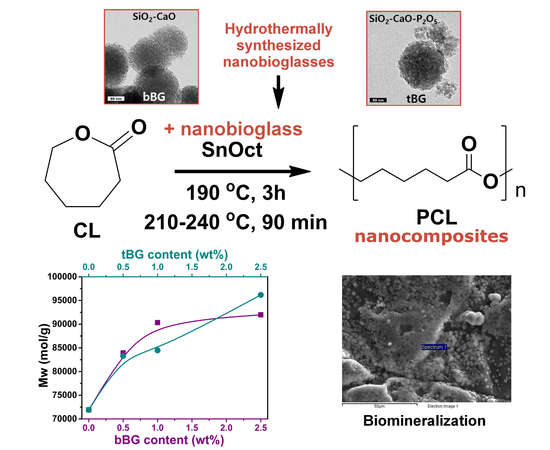
Biocompatible Nanobioglass Reinforced Poly(ε-Caprolactone) Composites Synthesized via In Situ Ring Opening Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Binary and Ternary Nanobioglasses
2.3. Synthesis of PCL Nanocomposites
2.4. Physicochemical Characterization
2.5. Cell Cultures
2.5.1. Isolation, Cultivation and Genetic Modification of Wharton Jelly-Derived Mesenchymal Stem Cells (WJ-MSCs)
2.5.2. Sterilization of the Materials and WJ-SCs Plating
2.5.3. 3-[4,5-Dimethylthiazole-2-yl]-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.5.4. Observation in a Fluorescence Microscope
3. Results and Discussion
3.1. Characterization of the Nanofillers
3.2. Synthesis and Characterization of PCL Nanocomposites
3.2.1. Synthesis of PCL Nanocomposites via In Situ ROP
3.2.2. Morphological Characterization
3.2.3. Structural Characterization
3.2.4. Mechanical Properties
3.2.5. Thermal Characterization
3.2.6 Wettability and Enzymatic Hydrolysis
3.2.7 In Vitro Bioactivity
3.2.8 Adhesion and Proliferation of WJ-SCs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A



References
- Wan, C.; Chen, B. Poly(ε-caprolactone)/graphene oxide biocomposites: Mechanical properties and bioactivity. Biomed. Mater. 2011, 6, 55010. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Bikiaris, D.N. Nanocomposites of aliphatic polyesters: An overview of the effect of different nanofillers on enzymatic hydrolysis and biodegradation of polyesters. Polym. Degrad. Stab. 2012, 97, 2077–2089. [Google Scholar] [CrossRef]
- Nerantzaki, M.C.; Koliakou, I.G.; Kaloyianni, M.G.; Terzopoulou, Z.N.; Siska, E.K.; Karakassides, M.A.; Boccaccini, A.R.; Bikiaris, D.N. New N-(2-carboxybenzyl)chitosan composite scaffolds containing nanoTiO2 or bioactive glass with enhanced cell proliferation for bone-tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 71–81. [Google Scholar] [CrossRef]
- Ng, K.M.; Lau, Y.T.R.; Chan, C.M.; Weng, L.T.; Wu, J. Surface studies of halloysite nanotubes by XPS and ToF-SIMS. Surf. Interface Anal. 2011, 43, 795–802. [Google Scholar] [CrossRef]
- Alani, A.; Knowles, J.C.; Chrzanowski, W.; Ng, Y.L.; Gulabivala, K. Ion release characteristics, precipitate formation and sealing ability of a phosphate glass-polycaprolactone-based composite for use as a root canal obturation material. Dent. Mater. 2009, 25, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K.; Cheung, K.M.C. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Cunningham, E.; Lennon, A.; McCarthy, H.O.; Buchanan, F.; Clarke, S.A.; Dunne, N. Effects of poly(ε-caprolactone) coating on the properties of three-dimensional printed porous structures. J. Mech. Behav. Biomed. Mater. 2017, 70, 68–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Michalczyk, C.; Singer, F.; Virtanen, S.; Boccaccini, A.R. In vitro study of polycaprolactone/bioactive glass composite coatings on corrosion and bioactivity of pure Mg. Appl. Surf. Sci. 2015, 355, 832–841. [Google Scholar] [CrossRef]
