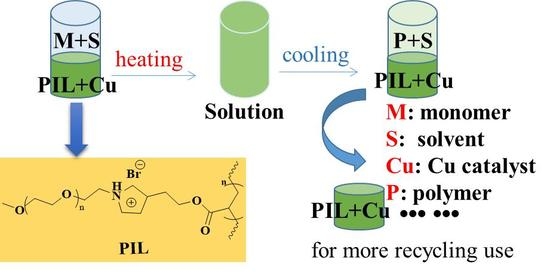
Poly(Ionic Liquid): A New Phase in a Thermoregulated Phase Separated Catalysis and Catalyst Recycling System of Transition Metal-Mediated ATRP
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
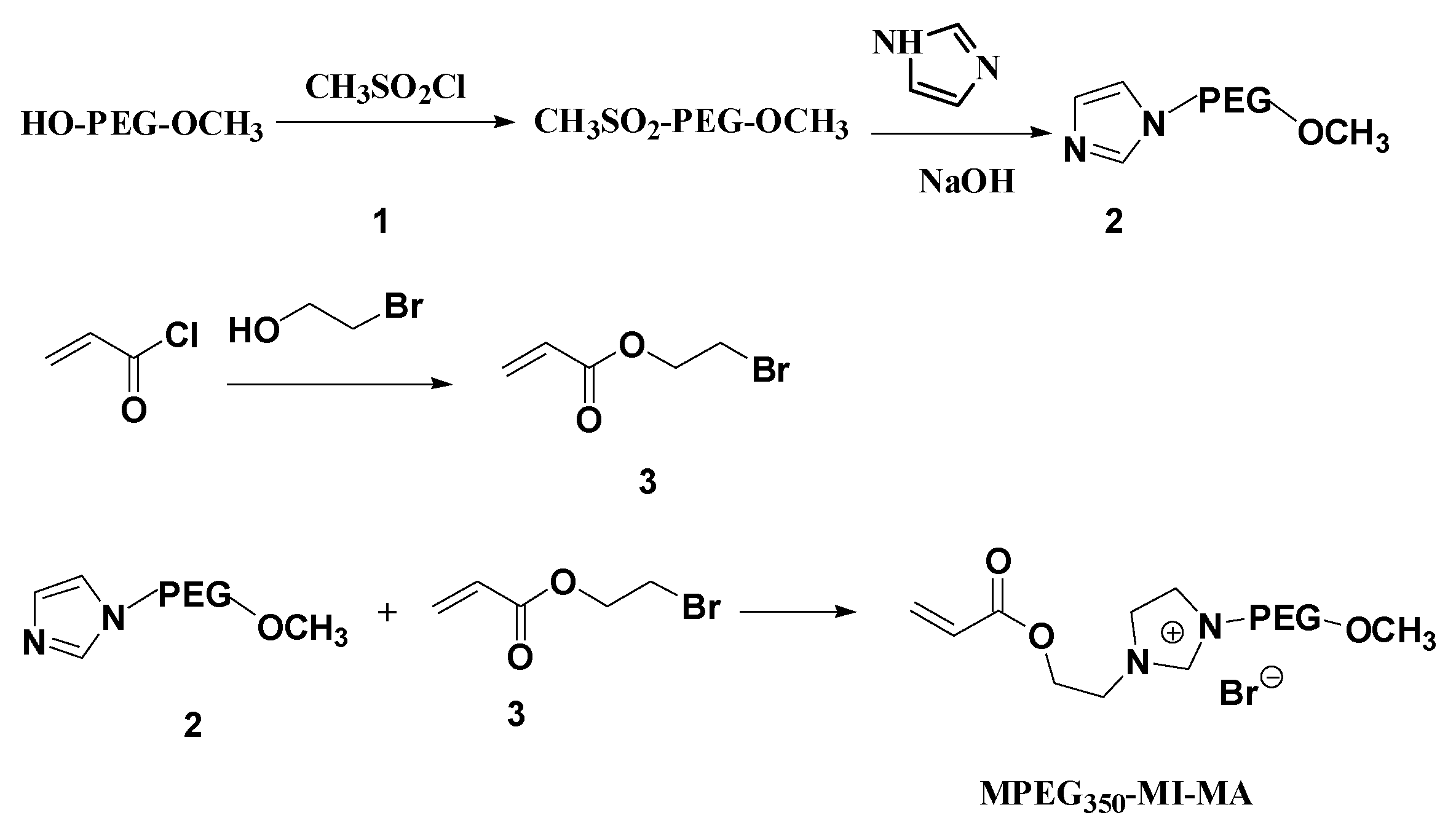
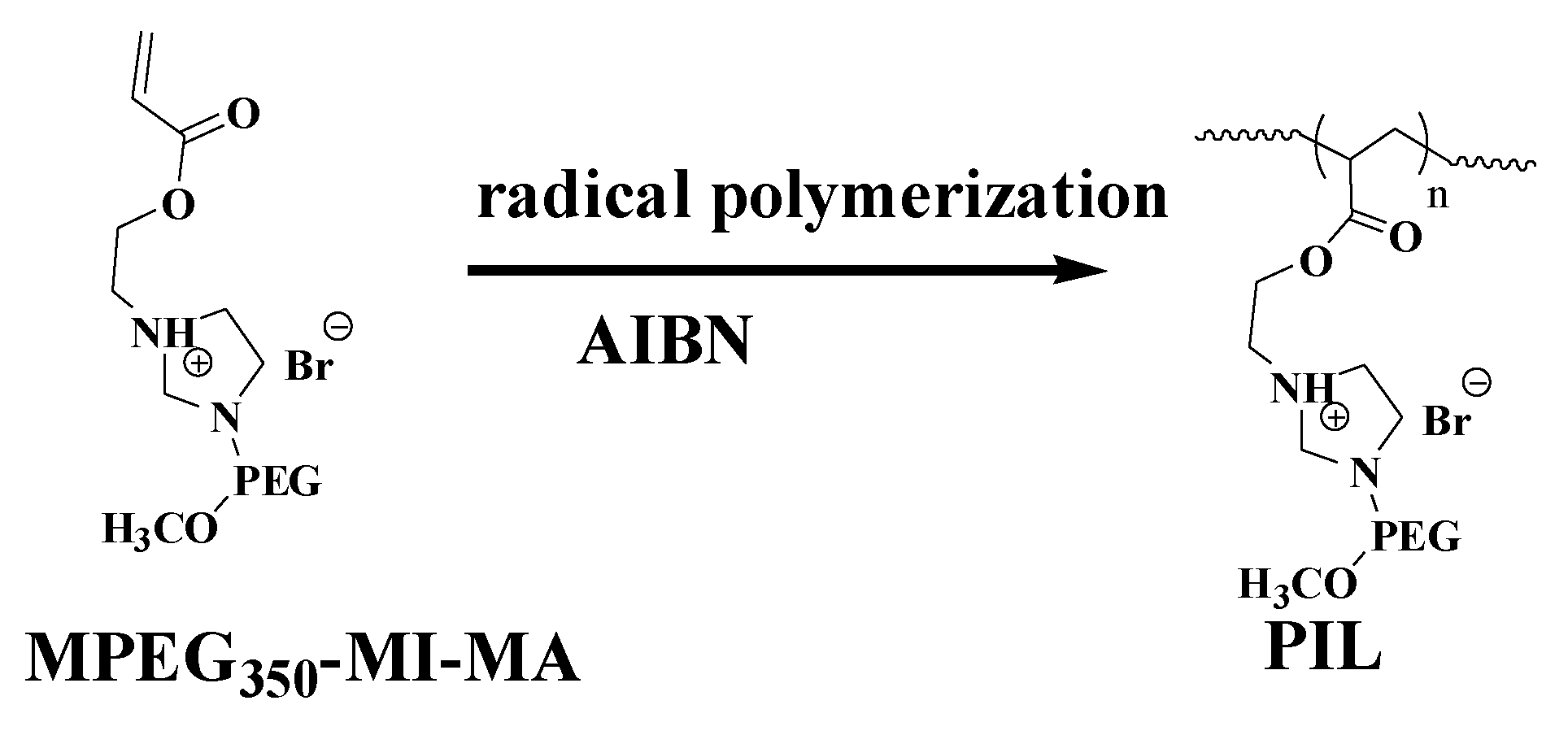
2.2. Synthesis of the Thermoregulated Poly(Ionic Liquid) (PIL)
2.3. Typical Procedure for the TPSC-Based ICAR ATRP of MMA and Catalyst Recycling
2.4. Typical Procedure for Chain Extension of PMMA
2.5. Characterizations
3. Results and Discussion
3.1. Selection of Solvent for TPSC-Based ICAR (Initiators for Continuous Activator Regeneration) ATRP System
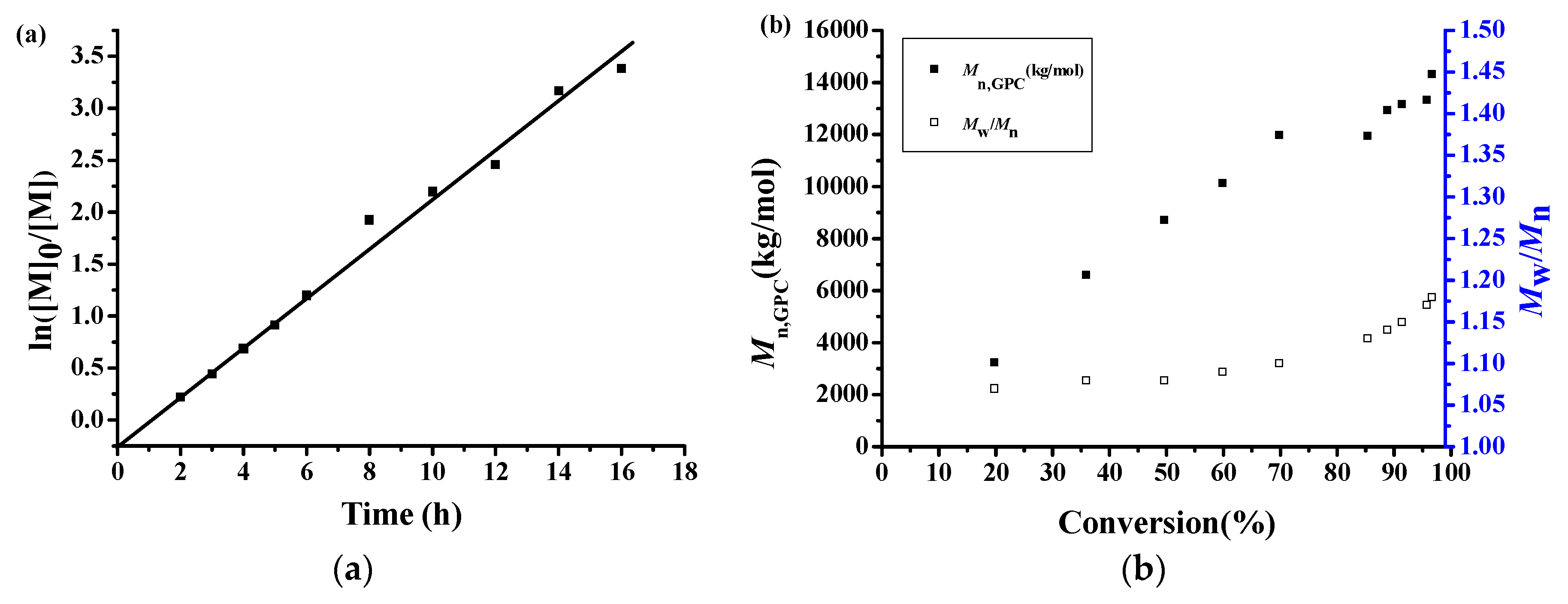
3.2. Polymerization Kinetics of MMA
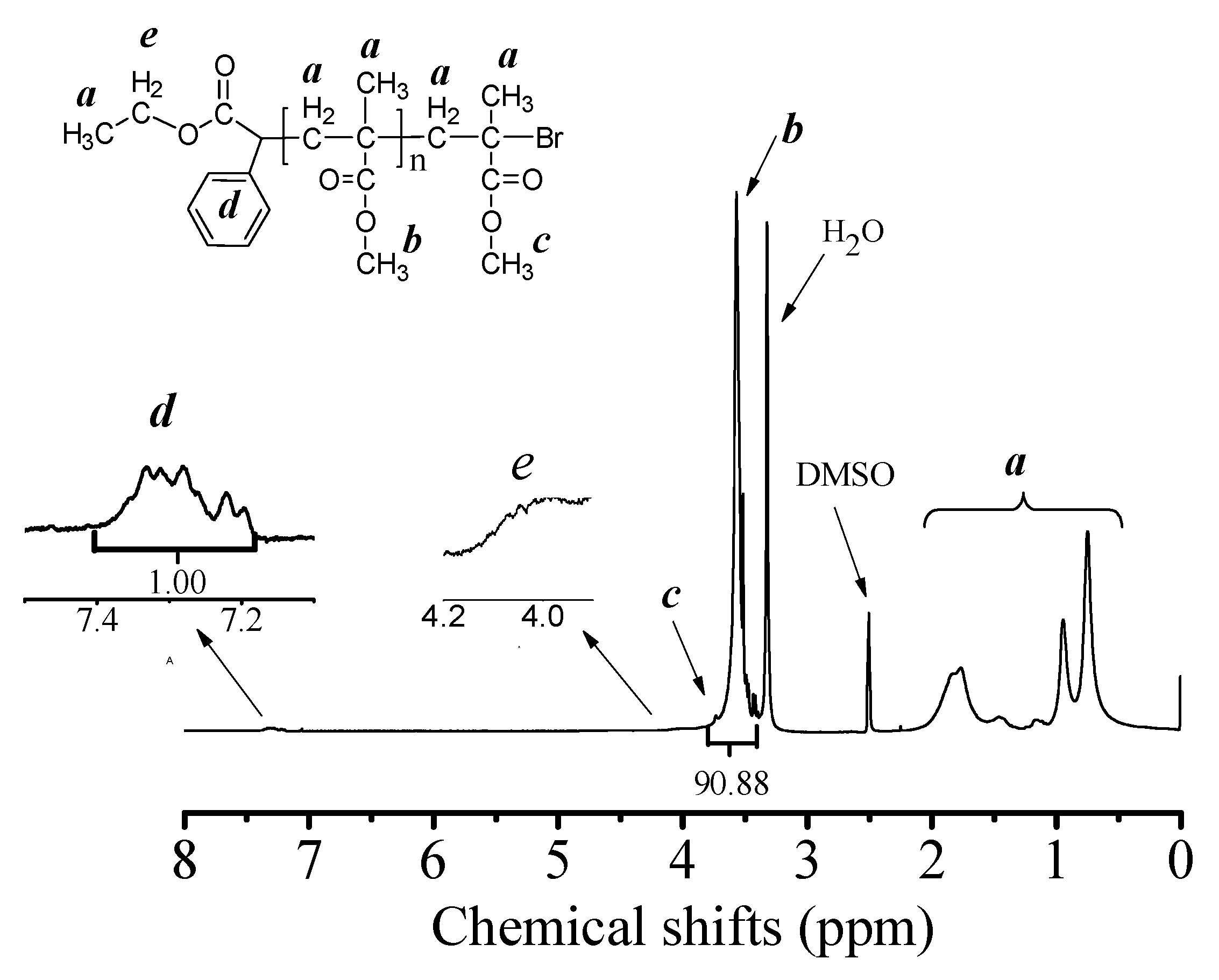
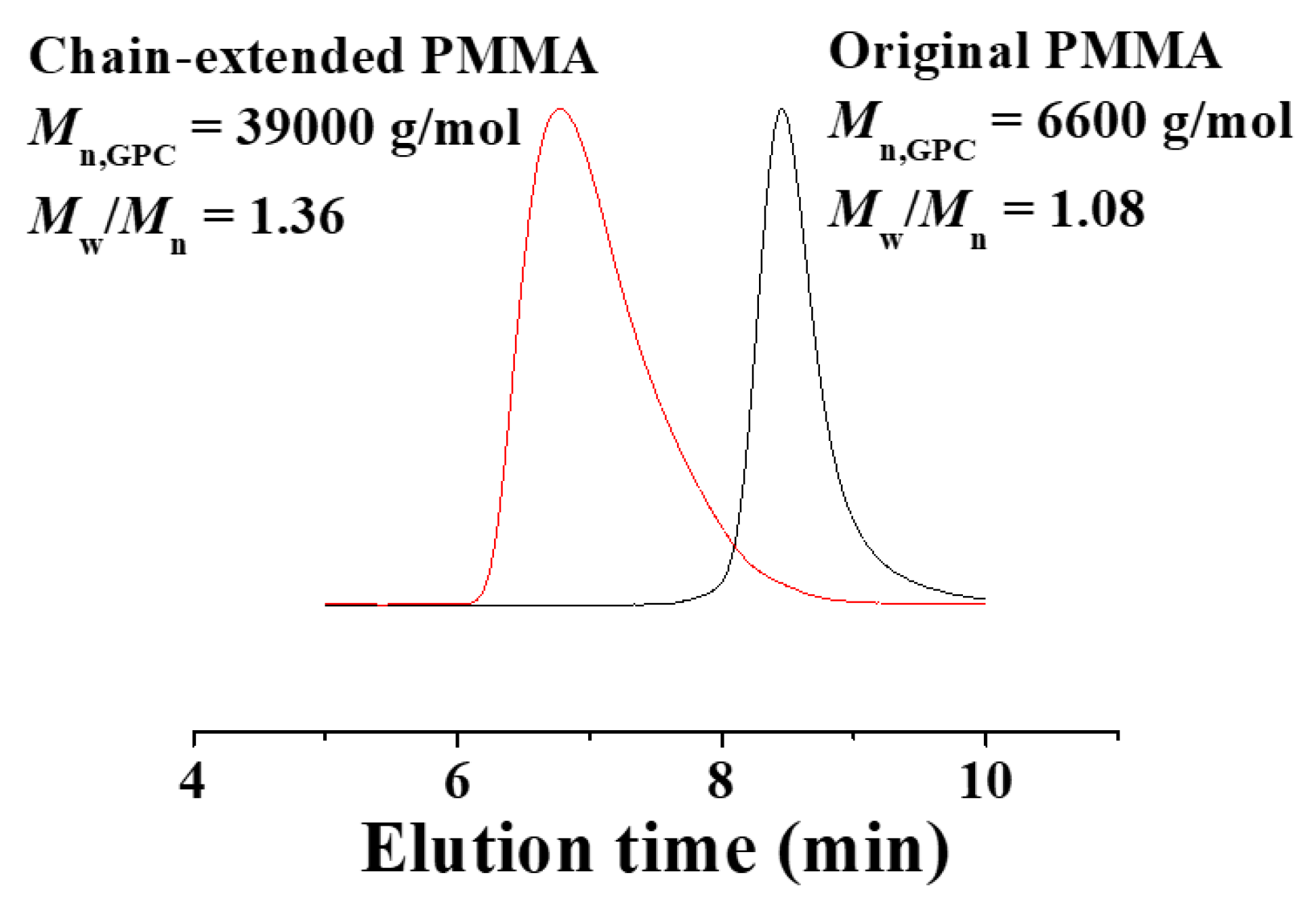
3.3. Chain-End Analysis and Chain Extension
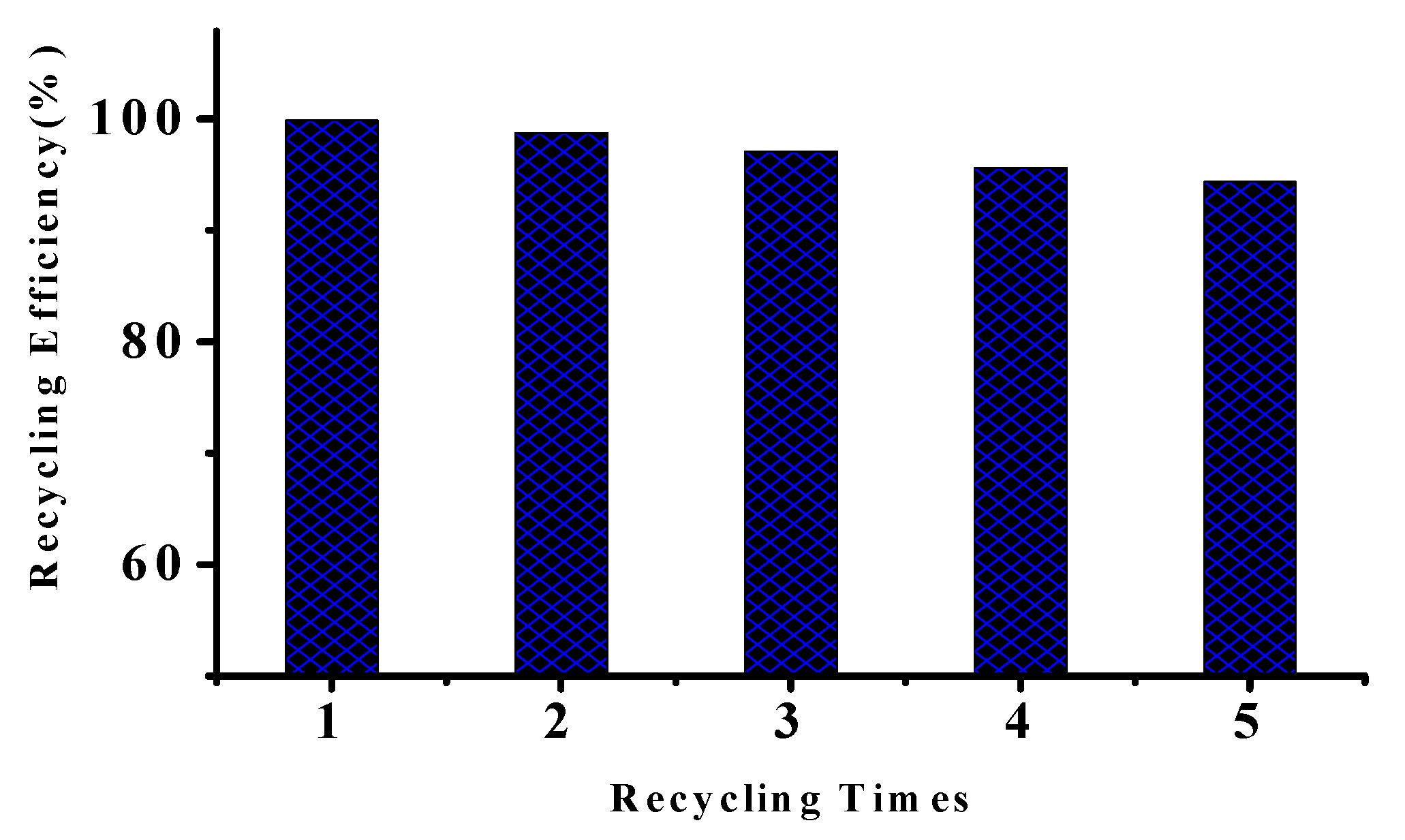
3.4. Catalyst Recycling and Reuse
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Gao, H.F.; Matyjaszewski, K. Synthesis of star polymers by a combination of ATRP and the “click” coupling method. Macromolecules 2006, 39, 4960–4965. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Fors, B.P.; Hawker, C.J. Control of a living radical polymerization of methacrylates by light. Angew. Chem. Int. Ed. 2012, 51, 8850–8853. [Google Scholar] [CrossRef] [PubMed]
- Konkolewicz, D.; Schroder, K.; Buback, J.; Bernhard, S.; Matyjaszewski, K. Visible light and sunlight photoinduced ATRP with ppm of Cu catalyst. ACS Macro Lett. 2012, 1, 1219–1223. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, B.J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Photoinduced iron-based water-induced phase separable catalysis (WPSC) ICAR ATRP of poly(ethylene glycol) methyl ether methacrylate. Macromol. Rapid Commun. 2017, 38, 1700116. [Google Scholar] [CrossRef] [PubMed]
