Effect of Chemical Disinfection on Chitosan Coated PMMA and PETG Surfaces—An In Vitro Study
Abstract
:1. Introduction
- -
- Chemical disinfectants have no influence on CS coating abrasion resistance.
- -
- There are no differences between the tested chemical disinfectants regarding abrasion resistance of CS coatings.
- -
- There are no differences between PMMA and PETG materials regarding the abrasion resistance of CS coatings.
2. Materials and Methods
2.1. Establishing Specimens
2.2. Disinfectants
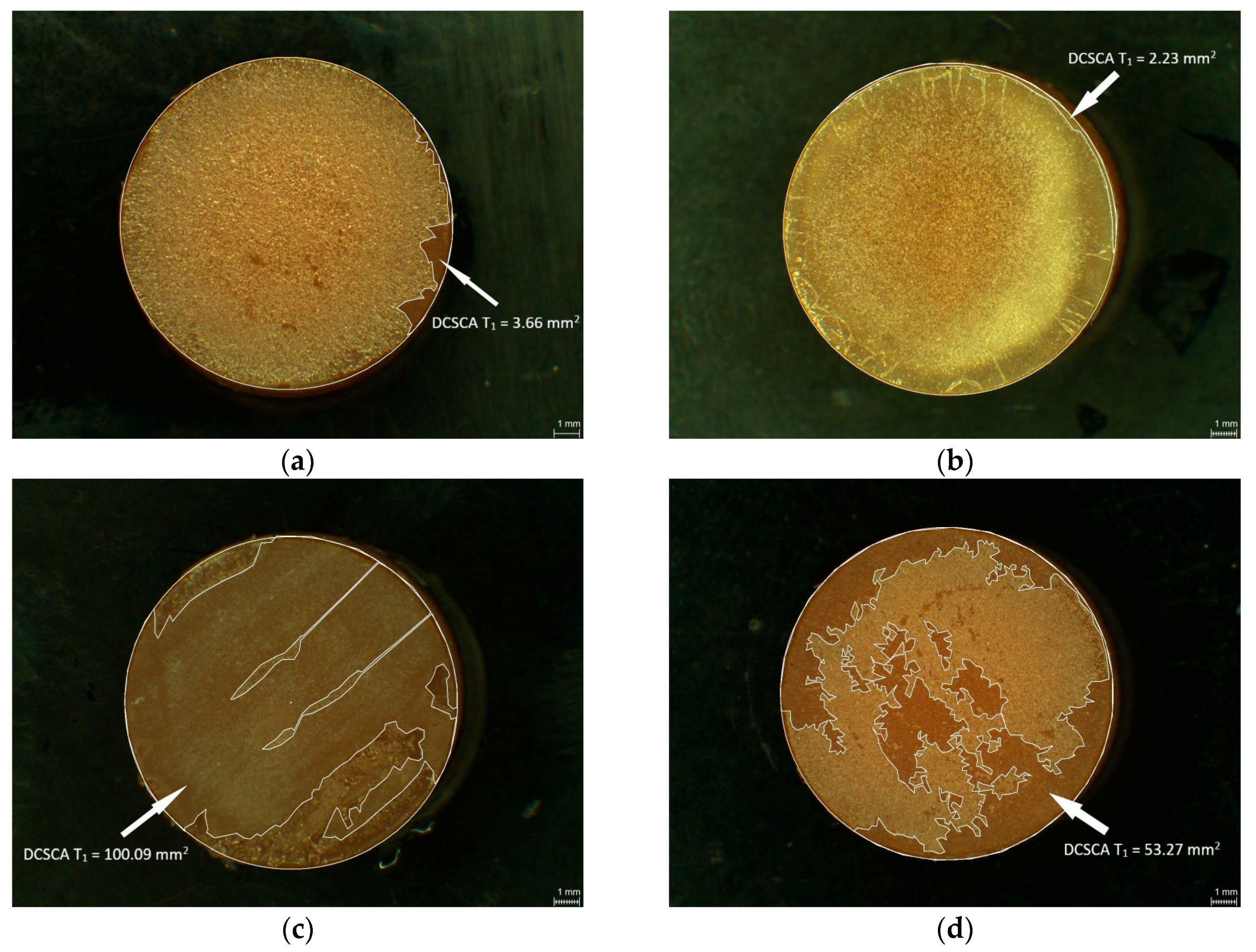
2.3. Measurement of Damaged Chitosan Coating Area (DCSCA)
2.4. Disinfection Procedure and Abrasion Test
2.5. Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis
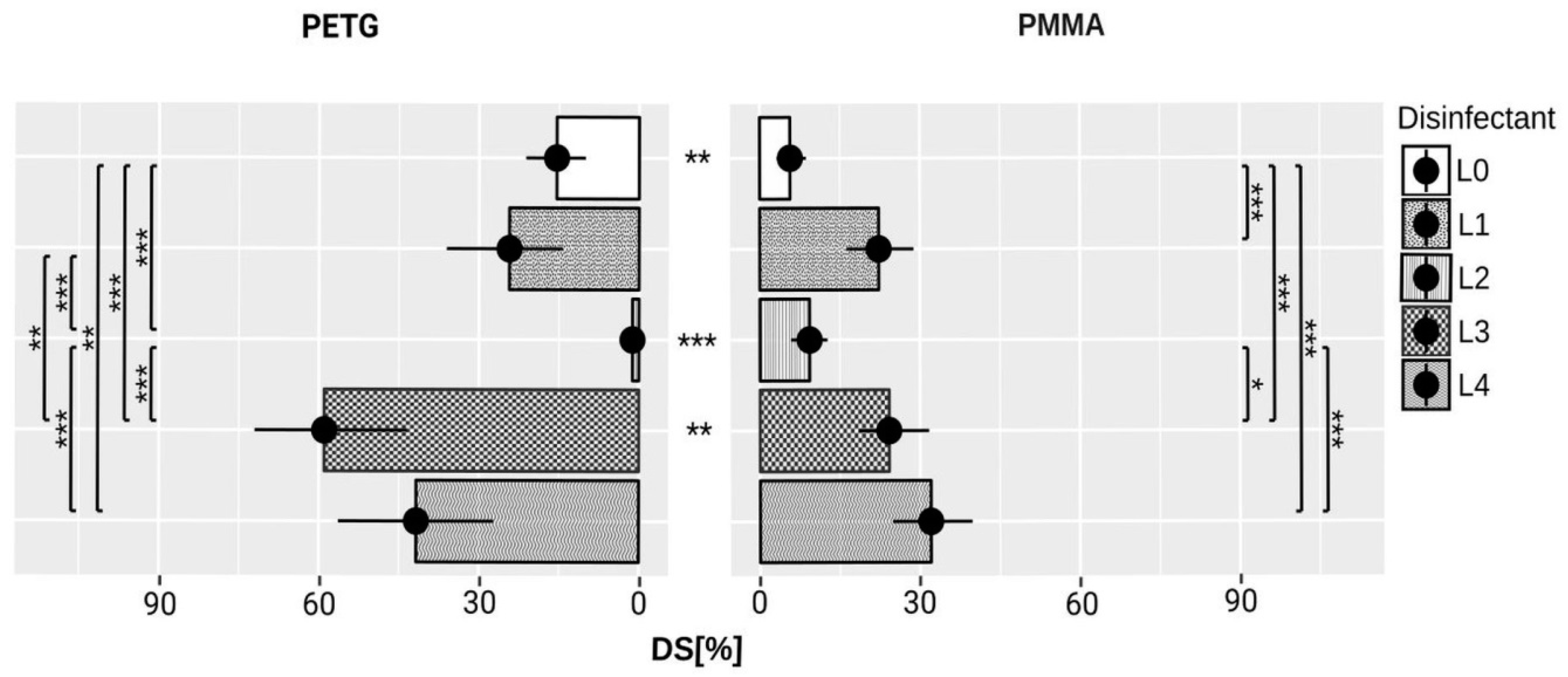
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Brand Name | Batch-No | Dosage | Exposure Time (min) |
|---|---|---|---|---|
| L1 | Printosept-ID | 294102 | Solution ready to use | 5 |
| L2 | MD 520 | 1401207 | Solution ready to use | 10 |
| L3 | Silosept® | 1241466 | 20 g of Silosept into 2 L of lukewarm water (for 2% solution) | 10 |
| L4 | Dentavon® | 1240192 | 40 g Dentavon into 2 L of lukewarm water (for 2% solution) | 10 |
| Ingredients | % |
|---|---|
| Aqua dest. | 82.93 |
| Hydroxyethyl cellulose | 12.5 |
| Sorbitol solution | 4.28 |
| Potassium chloride | 0.12 |
| Sodium chloride | 0.08 |
| Sodium monohydrogenphosphate 12 H2O | 0.06 |
| Calcium chloride 2 H2O | 0.02 |
| Magnesium chloride 6 H2O | 0.01 |
| Preservative Propyl 4-Hydroxybenzoate | <0.01 |
| Material | Disinfectant | T0 Mean (mm2) ± SD | T1 Mean (mm2) ± SD |
|---|---|---|---|
| L0 | 0.8 ± 1.5 | 7.2 ± 6.1 | |
| L1 | 3.1 ± 2.3 | 27.8 ± 13.5 | |
