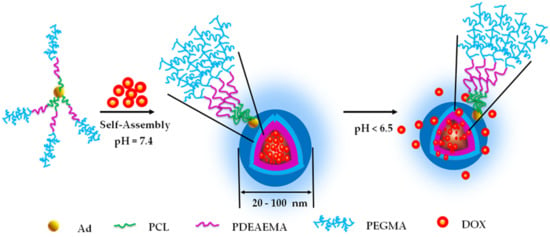
pH-Sensitive Micelles Based on Star Copolymer Ad-(PCL-b-PDEAEMA-b-PPEGMA)4 for Controlled Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
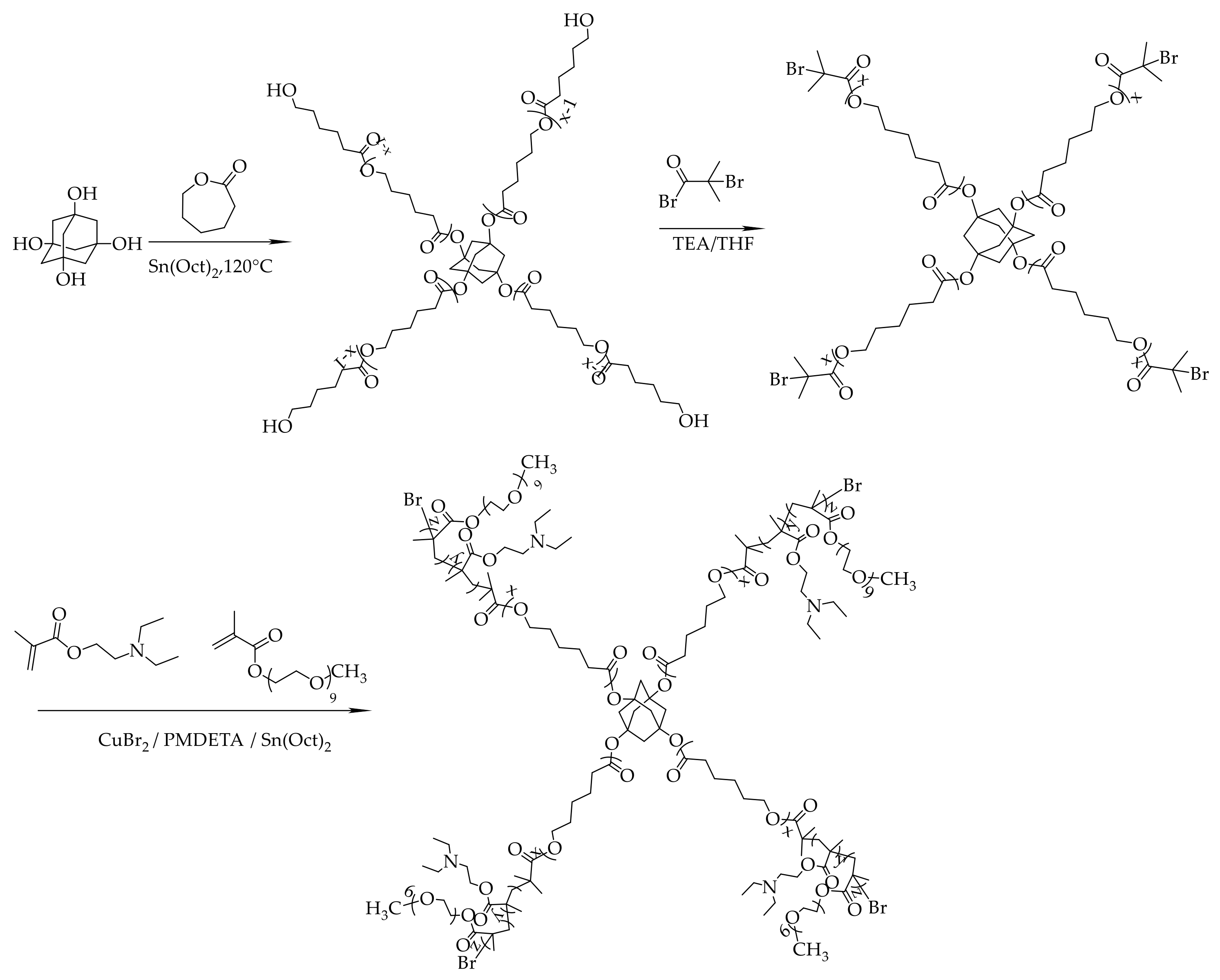
2.3. Synthesis of Ad-(PCL-b-PDEAEMA-b-PPEGMA)4 Copolymers
2.3.1. Synthesis of Ad-(PCL)4
2.3.2. Synthesis of Ad-(PCL-Br)4
2.3.3. Synthesis of Ad-(PCL-b-PDEAEMA-b-PPEGMA)4
2.3.4. Determination of CMC Values
2.3.5. Acid–Base Titration
2.3.6. Micelle Preparation and Characterization
2.3.7. Encapsulation and Release of DOX
3. Results and Discussion
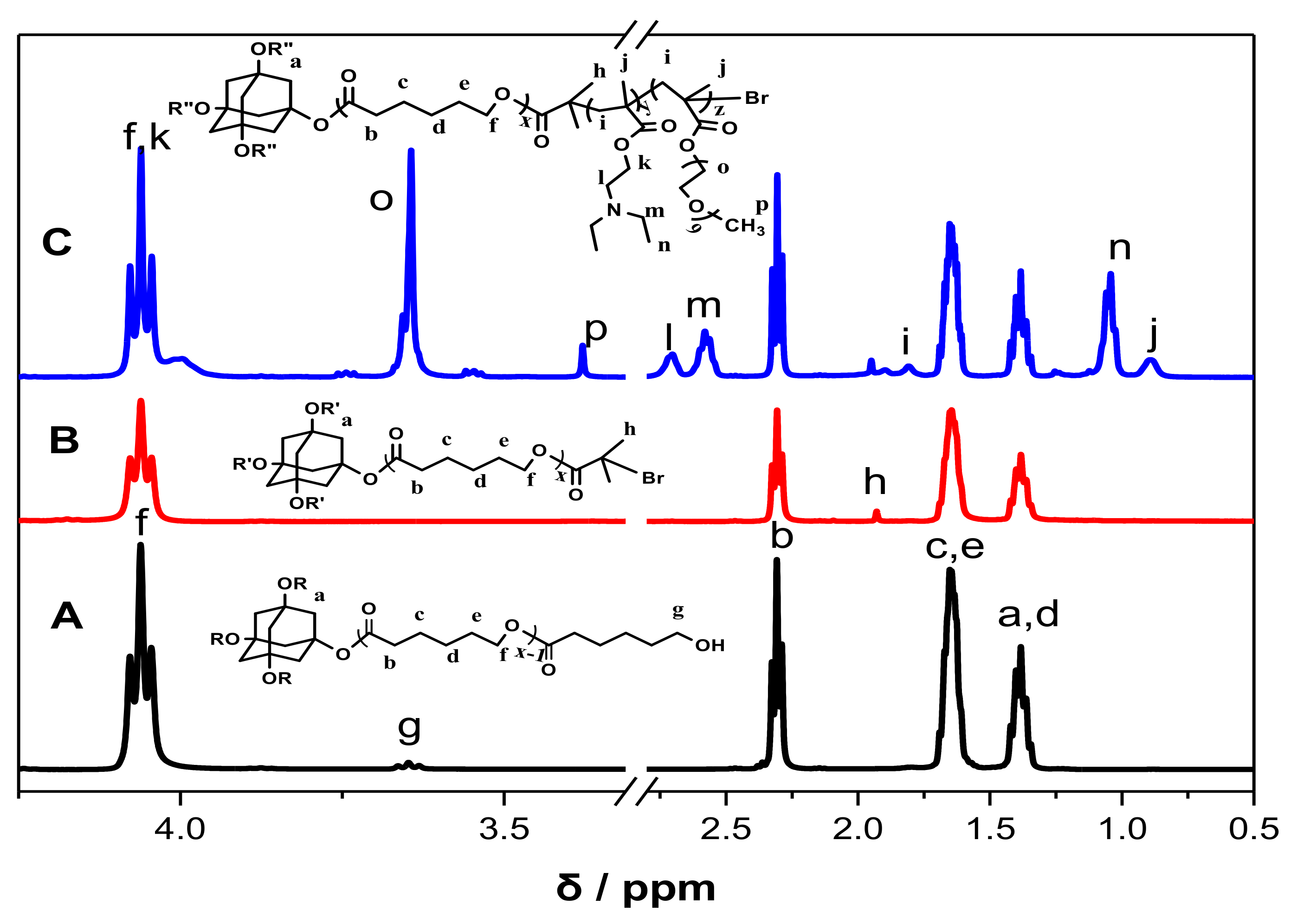
3.1. Synthesis and Characterization of Ad-(PCL-b-PDEAEMA-b-PPEGMA)4 Star Copolymers
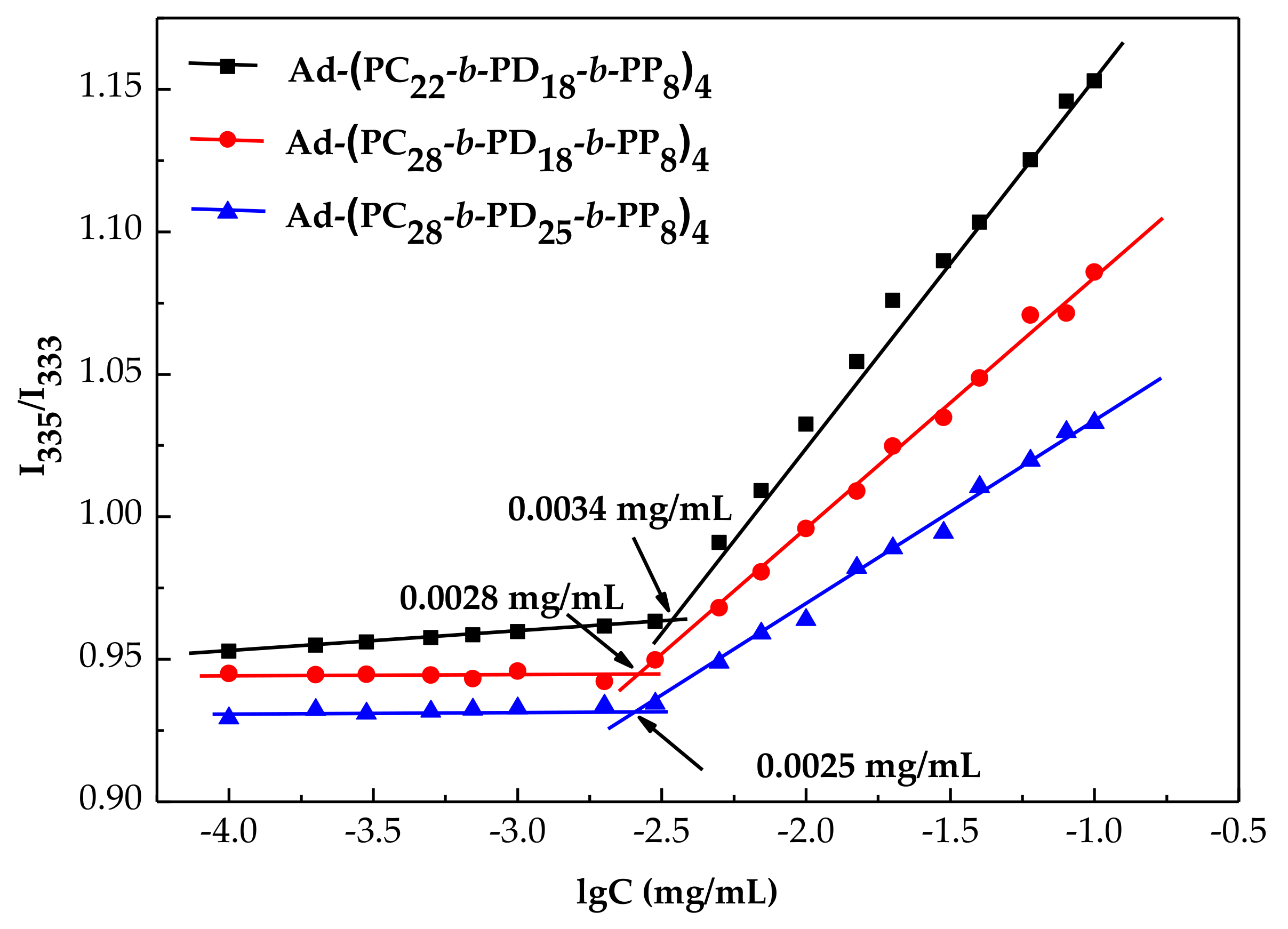
3.2. CMC Values Determined by Fuorescence Analysis
3.3. Titration of Ad-(PCL-b-PDEAEMA-b-PPEGMA)4 Copolymers
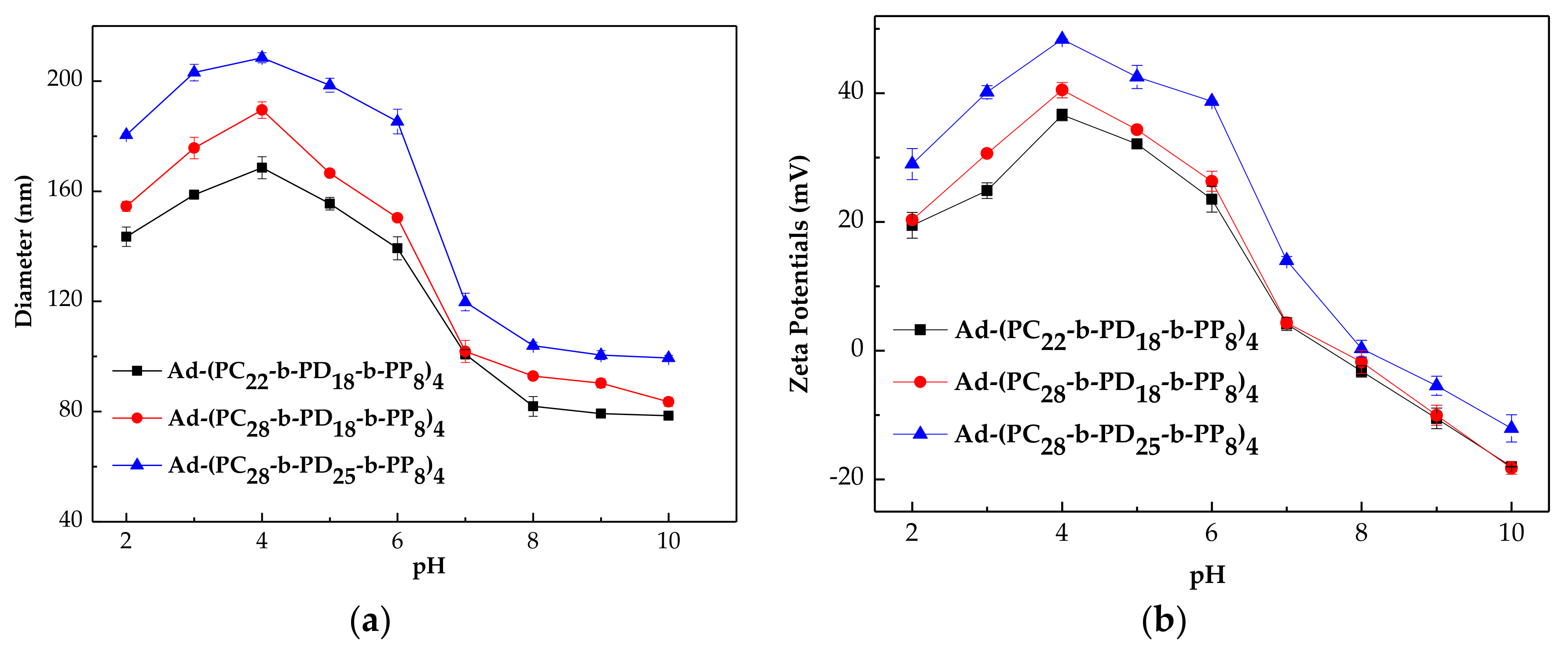
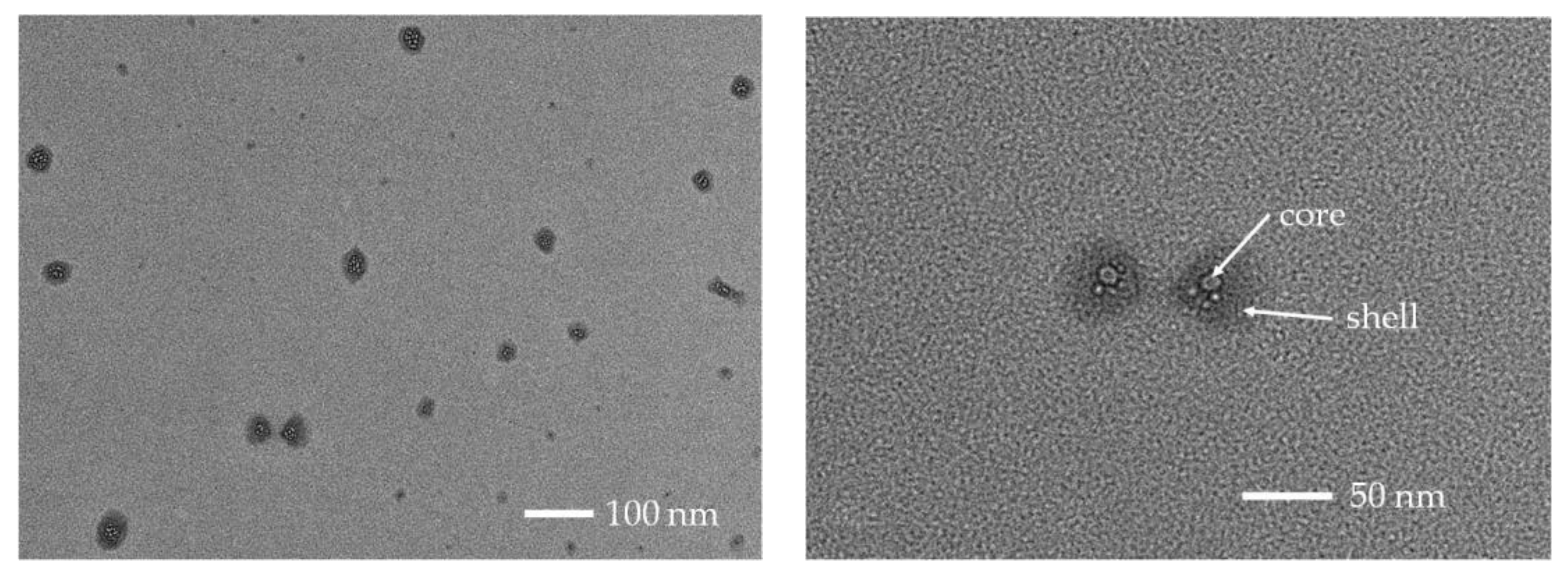
3.4. Particle Size and Zeta Potential of the Micelles at Different pH