- Shi, M.; Zhai, D.; Zhao, L.; Wu, C.; Chang, J. Nanosized Mesoporous Bioactive Glass/Poly(lactic-co-glycolic Acid) Composite-Coated CaSiO3 Scaffolds with Multifunctional Properties for Bone Tissue Engineering. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Mavis, B.; Demirtaş, T.T.; Gümüşderelioğlu, M.; Gündüz, G.; Çolak, Ü. Synthesis, characterization and osteoblastic activity of polycaprolactone nanofibers coated with biomimetic calcium phosphate. Acta Biomater. 2009, 5, 3098–3111. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, T.; Videira, A.C.; Bártolo, P.; Strauch, M.; Murch, G.E.; Ferreira, J.M.F. On the mechanical properties of PLC-bioactive glass scaffolds fabricated via BioExtrusion. Mater. Sci. Eng. C 2015, 57, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Menaszek, E.; Zagrajczuk, B.; Pawlik, J.; Cholewa-Kowalska, K. New generation poly(ε-caprolactone)/gel-derived bioactive glass composites for bone tissue engineering: Part I. Material properties. Mater. Sci. Eng. C 2015, 56, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Lin, Y.H.; Hsu, F.Y. Preparation and characterization of mesoporous bioactive glass/polycaprolactone nanofibrous matrix for bone tissues engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Hutmacher, D.W.; Stevens, M.M.; Woodruff, M.A. Fabrication and in vitro characterization of bioactive glass composite scaffolds for bone regeneration. Biofabrication 2014, 6, 29501. [Google Scholar] [CrossRef]
- Ródenas-Rochina, J.; Ribelles, J.L.G.; Lebourg, M. Comparative study of PCL-HAp and PCL-bioglass composite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Chrissafis, K.; Antoniadis, G.; Paraskevopoulos, K.M.; Vassiliou, A.; Bikiaris, D.N. Comparative study of the effect of different nanoparticles on the mechanical properties and thermal degradation mechanism of in situ prepared poly(ε-caprolactone) nanocomposites. Compos. Sci. Technol. 2007, 67, 2165–2174. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. In situ polymerization approach to graphene-reinforced nylon-6 composites. Macromolecules 2010, 43, 6716–6723. [Google Scholar] [CrossRef]
- Jiang, X.; Bin, Y.; Matsuo, M. Electrical and mechanical properties of polyimide–carbon nanotubes composites fabricated by in situ polymerization. Polymer 2005, 46, 7418–7424. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Filippousi, M.; Van Tendeloo, G.; Terzopoulou, Z.; Bikiaris, D.; Goudouri, O.M.; Detsch, R.; Gruenewald, A.; Boccaccini, A.R. Novel poly(butylene succinate) nanocomposites containing strontium hydroxyapatite nanorods with enhanced osteoconductivity for tissue engineering applications. Express Polym. Lett. 2015, 9, 773–789. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Mkhabela, V.J.; Ray, S.S. Poly(ε-caprolactone) Nanocomposite Scaffolds for Tissue Engineering: A Brief Overview. J. Nanosci. Nanotechnol. 2014, 14, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Nerantzaki, M.; Koliakou, I.; Kaloyianni, M.G.; Koumentakou, I.; Siska, E.; Diamanti, E.; Karakassides, M.A.; Boccaccini, A.R.; Bikiaris, D.N. A biomimetic approach for enhancing adhesion and osteogenic differentiation of adipose-derived stem cells on poly(butylene succinate) composites with bioactive ceramics and glasses. Eur. Polym. J. 2017, 87, 159–173. [Google Scholar] [CrossRef]
- Baciu, D.; Th, S.; Charalambopoulou, G.; Stubos, A. Synthesis and characterization of well-ordered mesoporous bioglass nanospheres for biomedical applications. In Proceedings of the 10th Anniversary Conference of the Hellenic Society for Biomaterials, Athens, Greece, 26–28 November 2015. [Google Scholar]
- Baciu, D.; Ioannou, Z.; Th, S.; Charalambopoulou, G.; Stubos, A. Removal of methylene blue from aqueous solutions using an ordered mesoporous SiO2–CaO ceramic sorbent. In Proceedings of the 14th International Conference on Environmental Science and Technology (CEST 2015), Rhodes, Greece, 3–5 September 2015. [Google Scholar]