| PMMA | L2 | 3.5 ± 4 | 11.4 ± 6.8 |
| L3 | 4.2 ± 2.8 | 29.9 ± 14.6 | |
| L4 | 2.6 ± 1.3 | 40 ± 16.1 | |
| L0 | 0.2 ± 0.5 | 19.7 ± 12.6 | |
| L1 | 0 ± 0 | 31 ± 24.9 | |
| PETG | L2 | 0 ± 0 | 1.5 ± 2.5 |
| L3 | 0.1 ± 0.3 | 75.3 ± 31.6 | |
| L4 | 0.4 ± 1 | 53 ± 31.1 |
References
- Dash, M.; Chiellini, F.; Ottenbrite, R.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan-as a biomaterial. Biomater. Artif. Cells Artif. Org. 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Whang, H.S.; Kirsch, W.; Zhu, Y.H.; Yang, C.Z.; Hudson, S.M. Hemostatic agents derived from chitin and chitosan. J. Macromol. Sci. Polym. Rev. 2005, 45, 309–323. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti. Infect Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 69B, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Marreco, P.R.; Moreira, P.D.L.; Genari, S.C.; Moraes, Â.M. Effects of different sterilization methods on the morphology, mechanical properties, and cytotoxicity of chitosan membranes used as wound dressings. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Bumgardner, J.D.; Wiser, R.; Gerard, P.D.; Bergin, P.; Chestnutt, B.; Marini, M.; Ramsey, V.; Elder, S.H.; Gilbert, J.A. Chitosan: Potential use as a bioactive coating for orthopaedic and craniofacial/dental implants. J. Biomater. Sci. Polym. Ed. 2003, 14, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Boening, W.K.; Grychowska, N.; Paradowska-Stolarz, A. Clinical application of chitosan in dental specialities. Mini Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Skoskiewicz-Malinowska, K.; Kaczmarek, U.; Malicka, B.; Walczak, K.; Zietek, M. Application of chitosan and propolis in endodontic treatment: A review. Mini Rev. Med. Chem. 2017, 17, 410–434. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Wolf, E.; Walczak, K.; Meissner, H.; Boening, K. Chitosan coating on silica-modified polymethyl methacrylate for dental applications. Coatings 2017, 7, 168. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Wolf, E.; Richter, G.; Meissner, H.; Boening, K. New concept of polymethyl methacrylate (PMMA) and polyethylene terephthalate (PET) surface coating by chitosan. Polymers 2016, 8, 132. [Google Scholar] [CrossRef]
- Jordan, A.R.; Micheelis (Gesamtbearbeitung), W. Fünfte Deutsche Mundgesundheitsstudie-(DMS V); Institut der Deutschen Zahnärzte (IDZ): Köln, Germany, 2016; ISBN 978-3-7691-0020-4. [Google Scholar]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, W.; Burkert, J.; Vorwig, O.; Blessmann, M.; Cachovan, G.; Zeuch, J.; Eichhorn, M.; Heiland, M. Bleeding incidence after oral surgery with continued oral anticoagulation. Clin. Oral. Investig. 2012, 16, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, A.M.; Miyoshi, P.R.; Gnoatto, N.; Paranhos, H.D.F.O.; Figueiredo, L.C.D.; Salvador, S.L. Cross-contamination in the dental laboratory through the polishing procedure of complete dentures. Braz. Dent. J. 2004, 15, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.G.; Collins, A.S.; Cleveland, J.L.; Harte, J.A.; Eklund, K.J.; Malvitz, D.M. Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings-2003. MMWR 2003, 52, 1–68. [Google Scholar] [PubMed]
- Deutscher Arbeitskreis für Hygiene in der Zahnmedizin (hrsg.): Hygieneleitfaden, 11 Ausgabe 2017. Available online: https://www.bzaek.de/fileadmin/PDFs/za/hygieneplan/hygieneleitfaden.pdf (accessed on 12 February 2018).
