Effect of Counterion Valence on Conformational Behavior of Spherical Polyelectrolyte Brushes Confined between Two Parallel Walls
Abstract
:1. Introduction
2. Simulation Model and Method
3. Results and Discussion
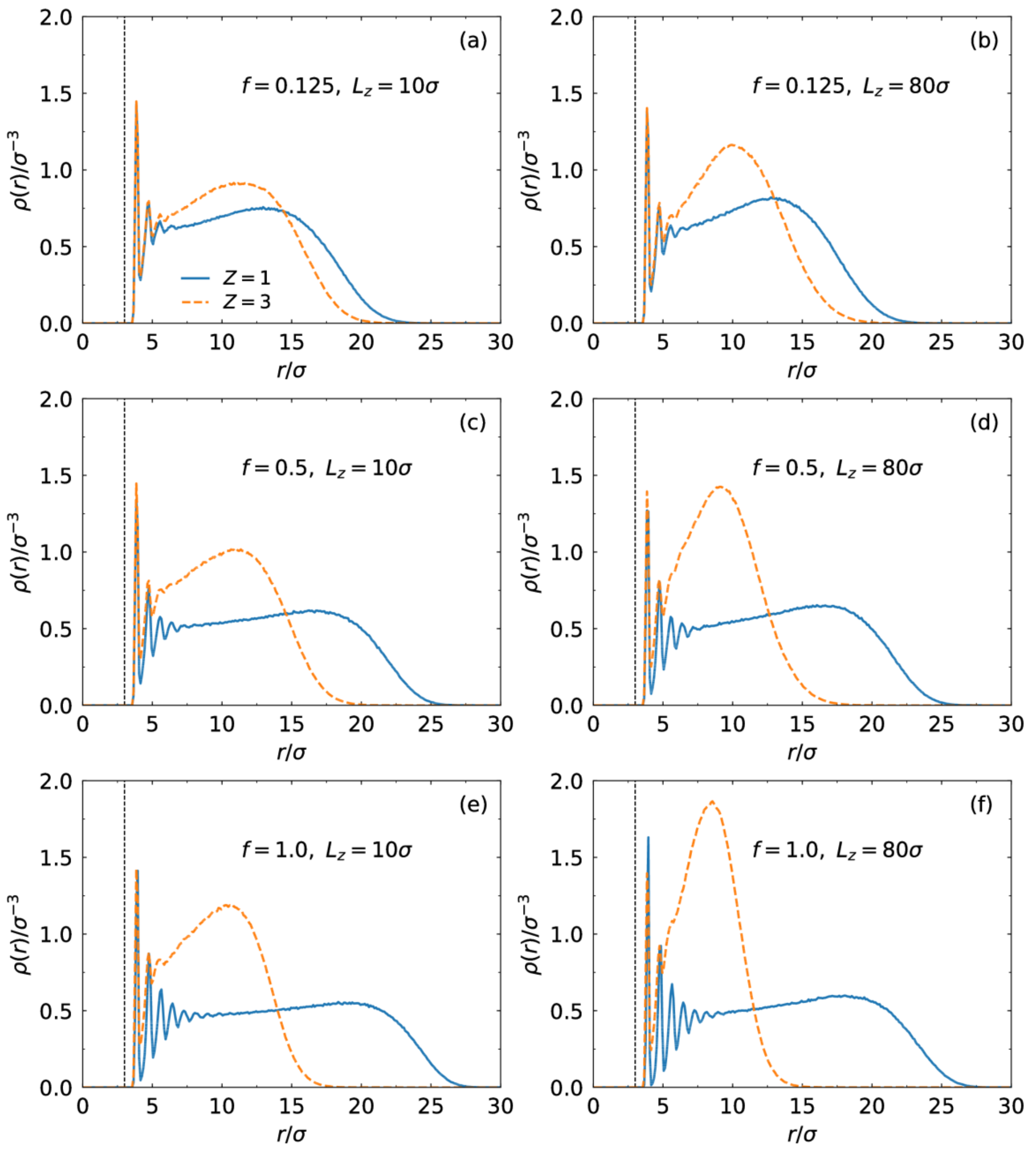
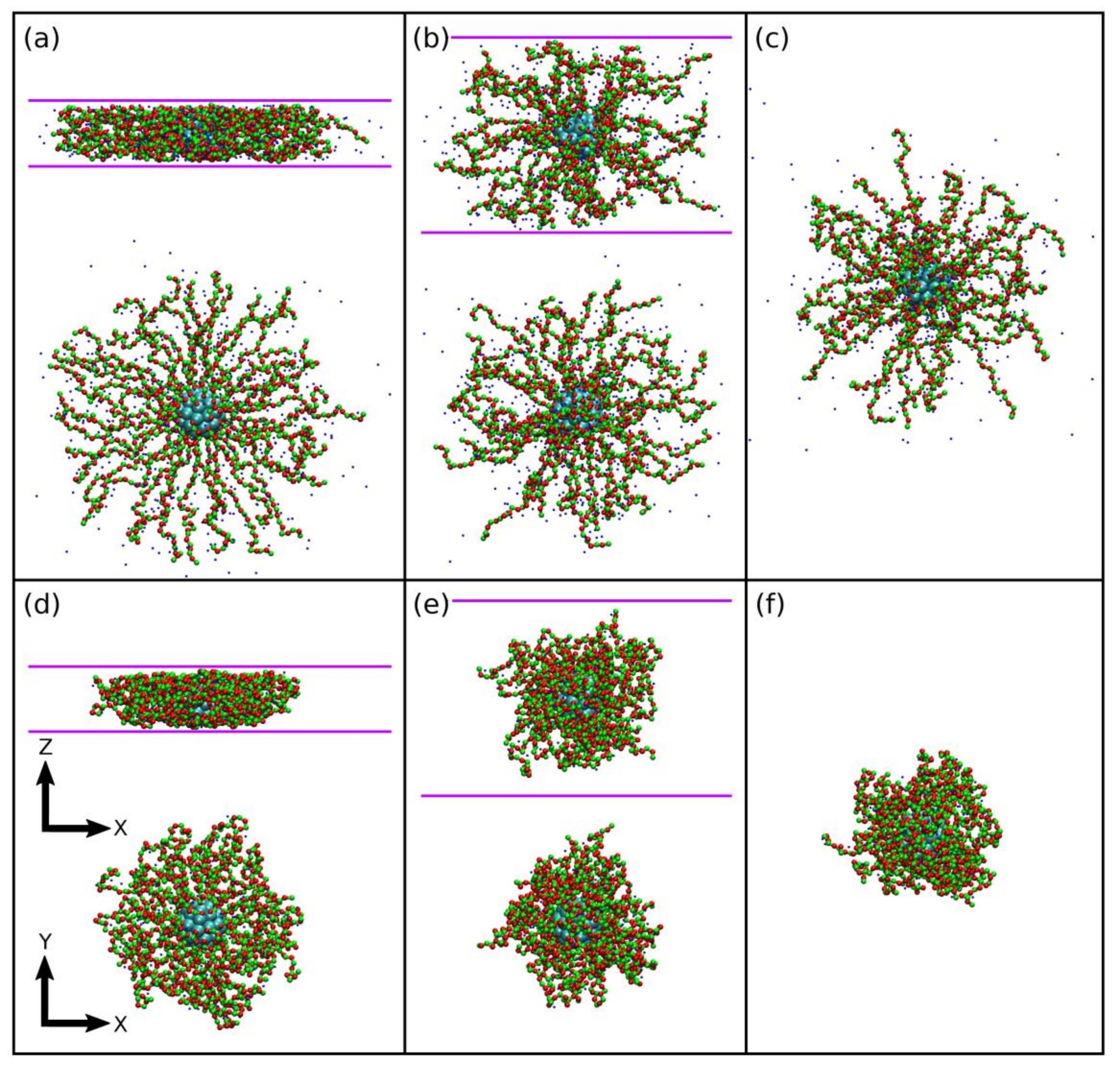
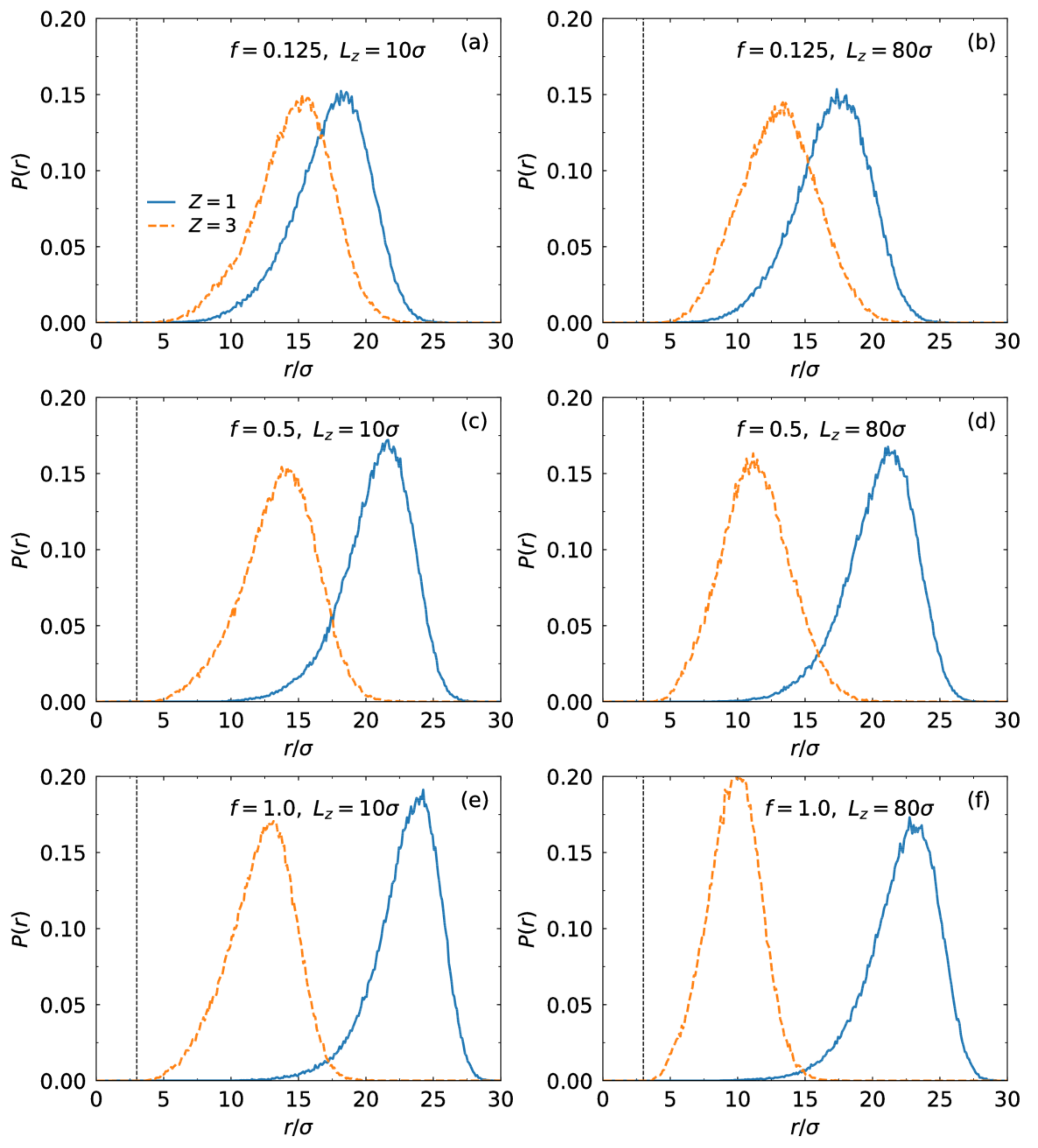
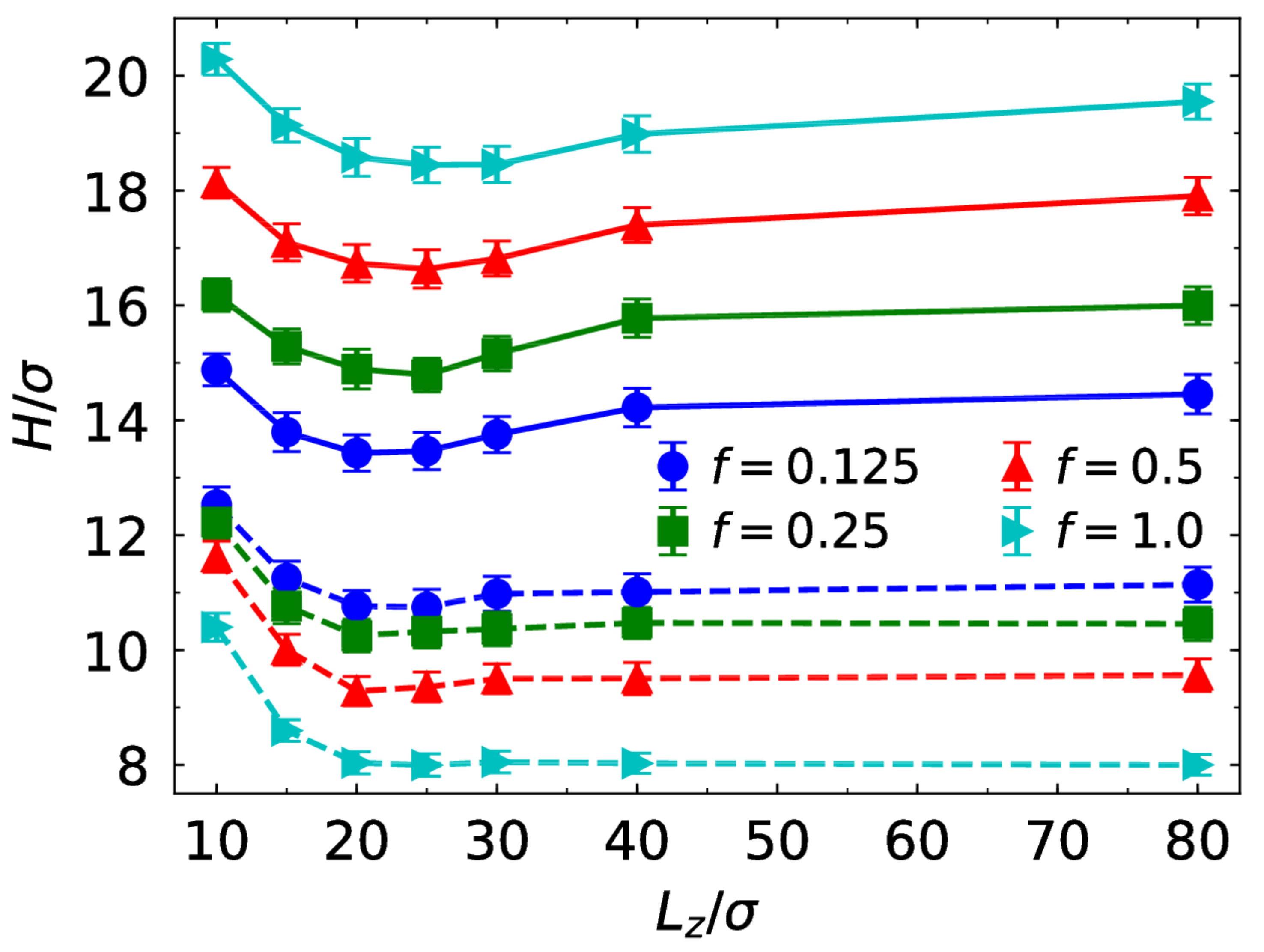
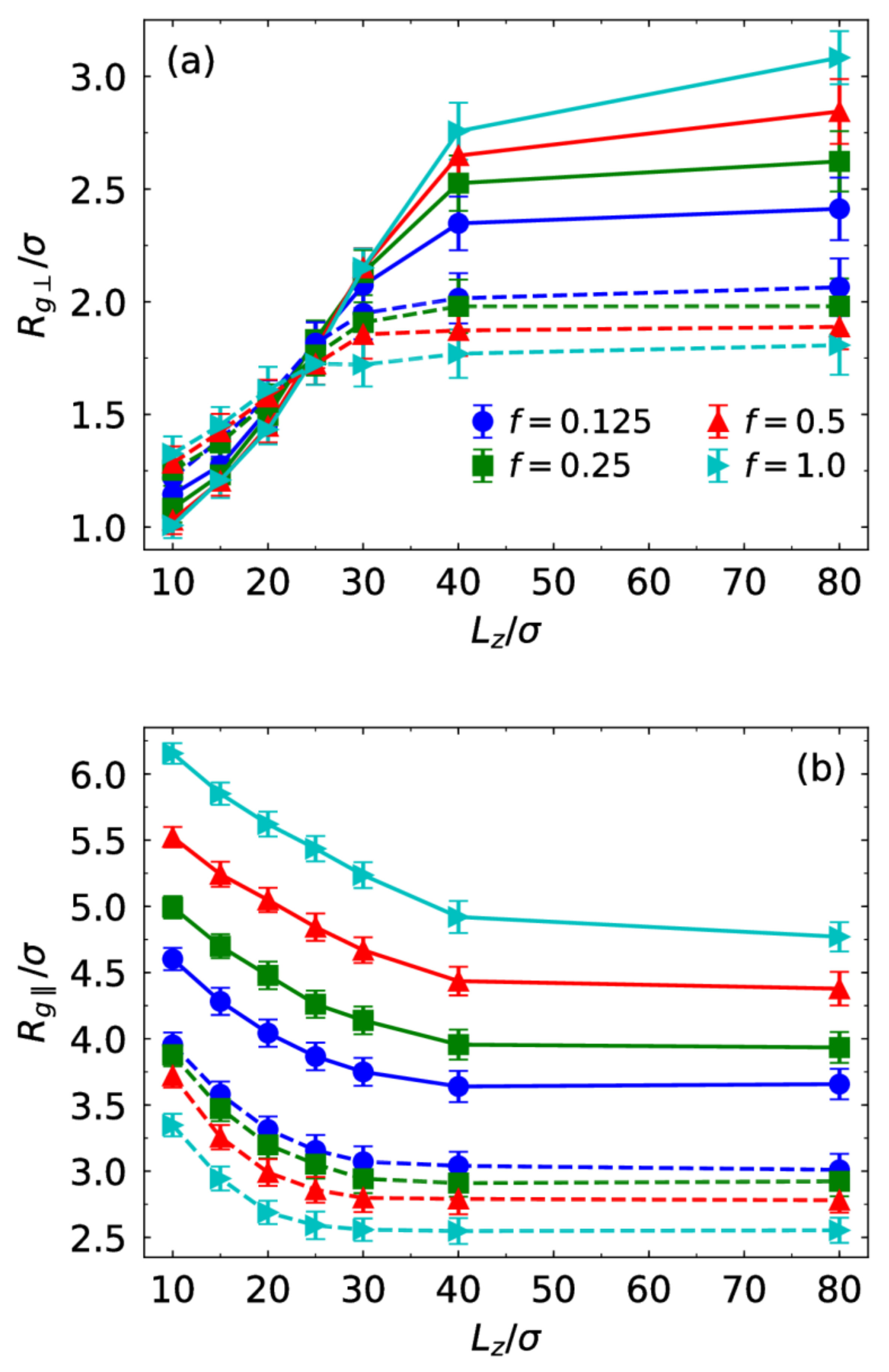
3.1. Brush Conformation
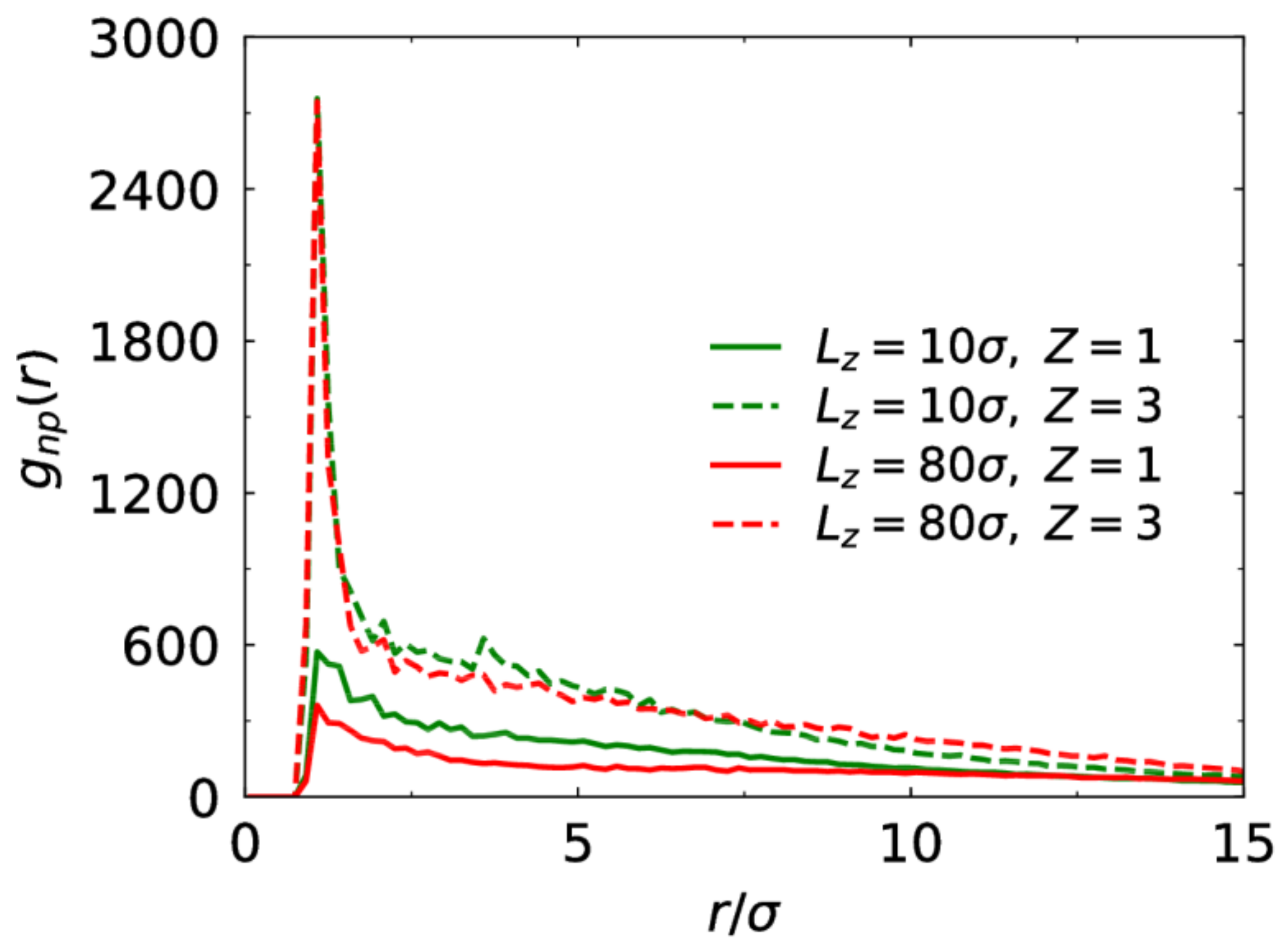
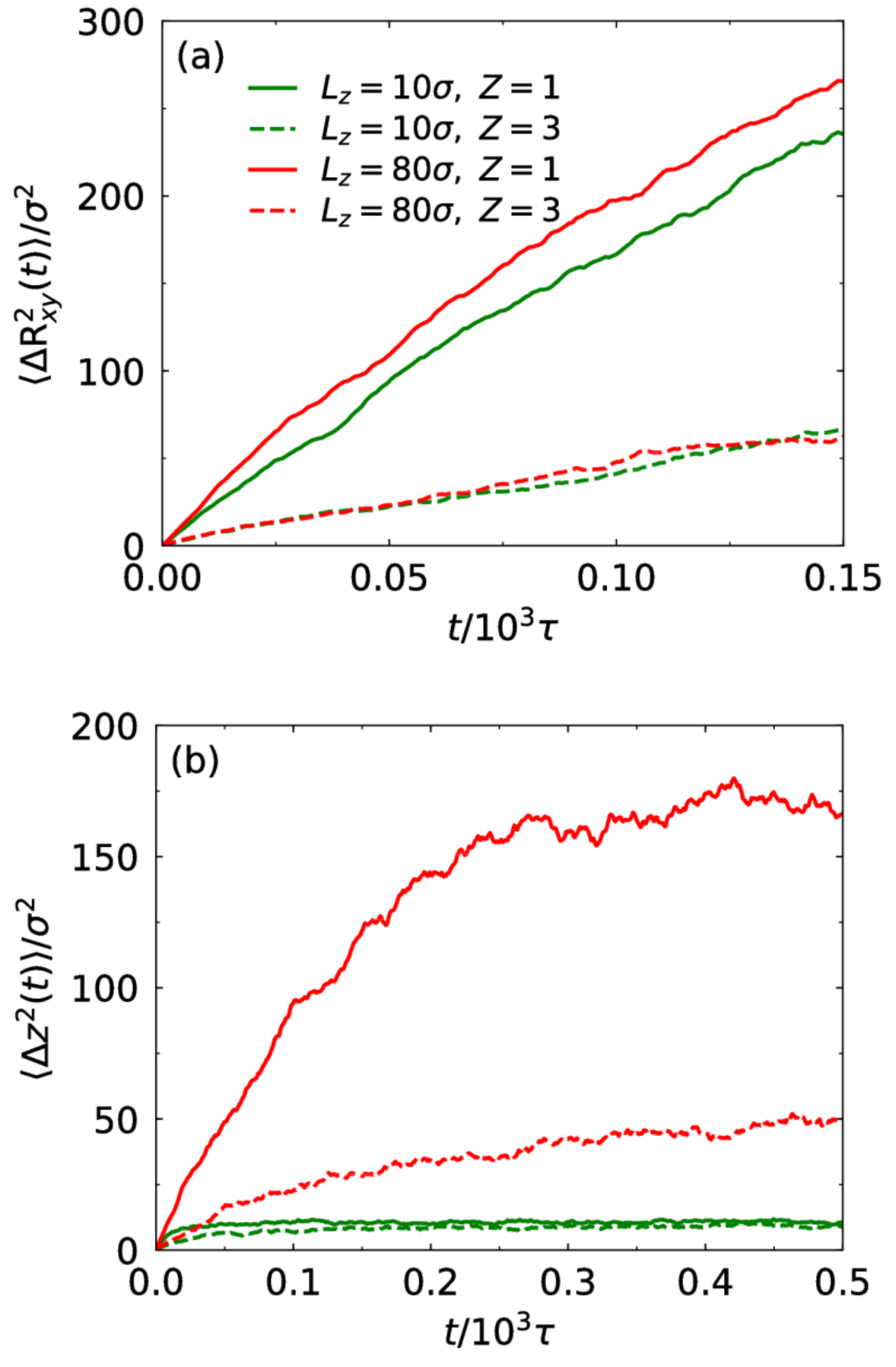
3.2. Electrostatic Correlation and Counterion Diffusion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rühe, J.; Ballauff, M.; Biesalski, M.; Dziezok, P.; Grohn, F.; Johannsmann, D.; Houbenov, N.; Hugenberg, N.; Konradi, R.; Minko, S.; et al. Polyelectrolyte brushes. Adv. Polym. Sci. 2004, 165, 79–150. [Google Scholar]
- Ballauff, M.; Borisov, O. Polyelectrolyte brushes. Curr. Opin. Colloid Interface Sci. 2006, 11, 316–323. [Google Scholar] [CrossRef]
- Ballauff, M. Spherical polyelectrolyte brushes. Prog. Polym. Sci. 2007, 32, 1135–1151. [Google Scholar] [CrossRef]