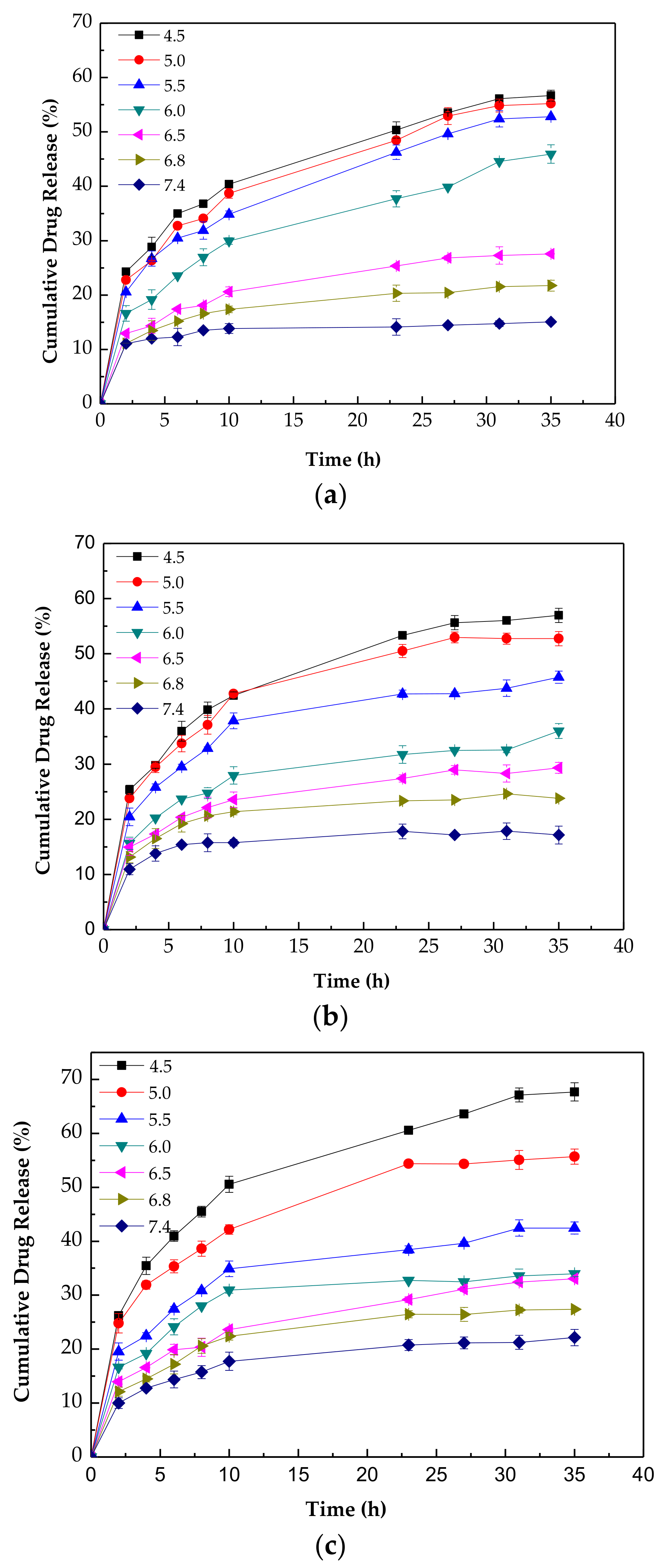
3.5. Encapsulation and pH-Triggered Release of DOX∙HCl
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiagge, E.; Bensenhaver, J.M.; Oppong, J.K.; Awuah, B.; Newman, L.A. Global surgical nncology disease burden: addressing disparities via global surgery initiatives: the University of Michigan International Breast Cancer Registry. Ann. Surg. Oncol. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. World cancer report 2014. Geneva, Switzerland: World Health Organization, international agency for research on cancer. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Mottet, N.; Schmid, H.P.; van der Kwast, T.; Wiegel, T.; et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 2011, 59, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.W.; Gao, X.L.; Su, L.N.; Xia, H.M.; Gu, G.Z.; Pang, Z.Q.; Jiang, X.G.; Yao, L.; Chen, J.; Chen, H.Z. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 2011, 32, 8010–8020. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; FaroKHzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Haag, R. Supramolecular drug-delivery systems based on polymeric core-shell architectures. Angew. Chem. Int. Edit. 2004, 43, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. J. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Yang, Y.Q.; Huang, T.X.; Zhao, B.; Guo, X.D.; Wang, J.F.; Zhang, L.J. Self-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug delivery. Biomaterials 2012, 33, 6273–6283. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Zhao, B.; Li, Z.D.; Lin, W.J.; Zhang, C.Y.; Guo, X.D.; Wang, J.F.; Zhang, L.J. pH-sensitive micelles self-assembled from multi-arm star triblock co-polymers poly(epsilon-caprolactone)-b-poly(2-(diethylamino)ethyl methacrylate)-b-poly(poly(ethylene glycol) methyl ether methacrylate) for controlled anticancer drug delivery. Acta Biomater. 2013, 9, 7679–7690. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fay, F.; Hak, S.; Perez-Aguilar, J.M.; Sanchez-Gaytan, B.L.; Goode, B.; Duivenvoorden, R.; Davies, C.d.; Bjørkøy, A.; Weinstein, H.; et al. Augmenting drug-carrier compatibility improves tumour nanotherapy efficacy. Nat. Commun. 2016, 7, 11221. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agarwal, P.; Zhao, S.T.; Yu, J.H.; Lu, X.B.; He, X.M. A biomimetic hybrid nanoplatform for encapsulation and precisely controlled delivery of theranostic agents. Nat. Commun. 2016, 7, 10350. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. The First Targeted delivery of siRNA in humans via a self-Assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Fort, R.C.; Schleyer, P.V.R. Adamantane: consequences of the diamondoid structure. Chem. Rev. 1964, 64, 277–300. [Google Scholar] [CrossRef]
- Fu, S.Q.; Guo, J.W.; Zhu, D.Y.; Yang, Z.; Yang, C.F.; Xian, J.X.; Li, X. Novel halogen-free flame retardants based on adamantane for polycarbonate. RSC Adv. 2015, 5, 67054–67065. [Google Scholar] [CrossRef]