- Magenau, A.J.D.; Strandwitz, N.C.; Gennaro, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization. Science 2011, 332, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.K.; Zhou, Y.N.; Luo, Z.H. Kinetic insight into electrochemically mediated ATRP gained through modeling. AICHE J. 2015, 61, 4347–4357. [Google Scholar] [CrossRef]
- Li, B.; Yu, B.; Huck, W.T.S.; Zhou, F.; Liu, W.M. Electrochemically induced surface-initiated atom-transfer radical polymerization. Angew. Chem. 2012, 124, 5182–5185. [Google Scholar] [CrossRef]
- Chmielarz, P. Cellulose-based graft copolymers prepared by simplified electrochemically mediated ATRP. Express. Polym. Lett. 2017, 11, 140–151. [Google Scholar] [CrossRef]
- Cheng, Z.P.; Zhu, X.L.; Chen, G.J.; Xu, W.J.; Lu, J.M. Reverse atom transfer radical solution polymerization of methyl methacrylate under pulsed microwave irradiation. J. Polym. Sci. 2002, 40, 3823–3834. [Google Scholar] [CrossRef]
- Cheng, Z.P.; Zhu, X.L.; Chen, M.; Chen, J.Y.; Zhang, L.F. Atom transfer radical polymerization of methyl methacrylate with low concentration of initiating system under microwave irradiation. Polymer 2003, 44, 2243–2247. [Google Scholar] [CrossRef]
- Cheng, Z.P.; Zhu, X.L.; Zhou, N.C.; Zhu, J.; Zhang, Z.B. Solution ATRP of styrene under pulsed microwave irradiation. Radiat. Phys. Chem. 2005, 72, 695–701. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Tang, H.D.; Ding, S.J. Catalyst separation in atom transfer radical polymerization. Prog. Polym. Sci. 2004, 29, 1053–1078. [Google Scholar] [CrossRef]