- Khambatta, F.B.; Warner, F.; Russell, T.; Stein, R.S. Small-angle X-ray and light scattering studies of the morphology of blends of poly(ε-caprolactone) with poly (vinyl chloride). J. Polym. Sci. Part B Polym. Phys. 1976, 14, 1391–1424. [Google Scholar] [CrossRef]
- Vazquez, N.I.; Gonzalez, Z.; Ferrari, B.; Castro, Y. Synthesis of mesoporous silica nanoparticles by sol–gel as nanocontainer for future drug delivery applications. Bol. Soc. Esp. Cerám. Vidr. 2017, 56, 139–145. [Google Scholar] [CrossRef]
- Liang, Y.; Lu, S.; Wu, D.; Sun, B.; Xu, F.; Fu, R. Polyethylene glycol-induced self-assembly to synthesize an ordered mesoporous polymer with a two-dimensional hexagonal structure. J. Mater. Chem. A 2013, 1, 3061–3067. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. Part A 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Aiyathurai, L.; Roether, J.A.; Luklinska, Z.B. Development of high-viscosity, two-paste bioactive bone cements. Biomaterials 2005, 26, 3713–3718. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Ragel, C.; Salinas, A.J. Glasses with medical applications. Eur. J. Inorg. Chem. 2003, 2003, 1029–1042. [Google Scholar] [CrossRef]
- Bizari, D.; Rabiee, M.; Moztarzadeh, F.; Tahriri, M.; Alavi, S.H.; Masaeli, R. Synthesis, characterization and biological evaluation of sol–gel derived nanomaterial in the ternary system 64% SiO2-31% CaO-5% P2O5 as a bioactive glass: In vitro study. Ceram. Silik. 2013, 57, 201–209. [Google Scholar]
- Pappas, G.S.; Liatsi, P.; Kartsonakis, I.A.; Danilidis, I.; Kordas, G. Synthesis and characterization of new SiO2–CaO hollow nanospheres by sol–gel method: Bioactivity of the new system. J. Non-Cryst. Solids 2008, 354, 755–760. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, D.; Li, N.; Wen, J.; Jiang, X.; Liu, C.; Li, Y. Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci. Rep. 2016, 6, 19361. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiorilli, S.L.; Mortera, R.S.; Onida, B.; Saino, E.; Visai, L.; Verné, E.; Vitale-Brovarone, C. Mesoporous bioactive glass as a multifunctional system for bone regeneration and controlled drug release. J. Appl. Biomater. Funct. Mater. 2012, 10, 12–21. [Google Scholar] [PubMed]
- Arcos, D.; Vila, M.; López-Noriega, A.; Rossignol, F.; Champion, E.; Oliveira, F.J.; Vallet-Regí, M. Mesoporous bioactive glasses: Mechanical reinforcement by means of a biomimetic process. Acta Biomater. 2011, 7, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Moztarzadeh, F.; Tahriri, M. Investigation of the physico-chemical reactivity of a mesoporous bioactive SiO2–CaO–P2O5 glass in simulated body fluid. J. Non-Cryst. Solids 2010, 356, 1470–1478. [Google Scholar] [CrossRef]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Alsonso-Moreno, C.; Garcia-Martinez, J.; Fernandez-Baeza, J.; Sanchez-Barba, L.; Lara-Sanchez, A.; Otero, A. Ring-Opening (ROP) versus Ring-Expansion (REP) Polymerization of ε-Caprolactone To Give Linear or Cyclic Polycaprolactones. Macromolecules 2013, 46, 6388–6394. [Google Scholar]
