. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Zhang, W.; Zhao, X.; Cao, G.; Liu, F.; Di, X.; Yang, H.; Wang, Y.; Wang, C. A Simple, Green Method to Fabricate Composite Membranes for Effective Oil-in-Water Emulsion Separation. Polymers 2018, 10, 323. https://doi.org/10.3390/polym10030323
Yu Q, Zhang W, Zhao X, Cao G, Liu F, Di X, Yang H, Wang Y, Wang C. A Simple, Green Method to Fabricate Composite Membranes for Effective Oil-in-Water Emulsion Separation. Polymers. 2018; 10(3):323. https://doi.org/10.3390/polym10030323
Chicago/Turabian StyleYu, Qianqian, Wenbo Zhang, Xinyue Zhao, Guoliang Cao, Feng Liu, Xin Di, Haiyue Yang, Yazhou Wang, and Chengyu Wang. 2018. "A Simple, Green Method to Fabricate Composite Membranes for Effective Oil-in-Water Emulsion Separation" Polymers 10, no. 3: 323. https://doi.org/10.3390/polym10030323