- Toomey, R.; Tirrell, M. Functional polymer brushes in aqueous media from self-assembled and surface-initiated polymers. Annu. Rev. Phys. Chem. 2008, 59, 493–517. [Google Scholar] [CrossRef] [PubMed]
- Mercurieva, A.A.; Birshtein, T.M.; Zhulina, E.B.; Iakovlev, P.; van Male, J.; Leermakers, F.A.M. An annealed polyelectrolyte brush in a polar-nonpolar binary solvent: Effect of pH and ionic strength. Macromolecules 2002, 35, 4739–4752. [Google Scholar] [CrossRef]
- Israels, R.; Leermakers, F.A.M.; Fleer, G.J.; Zhulina, E.B. Charged Polymeric Brushes: Structure and Scaling Relations. Macromolecules 1994, 27, 3249–3261. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V.; van Male, J.; Leermakers, F.A.M. Adsorption of tethered polyelectrolytes onto oppositely charged solid-liquid interfaces. Langmuir 2001, 17, 1277–1293. [Google Scholar] [CrossRef]
- Pincus, P. Colloid stabilization with grafted polyelectrolytes. Macromolecules 1991, 24, 2912–2919. [Google Scholar] [CrossRef]
- Csajka, F.S.; Netz, R.R.; Seidel, C.; Joanny, J.F. Collapse of polyelectrolyte brushes: scaling theory and simulations. Eur. Phys. J. E 2001, 4, 505–513. [Google Scholar] [CrossRef]
