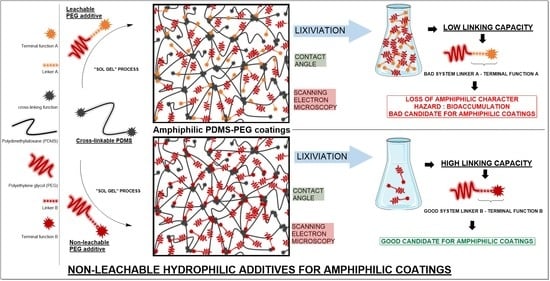
Non-Leachable Hydrophilic Additives for Amphiphilic Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Coating
2.2.1. Synthesis of Bis(Trimethoxysilane)-Terminated Poly(Dimethyl Siloxane) (PDMS28)
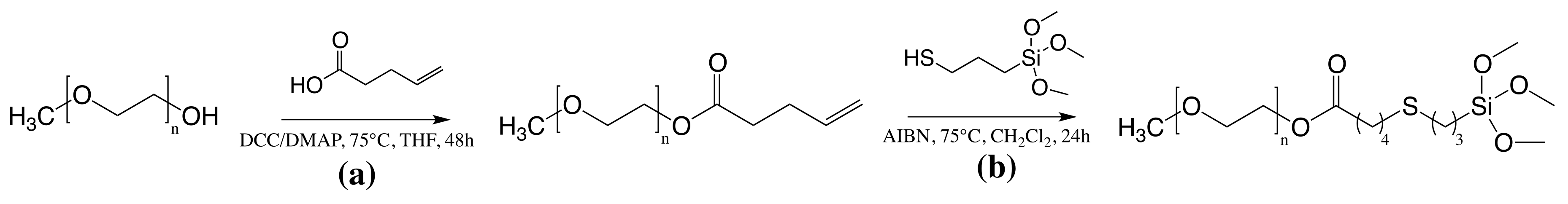
2.2.2. Functionalization of PEG45 with Short Alkyl Chain (C8) and Trimethoxysilane End Groups (45-Alk8-TMS)
2.2.3. Functionalization of PEG45 with Alkyl Chain C14 and Trimethoxysilane End Groups (45-Alk14-TMS)
2.2.4. Film Preparation
2.3. Characterizations
2.3.1. Nuclear Magnetic Resonance
2.3.2. Scanning Electron Microscopy
2.3.3. Soxhlet Extraction
2.3.4. Contact Angle Measurement
3. Results
3.1. Synthesis
3.1.1. Synthesis of Dihydroxylalkyl Polydimethylsiloxane (PDMS28)
3.1.2. Poly(Ethylene Glycol) End-Group Modifications
3.2. Film Preparation
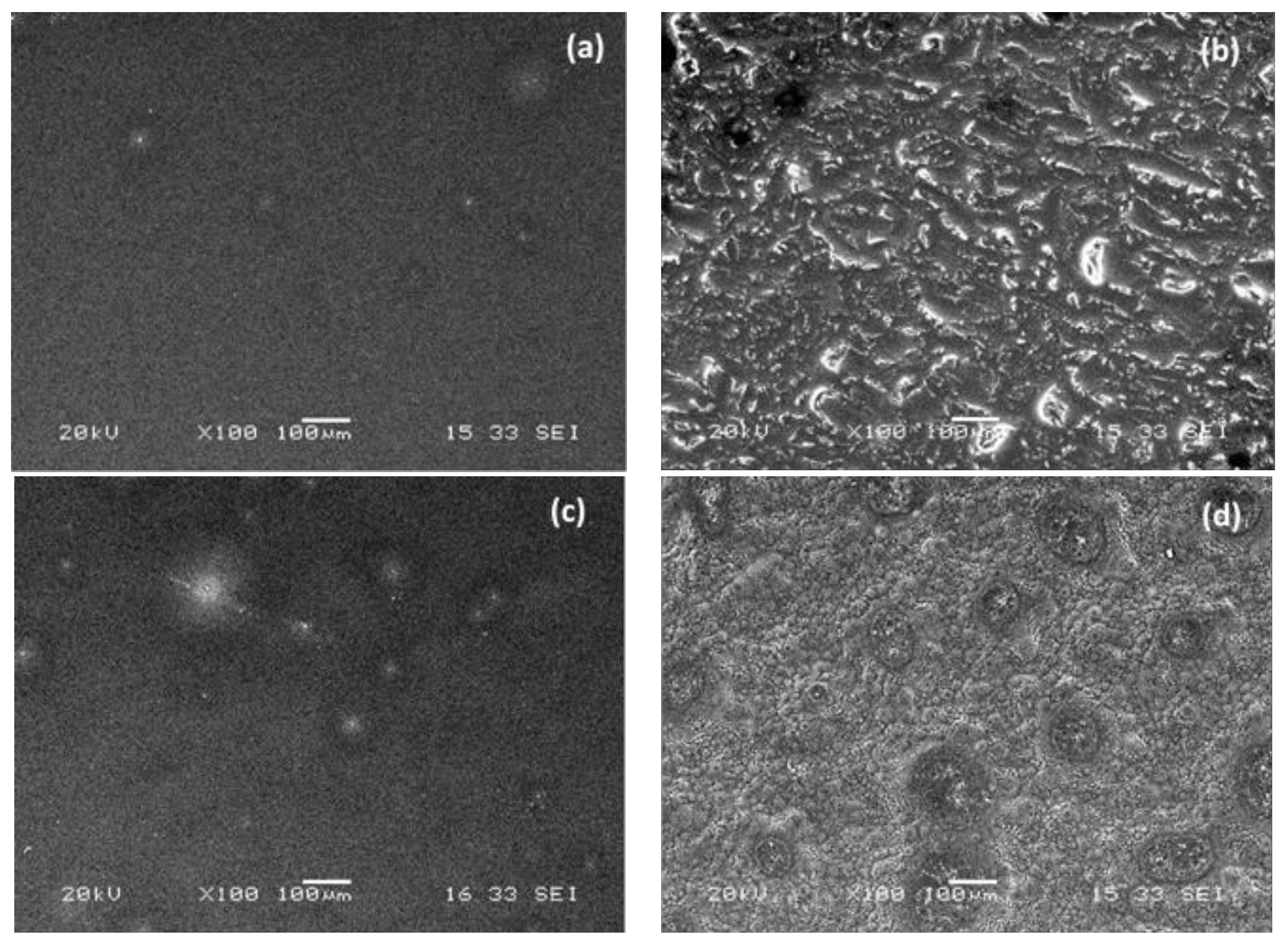
3.3. Scanning Electron Microscopy
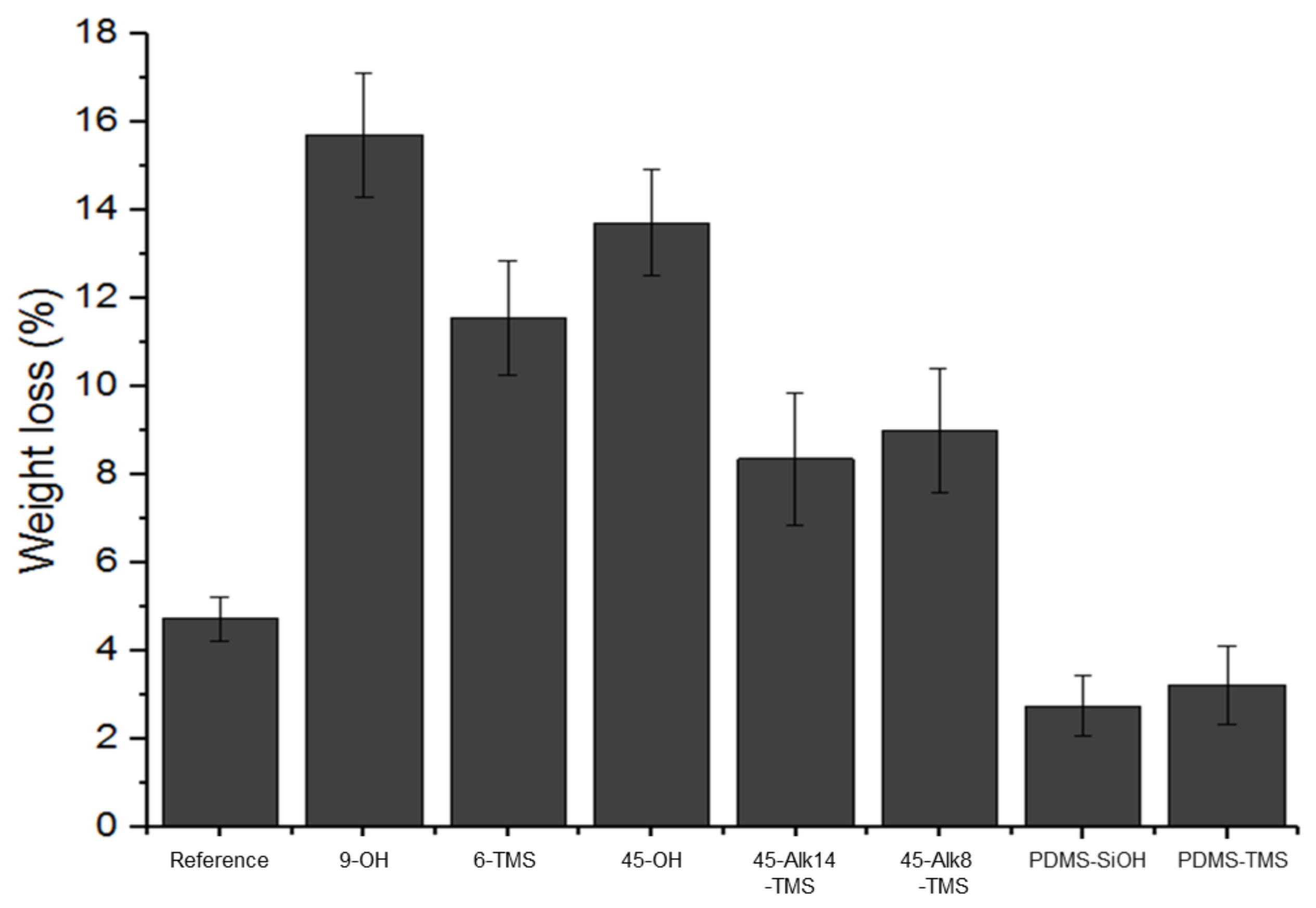
3.4. Soxhlet Extraction
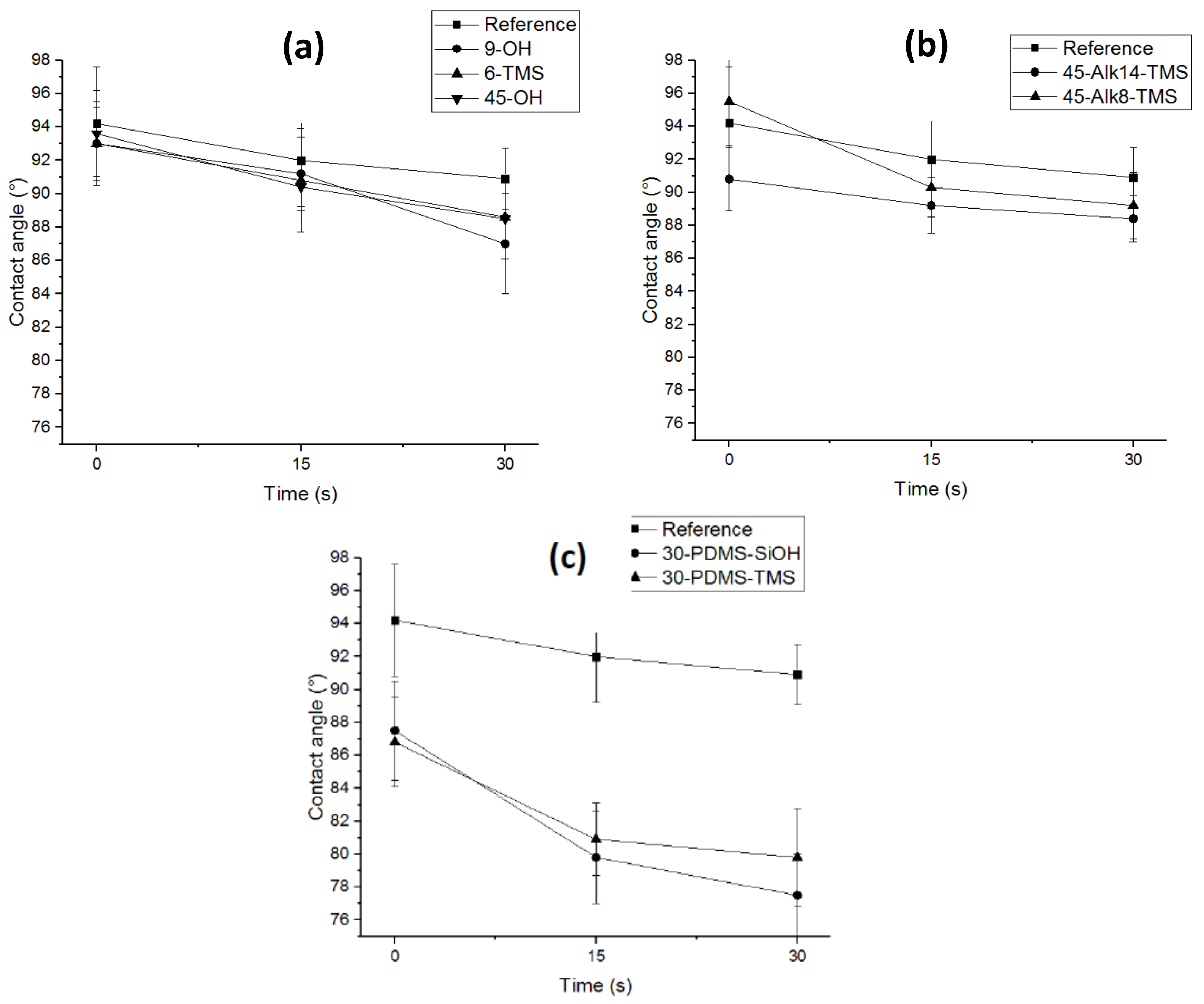
3.5. Contact Angle Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Callow, M.E.; Fletcher, R.L. The Influence of Low Surface Energy Materials on Bioadhesion—A Review. Int. Biodeterior. Biodegrad. 1994, 34, 333–348. [Google Scholar] [CrossRef]