- Haddleton, D.M.; Kukulj, D.; Radigue, A.P. Atom transfer polymerization of methyl methacrylate mediated by solid supported copper catalysts. Chem. Commun. 1999, 1, 99–100. [Google Scholar] [CrossRef]
- Ding, S.J.; Xing, Y.C.; Radosz, A.M.; Shen, Y.Q. Magnetic nanoparticle supported catalyst for atom transfer radical polymerization. Macromolecules 2006, 39, 6399–6405. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Zhu, S.P.; Zeng, F.Q.; Pelton, R. Supported atom transfer radical polymerization of methyl methacrylate mediated by CuBr–tetraethyldiethylenetriamine grafted onto silica gel. J. Polym. Sci. A 2015, 39, 1051–1059. [Google Scholar] [CrossRef]
- Kickelbick, G.; Hyunjong, P.A.; Matyjaszewski, K. Immobilization of the copper catalyst in atom transfer radical polymerization. Macromolecules 1999, 32, 2941–2947. [Google Scholar] [CrossRef]
- Hong, S.C.; Matyjaszewski, K. Fundamentals of supported catalysts for atom transfer radical polymerization (ATRP) and application of an immobilized/soluble hybrid catalyst system to ATRP. Macromolecules 2002, 35, 7592–7605. [Google Scholar] [CrossRef]
- Honigfort, M.E.; Brittain, W.J. Use of jandaJel resins for copper removal in atom transfer radical polymerization. Macromolecules 2003, 36, 3111–3114. [Google Scholar] [CrossRef]
- Hong, S.C.; Hyunjong, P.A.; Matyjaszewski, K. An immobilized/soluble hybrid catalyst system for atom transfer radical polymerization. Macromolecules 2001, 34, 5099–5102. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Li, H.; Liu, Y. A novel immobilized cobalt(II)/copper(II) bimetallic catalyst for atom transfer radical polymerization (ATRP) of methyl methacrylate. Appl. Catal. 2007, 332, 192–199. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Wu, X. Thermoregulated phase-separable phosphine rhodium complex catalyst for hydroformylation of cyclohexene. Catal. Lett. 2002, 79, 55–57. [Google Scholar] [CrossRef]