- Borisov, O.V.; Birshtein, T.M.; Zhulina, E.B. Collapse of grafted polyelectrolyte layer. J. Phys. II 1991, 1, 521–526. [Google Scholar] [CrossRef]
- Nap, R.; Gong, P.; Szleifer, I. Weak polyelectrolytes tethered to surfaces: effect of geometry, acid-base equilibrium and electrical permittivity. J. Polym. Sci., Part B: Polym. Phys. 2006, 44, 2638–2662. [Google Scholar] [CrossRef]
- Gong, P.; Genzer, J.; Szleifer, I. Phase behavior and charge regulation of weak polyelectrolyte grafted layers. Phys. Rev. Lett. 2007, 98, 018302. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wu, T.; Genzer, J.; Szleifer, I. Behavior of surface-anchored poly(acrylic acid) brushes with grafting density gradients on solid substrates: 2. Theory. Macromolecules 2007, 40, 8765–8773. [Google Scholar] [CrossRef]
- Csajka, F.S.; Seidel, C. Strongly charged polyelectrolyte brushes: a molecular dynamics study. Macromolecules 2000, 33, 2728–2739. [Google Scholar] [CrossRef]
- Seidel, C. Strongly stretched polyelectrolyte brushes. Macromolecules 2003, 36, 2536–2543. [Google Scholar] [CrossRef]