- Yang, C.F.; Xiao, J.Y.; Xiao, W.F.; Lin, W.J.; Chen, J.R.; Chen, Q.; Zhang, L.J.; Zhang, C.Y.; Guo, J.W. Fabrication of PDEAEMA-based pH-responsive mixed micelles for application in controlled doxorubicin release. RSC Adv. 2017, 7, 27564–27573. [Google Scholar] [CrossRef]
- Lin, W.J.; Nie, S.Y.; Zhong, Q.; Yang, Y.Q.; Cai, C.Z.; Wang, J.F.; Zhang, L.J. Amphiphilic miktoarm star copolymer (PCL)3-(PDEAEMA-b-PPEGMA)3 as pH-sensitive micelles in the delivery of anticancer drug. J. Mater. Chem. B 2014, 2, 4008–4020. [Google Scholar] [CrossRef]
- Xu, J.; Ge, Z.; Zhu, Z.; Luo, S.; Liu, H.; Liu, S. Synthesis and micellization properties of double hydrophilic A2BA2 and A4BA4 non-linear block copolymers. Macromolecules 2006, 39, 8178–8185. [Google Scholar] [CrossRef]
- Feng, C.; Shen, Z.; Li, Y.G.; Gu, L.N.; Zhang, Y.Q.; Lu, G.L.; Huang, X.Y. PNIPAM-b-(PEA-g-PDMAEA) double-hydrophilic graft copolymer: synthesis and its application for preparation of gold nanoparticles in aqueous media. J. Polym. Sci., Part A: Polym. Chem. 2009, 47, 1811–1824. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Torchilin, V.P. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 2013, 34, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Van Vlerken, L.E.; Duan, Z.F.; Seiden, M.V.; Amiji, M.M. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007, 67, 4843–4850. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.S.; Liu, S.Y. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 2013, 42, 7289–7325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, J.; Wang, Q.; Zhong, Z.; Zhuo, R. Miktoarm copolymers bearing one poly(ethylene glycol) chain and several poly(ε-caprolactone) chains on a hyperbranched polyglycerol core. Macromolecules 2010, 43, 6671–6677. [Google Scholar] [CrossRef]
- Hong, H.Y.; Mai, Y.Y.; Zhou, Y.F.; Yan, D.Y.; Cui, J. Self-assembly of large multimolecular micelles from hyperbranched star copolymers. Macromol. Rapid Commun. 2007, 28, 591–596. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Y.; Gong, Y.; Shu, Q.; Yin, M.; Liu, X.; Chen, M. pH-induced outward movement of star centers within coumarin-centered star-block polymer micelles. Chem. Commun. 2012, 48, 10883–10885. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kang, H.C.; Huh, K.M.; Bae, Y.H. Biocompatible, pH-sensitive AB2 miktoarm polymer-based polymersomes: preparation, characterization, and acidic pH-activated nanostructural transformation. J. Mater. Chem. 2012, 22, 19168–19178. [Google Scholar] [CrossRef] [PubMed]


| Sample 1 | Mn,th 2 | Mn,GPC 3 | Mw/Mn 3 | Mn,NMR 4 |
|---|---|---|---|---|
| Ad-(PC22-b-PD18-b-PP8)4 | 40,495 | 34,524 | 1.37 | 36,209 |
| Ad-(PC28-b-PD18-b-PP8)4 | 43,234 | 36,735 | 1.42 | 38,823 |
| Ad-(PC28-b-PD25-b-PP8)4 | 48,422 | 41,793 | 1.46 | 44,826 |
| Micelle | DOX/Polymer | Size (nm) | DLC (%) | EE (%) |
|---|---|---|---|---|