- Du, X.Y.; Pan, J.L.; Chen, M.T.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Thermo-regulated phase separable catalysis (TPSC)-based atom transfer radical polymerization in a thermo-regulated ionic liquid. Chem. Commun. 2014, 50, 9266–9269. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.L.; Zhang, B.J.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Cu(II)-mediated atom transfer radical polymerization of methyl methacrylate via a strategy of thermo-regulated phase-separable catalysis in a liquid/liquid biphasic system: Homogeneous catalysis, facile heterogeneous separation, and recycling. Macromol. Rapid Commun. 2014, 35, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.Q.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Recent progress on transition metal catalyst separation and recycling in ATRP. Macromol. Rapid Commun. 2015, 36, 1702–1721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.J.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Fe(III)-mediated ICAR ATRP in a p-xylene/PEG-200 biphasic system: Facile and highly efficient separation and recycling of an iron catalyst. Polym. Chem. 2015, 6, 6616–6622. [Google Scholar] [CrossRef]
- Zhang, B.J.; Yao, L.; Liu, X.D.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Facilely recyclable Cu(II) macrocomplex with thermoregulated poly(ionic liquid) macroligand: Serving as a highly efficient atom transfer radical polymerization catalyst. ACS Sustain. Chem. Eng. 2016, 4, 7066–7073. [Google Scholar] [CrossRef]