- Naji, A.; Netz, R.R.; Seidel, C. Non-linear osmotic brush regime: Simulations and mean-field theory. Eur. Phys. J. E 2003, 12, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Hehmeyer, O.J.; Arya, G.; Panagiotopoulos, A.Z.; Szleifer, I. Monte Carlo simulation and molecular theory of tethered polyelectrolytes. J. Chem. Phys. 2007, 126, 244902. [Google Scholar] [CrossRef] [PubMed]
- Guptha, V.S.; Hsiao, P.Y. Polyelectrolyte brushes in monovalent and multivalent salt solutions. Polymer 2014, 55, 2900–2912. [Google Scholar] [CrossRef]
- Yan, L.T.; Xu, Y.Y.; Ballauff, M.; Muller, A.H.E.; Boker, A. Influence of Counterion Valency on the Conformational Behavior of Cylindrical Polyelectrolyte Brushes. J. Phys. Chem. B 2009, 113, 5104–5110. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Hoffmann, M.; Ballauff, M.; Jusufi, A. Spherical polyelectrolyte brushes in the presence of multivalent counterions: The effect of fluctuations and correlations as determined by molecular dynamics simulations. Phys. Rev. E 2008, 77, 031805. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Cao, D.; Wang, W.; Jusufi, A. Conformation of a Spherical Polyelectrolyte Brush in the Presence of Oppositely Charged Linear Polyelectrolytes. Macromolecules 2008, 41, 5477–5484. [Google Scholar] [CrossRef]
- Cao, Q.; Zuo, C.; Li, L. Electrostatic binding of oppositely charged surfactants to spherical polyelectrolyte brushes. Phys. Chem. Chem. Phys. 2011, 13, 9706–9715. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.Q.; Zuo, C.C.; Li, L.J.; Zhang, Y.H. Modulation of electroosmotic flow by electric field-responsive polyelectrolyte brushes: A molecular dynamics study. Microfluid. Nanofluid. 2012, 12, 649–655. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Zuo, C.C.; Li, L.J.; Yan, G. Effects of chain stiffness and salt concentration on responses of polyelectrolyte brushes under external electric field. Biomicrofluidics 2011, 5, 044119. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Xia, Z.H.; Zhe, J. Voltage-controlled flow regulating in nanofluidic channels with charged polymer brushes. Microfluid. Nanofluid. 2010, 9, 915–922. [Google Scholar] [CrossRef]