- Ng, J.M.K.; Gitlin, I.; Stroock, A.D.; Whitesides, G.M. Components for Integrated Poly(Dimethylsiloxane) Microfluidic Systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-Fouling Coatings of Poly(Dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Mirzadeh, H. Adhesion between Modified and Unmodified Poly(Dimethylsiloxane) Layers for a Biomedical Application. Int. J. Adhes. Adhes. 2004, 24, 247–257. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, S.A.; Pedraza, F.; Mahadik, S.S.; Relekar, B.P.; Thorat, S.S. Biocompatible Superhydrophobic Coating Material for Biomedical Applications. J. Sol-Gel Sci. Technol. 2017, 81, 791–796. [Google Scholar] [CrossRef]
- Martin, S.; Bhushan, B. Transparent, Wear-Resistant, Superhydrophobic and Superoleophobic Poly(Dimethylsiloxane) (PDMS) Surfaces. J. Colloid Interface Sci. 2017, 488 (Suppl. C), 118–126. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Sethuraman, S. Chapter 18—Biomedical Applications of Nondegradable Polymers. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 301–308. [Google Scholar]
- Sundaram, H.S.; Cho, Y.; Dimitriou, M.D.; Weinman, C.J.; Finlay, J.A.; Cone, G.; Callow, M.E.; Callow, J.A.; Kramer, E.J.; Ober, C.K. Fluorine-Free Mixed Amphiphilic Polymers Based on PDMS and PEG Side Chains for Fouling Release Applications. Biofouling 2011, 27, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Truby, K.; Wood, C.; Stein, J.; Cella, J.; Carpenter, J.; Kavanagh, C.; Swain, G.; Wiebe, D.; Lapota, D.; Meyer, A.; et al. Evaluation of the performance enhancement of silicone biofouling-release coatings by oil incorporation. Biofouling 2009, 15, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, C.J.; Swain, G.W.; Kovach, B.S.; Stein, J.; Darkangelo-Wood, C.; Truby, K.; Holm, E.; Montemarano, J.; Meyer, A.; Wiebe, D. The Effects of Silicone Fluid Additives and Silicone Elastomer Matrices on Barnacle Adhesion Strength. Biofouling 2003, 19, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Camós Noguer, A.; Olsen, S.M.; Hvilsted, S.; Kiil, S. Field Study of the Long-Term Release of Block Copolymers from Fouling-Release Coatings. Prog. Org. Coat. 2017, 112, 101–108. [Google Scholar] [CrossRef]
- Narrainen, A.P.; Hutchings, L.R.; Ansari, I.; Thompson, R.L.; Clarke, N. Multi-End-Functionalized Polymers: Additives to Modify Polymer Properties at Surfaces and Interfaces. Macromolecules 2007, 40, 1969–1980. [Google Scholar] [CrossRef]