- Jakubowski, W.; Matyjaszewski, K. Activator generated by electron transfer for atom transfer radical polymerization. Macromolecules 2005, 38, 4139–4146. [Google Scholar] [CrossRef]
- Jakubowski, W.; Min, K.; Matyjaszewski, K. Activators regenerated by electron transfer for atom transfer radical polymerization of styrene. Macromolecules 2006, 39, 39–45. [Google Scholar] [CrossRef]
- Bai, L.J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Activators generated by electron transfer for atom transfer radical polymerization: Recent advances in catalyst and polymer chemistry. Polym. Chem. 2012, 3, 2685–2697. [Google Scholar] [CrossRef]
- Abreu, C.M.R.; Mendonca, P.V.; Serra, A.C.; Popov, A.V.; Matyjaszewski, K.; Guliashvili, T.; Coelho, J.F.J. Inorganic sulfites: Efficient reducing agents and supplemental activators for atom transfer radical polymerization. ACS Macro Lett. 2012, 1, 1308–1311. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Matyjaszewski, K. ATRP of methyl acrylate with metallic zinc, magnesium, and iron as reducing agents and supplemental activators. Macromolecules 2011, 44, 683–685. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Jakubowski, W.; Min, K.; Tang, W.; Huang, J.Y.; Braunecker, W.A.; Tsarevsky, N.V. Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 15309–15314. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Zhang, L.F.; Zhang, Z.B.; Zhu, J.; Tu, Y.F.; Cheng, Z.P.; Zhu, X.L. Iron-mediated ICAR ATRP of methyl methacrylate. Macromolecules 2011, 44, 3233–3239. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids-new “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nat. 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Willauer, H.D.; Swatloski, R.P.; Visser, A.E.; Rogers, R.D. Room temperature ionic liquids as novel media for clean liquid−liquid extraction. Chem. Commun. 