- Miyata, N.; Fuke, K.I.; Chen, Q.; Kawashita, M.; Kokubo, T.; Nakamura, T. Apatite-forming ability and mechanical properties of PTMO-modified CaO–SiO2 hybrids prepared by sol–gel processing: Effect of CaO and PTMO contents. Biomaterials 2002, 23, 3033–3040. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Mechanism of Cyclic Ester Polymerization Initiated with Tin(II) Octoate. 2. Macromolecules Fitted with Tin(II) Alkoxide Species Observed Directly in MALDI−TOF Spectra. Macromolecules 2000, 33, 689–695. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Helwig, E.; Sandner, B.; Gopp, U.; Vogt, F.; Wartewig, S.; Henning, S. Ring-opening polymerization of lactones in the presence of hydroxyapatite. Biomaterials 2001, 22, 2695–2702. [Google Scholar] [CrossRef]
- Vassiliou, A.A.; Chrissafis, K.; Bikiaris, D.N. In situ prepared PBSu/SiO2 nanocomposites. Study of thermal degradation mechanism. Thermochim. Acta 2009, 495, 120–128. [Google Scholar] [CrossRef]
- Vasileiou, A.A.; Papageorgiou, G.Z.; Kontopoulou, M.; Docoslis, A.; Bikiaris, D. Covalently bonded poly(ethylene succinate)/SiO2 nanocomposites prepared by in situ polymerisation. Polymer 2013, 54, 1018–1032. [Google Scholar] [CrossRef]
- Vassiliou, A.A.; Bikiaris, D.; El Mabrouk, K.; Kontopoulou, M. Effect of evolved interactions in poly (butylene succinate)/fumed silica biodegradable in situ prepared nanocomposites on molecular weight, material properties, and biodegradability. J. Appl. Polym. Sci. 2011, 119, 2010–2024. [Google Scholar] [CrossRef]
- Fan, R.R.; Zhou, L.X.; Li, D.X.; Zhang, D.M.; Wu, M.; Guo, G. Preparation and Characterization of Composites Based on Poly (Butylene Succinate) and Poly (Lactic Acid) Grafted Tetracalcium Phosphate. J. Macromol. Sci. Part B 2014, 53, 296–308. [Google Scholar] [CrossRef]
- Tamjid, E.; Bagheri, R.; Vossoughi, M.; Simchi, A. Effect of particle size on the in vitro bioactivity, hydrophilicity and mechanical properties of bioactive glass-reinforced polycaprolactone composites. Mater. Sci. Eng. C 2011, 31, 1526–1533. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of surface functionalization of halloysite nanotubes on synthesis and thermal properties of poly(ε-caprolactone). J. Mater. Sci. 2018, 53, 6519–6541. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of catalyst type on recyclability and decomposition mechanism of poly(ethylene furanoate) biobased polyester. J. Anal. Appl. Pyrolysis 2017, 126, 357–370. [Google Scholar] [CrossRef]
- Hafezi, M.; Safarian, S.; Khorasani, M.T.; Abu Osman, N.A. Polyurethane/58S bioglass nanofibers: Synthesis, characterization, and in vitro evaluation. RSC Adv. 2016, 6, 35815–35824. [Google Scholar] [CrossRef]
- Asefnejad, A. Polyurethane/fluor-hydroxyapatite nanocomposite scaffolds for bone tissue engineering. Part I: Morphological, physical, and mechanical characterization. Int. J. Nanomed. 2011, 6, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, X.S. Preparation and characterization of polymer-Inorganic nanocomposites by in situ melt polycondensation of l-Lactic acid and surface-hydroxylated MgO. Biomacromolecules 2010, 11, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Dell’Era, A.; Vecchio Ciprioti, S. Synthesis, structural, spectroscopic and thermoanalytical study of sol–gel derived SiO2–CaO–P2O5 gel and ceramic materials. Thermochim. Acta 2016, 625, 20–27. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, M.; Huang, H.; Xu, H.M.; Hong, R.J.; Shen, H. Multifunctional TiO2 nanowires-modified nanoparticles bilayer film for 3D dye-sensitized solar cells. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 1166–1169. [Google Scholar] [CrossRef]