| Ad-(PC22-b-PD18-b-PP8)4 | 10/40 | 110.8 | 7.7 | 37.8 |
| 20/40 | 122.5 | 10.5 | 31.6 | |
| Ad-(PC28-b-PD18-b-PP8)4 | 10/40 | 129.0 | 9.9 | 40.4 |
| 20/40 | 152.5 | 16.7 | 34.7 | |
| Ad-(PC28-b-PD25-b-PP8)4 | 10/40 | 180.7 | 11.6 | 56 |
| 20/40 | 256.0 | 22.4 | 44.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Guo, J.; Tong, R.; Yang, C.; Chen, J.-K. pH-Sensitive Micelles Based on Star Copolymer Ad-(PCL-b-PDEAEMA-b-PPEGMA)4 for Controlled Drug Delivery. Polymers 2018, 10, 443. https://doi.org/10.3390/polym10040443
Yang H, Guo J, Tong R, Yang C, Chen J-K. pH-Sensitive Micelles Based on Star Copolymer Ad-(PCL-b-PDEAEMA-b-PPEGMA)4 for Controlled Drug Delivery. Polymers. 2018; 10(4):443. https://doi.org/10.3390/polym10040443
Chicago/Turabian StyleYang, Huiyan, Jianwei Guo, Rui Tong, Chufen Yang, and Jem-Kun Chen. 2018. "pH-Sensitive Micelles Based on Star Copolymer Ad-(PCL-b-PDEAEMA-b-PPEGMA)4 for Controlled Drug Delivery" Polymers 10, no. 4: 443. https://doi.org/10.3390/polym10040443