1998, 16, 1765–1766. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Ambrogi, M.; Tiruye, G.A.; Cordella, D.; Fernandes, A.M. Innovative polyelectrolytes/poly(ionic liquid)s for energy and the environment. Polym. Int. 2017, 66, 1119–1128. [Google Scholar] [CrossRef]
- Salamone, J.C.; Israel, S.C.; Taylor, P.; Snider, B. Polyvinylimidazolium salts of varying hydrophilic-hydrophobic character. J. Polym. Sci. 1974, 45, 65–73. [Google Scholar] [CrossRef]
- Salamone, J.C.; Israel, S.C.; Taylor, P.; Snider, B. Synthesis and homopolymerization of vinylimidazolium salts. Polymer 1973, 14, 639–644. [Google Scholar] [CrossRef]
- Mu, X.D.; Meng, J.Q.; Li, Z.C.; Kou, Y. Rhodium nanoparticles stabilized by ionic copolymers in ionic liquids: Long lifetime nanocluster catalysts for benzene hydrogenation. J. Am. Chem. Soc. 2005, 127, 9694–9695. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Yin, D.; Yu, N.; Zhao, H.; Yin, D. Easily recyclable polymeric ionic liquid-functionalized chiral salen Mn(III) complex for enantioselective epoxidation of styrene. J. Catal. 2009, 263, 284–291. [Google Scholar] [CrossRef]
- Wang, J.; Song, G.; Peng, Y. Reusable Pd nanoparticles immobilized on functional ionic liquid co-polymerized with styrene for Suzuki reactions in water–ethanol solution. Tetrahedron Lett. 2011, 52, 1477–1480. [Google Scholar] [CrossRef]
- Graham, C.M.; Meng, Y.; Ho, T.; Anderson, J.L. Sorbent coatings for solid-phase microextraction based on mixtures of polymeric ionic liquids. J. Sep. Sci. 2011, 34, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Anderson, J.L. Highly selective GC stationary phases consisting of binary mixtures