- Faÿ, F.; Hawkins, M.L.; Réhel, K.; Grunlan, M.; Linossier, I. Non-Toxic, Anti-Fouling Silicones with Variable PEO-Silane Amphiphile Content. Green Mater. 2016, 4, 53–62. [Google Scholar] [CrossRef]
- Ciriminna, R.; Bright, F.V.; Pagliaro, M. Ecofriendly Antifouling Marine Coatings. ACS Sustain. Chem. Eng. 2015, 3, 559–565. [Google Scholar] [CrossRef]
- Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Polymer Brush Coatings for Combating Marine Biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Camós Noguer, A.; Olsen, S.M.; Hvilsted, S.; Kiil, S. Diffusion of Surface-Active Amphiphiles in Silicone-Based Fouling-Release Coatings. Prog. Org. Coat. 2017, 106 (Suppl. C), 77–86. [Google Scholar] [CrossRef]
- Wu, Z.; Hjort, K. Surface Modification of PDMS by Gradient-Induced Migration of Embedded Pluronic. Lab Chip 2009, 9, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Røn, T.; Javakhishvili, I.; Hvilsted, S.; Jankova, K.; Lee, S. Ultralow Friction with Hydrophilic Polymer Brushes in Water as Segregated from Silicone Matrix. Adv. Mater. Interfaces 2016, 3, 1500472. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xie, Z.; Wang, G.; Ding, C.; Jiang, H.; Wang, P. Preparation and Evaluation of Amphiphilic Polymer as Fouling-Release Coating in Marine Environment. J. Coat. Technol. Res. 2017, 14, 1237–1245. [Google Scholar] [CrossRef]