- Binder, K.; Horbach, J.; Vink, R.; De Virgiliis, A. Confinement effects on phase behavior of soft matter systems. Soft Matter 2008, 4, 1555–1568. [Google Scholar] [CrossRef]
- Fritsche, M.; Heermann, D.W. Confinement driven spatial organization of semiflexible ring polymers: Implications for biopolymer packaging. Soft Matter 2011, 7, 6906–6913. [Google Scholar] [CrossRef]
- Reisner, W.; Pedersen, J.N.; Austin, R.H. DNA confinement in nanochannels: physics and biological applications. Rep. Prog. Phys. 2012, 75, 106601. [Google Scholar] [CrossRef] [PubMed]
- Bakajin, O.B.; Duke, T.A.J.; Chou, C.F.; Chan, S.S.; Austin, R.H.; Cox, E.C. Electrohydrodynamic Stretching of DNA in Confined Environments. Phys. Rev. Lett. 1998, 80, 2737–2740. [Google Scholar] [CrossRef]
- Javidpour, L.; Sahimi, M. Confinement in nanopores can destabilize α-helix folding proteins and stabilize the β structures. J. Chem. Phys. 2011, 135, 125101. [Google Scholar] [CrossRef] [PubMed]
- Paturej, J.; Milchev, A.; Egorov, S.A.; Binder, K. Star polymers confined in a nanoslit: a simulation test of scaling and self-consistent field theories. Soft Matter 2013, 9, 10522–10531. [Google Scholar] [CrossRef]