of polymeric ionic liquids. J. Sep. Sci. 2010, 33, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wanigasekara, E.; Perera, S.; Crank, J.; Sidisky, L.; Shirey, R.; Berthod, A.; Armstrong, D.W. Bonded ionic liquid polymeric material for solidphase microextraction GC analysis. Anal. Bioanal. Chem. 2010, 396, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.D.; Mallik, A.K.; Takafuji, M.; Jiang, S.; Ihara, H. New poly(ionic liquid)-grafted silica multi-mode stationary phase for anionexchange/reversed-phase/hydrophilic interaction liquid chromatography. Analyst 2012, 137, 2553–2555. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T. Development of industrial processes for MMA production. Catal. Catal. 2003, 45, 366–371. [Google Scholar]
- Riisager, A.; Fehrmann, R.; Berg, R.W.; Van, H.R.; Wasserscheid, P. Thermomorphic phase separation in ionic liquid-organic liquid systems—Conductivity and spectroscopic characterization. Phys. Chem. Chem. Phys. 2005, 7, 3052–3058. [Google Scholar] [CrossRef] [PubMed]

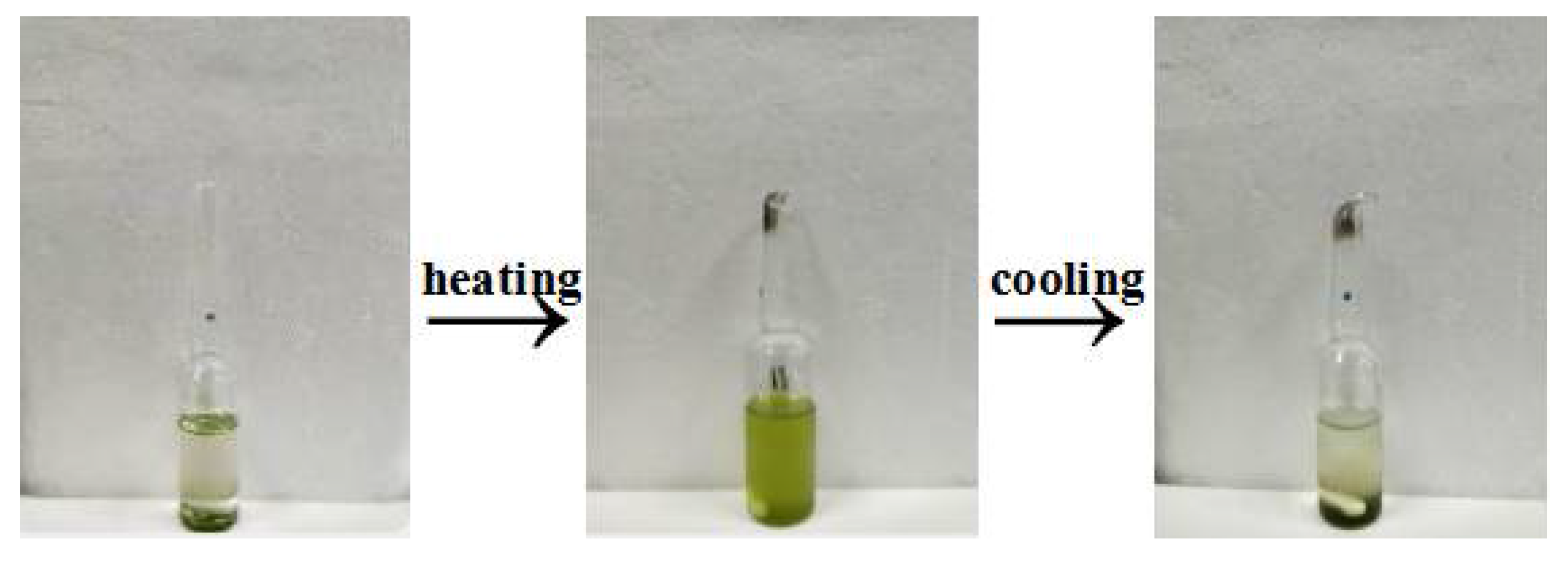
| Solvent (2.0 mL) | 25 °C | 70–90 °C | 25 °C |
|---|---|---|---|
| p-Xylene | I | S | I |
| o-Xylene | I | S | I |
| Toluene | I | S | I |
| Benzene | I | M | I |
| Cyclohexane | M | M | M |
| n-Hexane | I | S | I |
| Entry | Ligand | Reducing Agent | Time (h) | Conv. (%) | Mn,th a (g/mol) | Mn,GPC (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|---|
| 1 | TPMA | AIBN | 11.5 | 73.1 | 14,800 | 14,600 | 1.17 |
| 2 | PMDETA | AIBN | 10 | 81.9 | 16,400 | 10,300 | 1.39 |
| 3 | Me6TRAN | AIBN | 10 | 83.9 | 16,800 | 8700 | 1.51 |
| 4 | TDA-1 | AIBN | 10 | 72.5 | 14,500 | 29,100 | 1.58 |
| 5 | TPMA | AIBN | 5.5 | 69.9 | 14,000 | 12,000 | 1.31 |