- Bikiaris, D.; Karavelidis, V.; Karayannidis, G. A new approach to prepare poly(ethylene terephthalate)/silica nanocomposites with increased molecular weight and fully adjustable branching or crosslinking by SSP. Macromol. Rapid Commun. 2006, 27, 1199–1205. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, L.; Cook, W.D.; Mohn, D.; Lagerqvist, E.L.; Elliott, D.A.; Haynes, J.M.; Boyd, N.; Stark, W.J.; Pouton, C.W.; et al. Elastomeric nanocomposites as cell delivery vehicles and cardiac support devices. Soft Matter 2010, 6, 4715–4726. [Google Scholar] [CrossRef]
- Lei, B.; Shin, K.-H.; Noh, D.-Y.; Jo, I.-H.; Koh, Y.-H.; Kim, H.-E.; Kim, S.E. Sol–gel derived nanoscale bioactive glass (NBG) particles reinforced poly(ε-caprolactone) composites for bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Hong, Z.; Zhuang, X.; Chen, X.; Cui, Y.; Liu, Y.; Jing, X. Surface modification of bioactive glass nanoparticles and the mechanical and biological properties of poly(l-lactide) composites. Acta Biomater. 2008, 4, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Zagrajczuk, B.; Ziabka, M.; Dziadek, K.; Cholewa-Kowalska, K. The role of solvent type, size and chemical composition of bioactive glass particles in modulating material properties of poly(ε-caprolactone) based composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 90–99. [Google Scholar] [CrossRef]
- Dziadek, M.; Pawlik, J.; Menaszek, E.; Stodolak-Zych, E.; Cholewa-Kowalska, K. Effect of the preparation methods on architecture, crystallinity, hydrolytic degradation, bioactivity, and biocompatibility of PCL/bioglass composite scaffolds. J. Biomed. Mater. Res. - Part B Appl. Biomater. 2015, 103, 1580–1593. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Sinko, P.J. The role of crystallinity on differential attachment/proliferation of osteoblasts and fibroblasts on poly (caprolactone-co-glycolide) polymeric surfaces. Front. Mater. Sci. 2012, 6, 47–59. [Google Scholar] [CrossRef]
- Washburn, N.R.; Yamada, K.M.; Simon, C.G.; Kennedy, S.B.; Amis, E.J. High-throughput investigation of osteoblast response to polymer crystallinity: Influence of nanometer-scale roughness on proliferation. Biomaterials 2004, 25, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.; Petisco, S.; Sarasua, J.R. Improvement of thermal stability and mechanical properties of medical polyester composites by plasma surface modification of the bioactive glass particles. Polym. Degrad. Stab. 2013, 98, 1717–1723. [Google Scholar] [CrossRef]
- Blaker, J.J.; Maquet, V.; Jérôme, R.; Boccaccini, A.R.; Nazhat, S.N. Mechanical properties of highly porous PDLLA/Bioglass® composite foams as scaffolds for bone tissue engineering. Acta Biomater. 2005, 1, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Bismarck, A.; Boccaccini, A.R.; Young, A.M.; Nazhat, S.N. Premature degradation of poly(α-hydroxyesters) during thermal processing of Bioglass®-containing composites. Acta Biomater. 2010, 6, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass® composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of nanofiller’s type on the thermal properties and enzymatic degradation of poly(ε-caprolactone). Polym. Degrad. Stab. 2014, 108, 257–268. [Google Scholar] [CrossRef]
- Terzopoulou, Z.N.; Papageorgiou, G.Z.; Papadopoulou, E.; Athanassiadou, E.; Reinders, M.; Bikiaris, D.N. Development and study of fully biodegradable composite materials based on poly(butylene succinate) and hemp fibers or hemp shives. Polym. Compos. 2016, 37, 407–421. [Google Scholar] [CrossRef]