- Konieczny, M.; Likos, C.N. Polyelectrolyte stars in planar confinement. J. Chem. Phys. 2006, 124, 214904. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, M.; Likos, C.N. From sea-urchins to starfishes: controlling the adsorption of star-branched polyelectrolytes on charged walls. Soft Matter 2007, 3, 1130–1134. [Google Scholar] [CrossRef]
- Cao, Q.; You, H. Morphologies of spherical polyampholyte brushes: Effects of counterion valence and charged monomer sequence. Polymer 2017, 113, 233–246. [Google Scholar] [CrossRef]
- Kremer, K.; Grest, G.S. Dynamics of entangled linear polymer melts: A molecular-dynamics simulation. J. Chem. Phys. 1990, 92, 5057–5086. [Google Scholar] [CrossRef]
- Yeh, I.-C.; Berkowitz, M.L. Ewald summation for systems with slab geometry. J. Chem. Phys. 1999, 111, 3155–3162. [Google Scholar] [CrossRef]
- Mei, Y.; Lauterbach, K.; Hoffmann, M.; Borisov, O.V.; Ballauff, M.; Jusufi, A. Collapse of Spherical Polyelectrolyte Brushes in the Presence of Multivalent Counterions. Phys. Rev. Lett. 2006, 97, 158301. [Google Scholar] [CrossRef] [PubMed]
- Solis, F.J.; Cruz, M.O.d.l. Collapse of flexible polyelectrolytes in multivalent salt solutions. J. Chem. Phys. 2000, 112, 2030–2035. [Google Scholar] [CrossRef]
- He, S.; Arscott, P.G.; Bloomfield, V.A. Condensation of DNA by multivalent cations: Experimental studies of condensation kinetics. Biopolymers 2000, 53, 329–341. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Cao, Q.; Zuo, C. Effect of Counterion Valence on Conformational Behavior of Spherical Polyelectrolyte Brushes Confined between Two Parallel Walls. Polymers 2018, 10, 363. https://doi.org/10.3390/polym10040363
Li L, Cao Q, Zuo C. Effect of Counterion Valence on Conformational Behavior of Spherical Polyelectrolyte Brushes Confined between Two Parallel Walls. Polymers. 2018; 10(4):363. https://doi.org/10.3390/polym10040363
Chicago/Turabian StyleLi, Lujuan, Qianqian Cao, and Chuncheng Zuo. 2018. "Effect of Counterion Valence on Conformational Behavior of Spherical Polyelectrolyte Brushes Confined between Two Parallel Walls" Polymers 10, no. 4: 363. https://doi.org/10.3390/polym10040363