- Nendza, M. Hazard Assessment of Silicone Oils (Polydimethylsiloxanes, PDMS) Used in Antifouling-/Foul-Release-Products in the Marine Environment. Mar. Pollut. Bull. 2007, 54, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Brodus, D.; Hollimon, V.; Hu, H. A Brief Review of Recent Developments in the Designs that Prevent Bio-Fouling on Silicon and Silicon-Based Materials. Chem. Cent. J. 2017, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, R.; Alexander, K.S.; Riga, A.T. Physical Characterization of Polyethylene Glycols by Thermal Analytical Technique and the Effect of Humidity and Molecular Weight. Pharmazie 2010, 65, 343–347. [Google Scholar] [PubMed]
- Dantas, L.C.; da Silva-Neto, J.P.; Dantas, T.S.; Naves, L.Z.; das Neves, F.D.; da Mota, A.S. Bacterial Adhesion and Surface Roughness for Different Clinical Techniques for Acrylic Polymethyl Methacrylate. Int. J. Dent. 2016, 2016, 8685796. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.; Cox, C.D.; Hahn, M.S.; Grunlan, M.A. Protein-Resistant Silicones: Incorporation of Poly(ethylene oxide) via Siloxane Tethers. Biomacromolecules 2007, 8, 3244–3252. [Google Scholar] [CrossRef] [PubMed]