- Mochizuki, M.; Hirano, M.; Kanmuri, Y.; Kudo, K.; Tokiwa, Y. Hydrolysis of polycaprolactone fibers by lipase: Effects of draw ratio on enzymatic degradation. J. Appl. Polym. Sci. 1995, 55, 289–296. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Barone, D.-J.; Luklinska, Z.; Persenaire, O.; Belayew, A.; Eyckmans, J.; Schrooten, J.; Dubois, P. Osteoconductive and bioresorbable composites based on poly (l,l-lactide) and pseudowollastonite: From synthesis and interfacial compatibilization to in vitro bioactivity and in vivo osseointegration studies. Biomacromolecules 2011, 12, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Caridade, S.G.; Merino, E.G.; Alves, N.M.; de Zea Bermudez, V.; Boccaccini, A.R.; Mano, J.F. Chitosan membranes containing micro or nano-size bioactive glass particles: Evolution of biomineralization followed by in situ dynamic mechanical analysis. J. Mech. Behav. Biomed. Mater. 2013, 20, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaakkola, T.; Rich, J.; Tirri, T.; Närhi, T.; Jokinen, M.; Seppälä, J.; Yli-Urpo, A. In vitro Ca-P precipitation on biodegradable thermoplastic composite of poly(ε-caprolactone-co-dl-lactide) and bioactive glass (S53P4). Biomaterials 2004, 25, 575–581. [Google Scholar] [CrossRef]

















| Sample | n | w | ν | PDI |
|---|---|---|---|---|
| PCL | 48,400 | 71,900 | 68,300 | 1.49 |
| PCL/bBG 0.5% | 53,900 | 83,900 | 78,700 | 1.55 |
| PCL/bBG 1.0% | 59,200 | 90,300 | 85,300 | 1.53 |
| PCL/bBG 2.5% | 61,900 | 88,900 | 84,600 | 1.44 |
| PCL/tBG 0.5% | 49,900 | 83,300 | 77,900 | 1.67 |
| PCL/tBG 1.0% | 51,400 | 84,500 | 78,800 | 1.64 |
| PCL/tBG 2.5% | 61,200 | 96,200 | 90,300 | 1.57 |
| Sample | Tm (°C) | Tc (°C) | Xc (%) |
|---|---|---|---|
| PCL | 65.4 | 31.9 | 59.96 |
| PCL/bBG 0.5% | 66.4 | 32.1 | 62.57 |
| PCL/bBG 1.0% | 67.1 | 32.4 | 67.83 |
| PCL/bBG 2.5% | 64.8 | 31.4 | 50.31 |
| PCL/tBG 0.5% | 66.4 | 32.3 | 65.12 |
| PCL/tBG 1.0% | 66.8 | 32.3 | 72.02 |
| PCL/tBG 2.5% | 65.1 | 30.7 | 57.43 |
| Sample | Td,2%wt (°C) | Tmax (°C) |
|---|---|---|
| PCL | 329.9 | 438.2 |
| PCL/bBG 0.5% | 333.6 | 436.9 |
| PCL/bBG 1.0% | 335.8 | 434.8 |
| PCL/bBG 2.5% | 304.9 | 430.0 |
| PCL/tBG 0.5% | 330.1 | 436.9 |
| PCL/tBG 1.0% | 330.0 | 436.0 |
| PCL/tBG 2.5% | 311.8 | 431.9 |
| Sample | Contact angle (°) |
|---|---|
| PCL | 85.3 ± 0.68 |
| PCL/bBG 0.5% | 81.7 ± 0.3 |
| PCL/bBG 1.0% | 75.7 ± 0.41 |
| PCL/bBG 2.5% | 73.2 ± 0.3 |
| PCL/tBG 0.5% | 80 ± 2.87 |
| PCL/tBG 1.0% | 74.1 ± 0.48 |
| PCL/tBG 2.5% | 71.8 ± 0.21 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Bikiaris, D. Biocompatible Nanobioglass Reinforced Poly(ε-Caprolactone) Composites Synthesized via In Situ Ring Opening Polymerization. Polymers 2018, 10, 381. https://doi.org/10.3390/polym10040381
Terzopoulou Z, Baciu D, Gounari E, Steriotis T, Charalambopoulou G, Bikiaris D. Biocompatible Nanobioglass Reinforced Poly(ε-Caprolactone) Composites Synthesized via In Situ Ring Opening Polymerization. Polymers. 2018; 10(4):381. https://doi.org/10.3390/polym10040381
Chicago/Turabian StyleTerzopoulou, Zoi, Diana Baciu, Eleni Gounari, Theodore Steriotis, Georgia Charalambopoulou, and Dimitrios Bikiaris. 2018. "Biocompatible Nanobioglass Reinforced Poly(ε-Caprolactone) Composites Synthesized via In Situ Ring Opening Polymerization" Polymers 10, no. 4: 381. https://doi.org/10.3390/polym10040381