| 6 | TPMA | Glucose | 57 | 46.4 | 9500 | 11,400 | 1.11 |
| 7 | TPMA | AsAc | 57 | 62.5 | 12,700 | 16,200 | 1.11 |
| 8 | TPMA | AsAc-Na | 5.5 | 66.1 | 13,400 | 19,300 | 1.12 |
| Entry | y/x | Time (h) | Conv. (%) | Mn,th a (g/mol) | Mn,GPC (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | 0.5 | 5.5 | 19.8 | 4000 | 5600 | 1.10 |
| 2 | 0.8 | 5.5 | 30.2 | 6000 | 8100 | 1.11 |
| 3 | 1.0 | 5.5 | 39.4 | 7900 | 8900 | 1.11 |
| 4 | 1.5 | 5.5 | 43.6 | 8700 | 10,200 | 1.13 |
| 5 | 2.0 | 5.5 | 46.3 | 9300 | 10,300 | 1.14 |
| Entry | Recycling Times | Conv. (%) | Mn,th b (g/mol) | Mn,GPC (g/mol) | Mw/Mn | CR c (ppm) |
|---|---|---|---|---|---|---|
| 1 | 1 | 59.8 | 11,980 | 18,500 | 1.12 | 1.3 |
| 2 | 2 | 55.7 | 11,200 | 15,600 | 1.09 | 1.5 |
| 3 | 3 | 60.2 | 12,100 | 18,100 | 1.12 | 2.2 |
| 4 | 4 | 52.4 | 10,500 | 18,900 | 1.10 | 2.0 |
| 5 | 5 | 52.3 | 13,000 | 12,200 | 1.07 | 1.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Zhang, B.; Jiang, H.; Zhang, L.; Zhu, X. Poly(Ionic Liquid): A New Phase in a Thermoregulated Phase Separated Catalysis and Catalyst Recycling System of Transition Metal-Mediated ATRP. Polymers 2018, 10, 347. https://doi.org/10.3390/polym10040347
Yao L, Zhang B, Jiang H, Zhang L, Zhu X. Poly(Ionic Liquid): A New Phase in a Thermoregulated Phase Separated Catalysis and Catalyst Recycling System of Transition Metal-Mediated ATRP. Polymers. 2018; 10(4):347. https://doi.org/10.3390/polym10040347
Chicago/Turabian StyleYao, Lan, Bingjie Zhang, Hongjuan Jiang, Lifen Zhang, and Xiulin Zhu. 2018. "Poly(Ionic Liquid): A New Phase in a Thermoregulated Phase Separated Catalysis and Catalyst Recycling System of Transition Metal-Mediated ATRP" Polymers 10, no. 4: 347. https://doi.org/10.3390/polym10040347