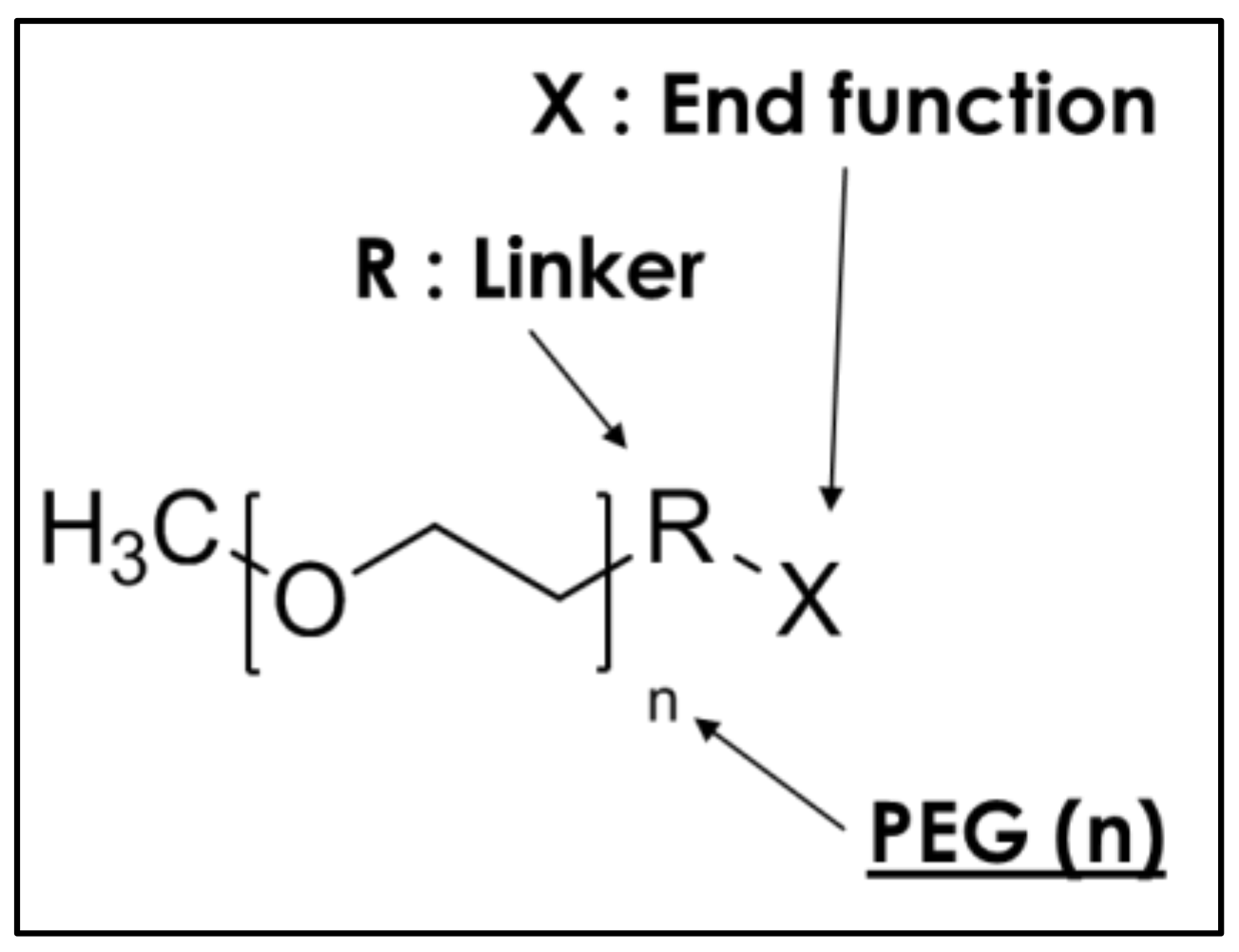
| n: PEG | R: Linker | X: End Function | Samples | States of Additive |
|---|---|---|---|---|
| 0 | - | Reference | - | |
| 6 | - | Trimethoxysilane | 6-TMS | Liquid |
| 9 | - | Hydroxy | 9-OH | Liquid |
| X | PDMS | Silanol | PDMS-SiOH | Liquid |
| X | PDMS | Trimethoxysilane | PDMS-TMS | Liquid |
| 45 | - | Hydroxy | 45-OH | White solid |
| 45 | Alkyl C8 | Trimethoxysilane | 45-Alk8-TMS | Yellow solid |
| 45 | Alkyl C14 | Trimethoxysilane | 45-Alk14-TMS | Yellow solid |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillet, G.; Azemar, F.; Faÿ, F.; Réhel, K.; Linossier, I. Non-Leachable Hydrophilic Additives for Amphiphilic Coatings. Polymers 2018, 10, 445. https://doi.org/10.3390/polym10040445
Gillet G, Azemar F, Faÿ F, Réhel K, Linossier I. Non-Leachable Hydrophilic Additives for Amphiphilic Coatings. Polymers. 2018; 10(4):445. https://doi.org/10.3390/polym10040445
Chicago/Turabian StyleGillet, Guillaume, Fabrice Azemar, Fabienne Faÿ, Karine Réhel, and Isabelle Linossier. 2018. "Non-Leachable Hydrophilic Additives for Amphiphilic Coatings" Polymers 10, no. 4: 445. https://doi.org/10.3390/polym10040445