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.C.; Kimpara, E.T.; Mancini, M.N.G.; Balducci, I.; Jorge, A.O.C.; Koga-Ito, C.Y. Effectiveness of six different disinfectants on removing five microbial species and effects on the topographic characteristics of acrylic resin. J. Prosthodont. 2008, 17, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Kotsiomiti, E.; Tzialla, A.; Hatjivasiliou, K. Accuracy and stability of impression materials subjected to chemical disinfection—A literature review. J. Oral. Rehabil. 2008, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Jnanadev, K.; Babu, C.S.; Shetty, S.S.; Kumar, G.S.; Sheetal, H. Disinfecting the acrylic resin plate using electrolyzed acid water and 2% glutaraldehyde: A comparative microbiological study. J. Indian Prosthodont. Soc. 2011, 11, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available online: https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines.pdf (accessed on 9 February 2018).
- Polyzois, G.L.; Zissis, A.J.; Yannikakis, S.A. The effect of glutaraldehyde and microwave disinfection on some properties of acrylic denture resin. Int. J. Prosthodont. 1995, 8, 150–154. [Google Scholar] [PubMed]
- Carvalho, C.F.; Vanderlei, A.D.; Salazar Marocho, S.M.; Pereira, S.; Nogueira, L.; Arruda Paes-Junior, T.J. Effect of disinfectant solutions on a denture base acrylic resin. Acta. Odontol. Latinoam. 2012, 25, 255–260. [Google Scholar] [PubMed]
- Orsi, I.A.; Andrade, V.G.; Bonato, P.S.; Raimundo, L.B.; Herzog, D.S.; Borie, E. Glutaraldehyde release from heat-polymerized acrylic resins after disinfection and chemical and mechanical polishing. Braz. Dent. J. 2011, 22, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Maris, P. Modes of action of disinfectants. Rev. Sci. Tech. 1995, 14, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P.; Stewart, G.S.A.B. Mechanisms of action of disinfectants. Int. Biodeterior. Biodegrad. 1998, 41, 261–268. [Google Scholar] [CrossRef]
- Reuter, G. Disinfection and hygiene in the field of food of animal origin. Int. Biodeterior. Biodegrad. 1998, 41, 209–215. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yoshida, S.; Ohashi, K.; Shinoda, Y.; Kato, M.; Mori, T.; Yoshimura, T.; Tanaka, K.; Sato, A.; Goto, T. Evaluation of efficacy and clinical utility of potassium peroxymonosulfate-based disinfectants. Can. J. Infect Control 2017, 32, 93–97. [Google Scholar]
- Tsiarta, N.; Schuurmans, J.; Matthijs, H.; Antoniou, M. Mode of action of hydrogen peroxide, peroxymonosulfate and persulfate on microcystis aeruginosa strain pcc 7806. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodos, Greece, 31 August–2 September 2017. [Google Scholar]
- Wilcox, R.R. Introduction to Robust Estimation and Hypothesis Testing; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Makuuchi, K. Critical review of radiation processing of hydrogel and polysaccharide. Radiat. Phys. Chem. 2010, 79, 267–271. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Don, T.-M.; Chiu, W.-Y. Free radical degradation of chitosan with potassium persulfate. Polym. Degrad. Stab. 2002, 75, 73–83. [Google Scholar] [CrossRef]
- Chang, K.L.B.; Tai, M.-C.; Cheng, F.-H. Kinetics and products of the degradation of chitosan by hydrogen peroxide. J. Agric. Food. Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Du, Y.; Xiao, L. Effect of hydrogen peroxide treatment on the molecular weight and structure of chitosan. Polym. Degrad. Stab. 2002, 76, 211–218. [Google Scholar] [CrossRef]
- Tian, F.; Liu, Y.; Hu, K.; Zhao, B. Study of the depolymerization behavior of chitosan by hydrogen peroxide. Carbohydr. Polym. 2004, 57, 31–37. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.-L.; Zhou, Q.; Fang, Y.; Wang, Y.-L.; Xu, F.; Han, L.-R.; Ibrahim, M.; Guo, L.-B.; Xie, G.-L.; et al. Synthesis, characterization, and antibacterial activity of cross-linked chitosan-glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Firdous, J.; Choi, Y.J.; Yun, C.H.; Cho, C.S. Design and application of chitosan microspheres as oral and nasal vaccine carriers: An updated review. Int. J. Nanomed. 2012, 7, 6077–6093. [Google Scholar] [CrossRef]
- Silva, R.; Silva, G.; Coutinho, O.; Mano, J.; Reis, R. Preparation and characterisation in simulated body conditions of glutaraldehyde crosslinked chitosan membranes. J. Mater. Sci. Mater. Med. 2004, 15, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Mano, J.F. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2004, 12, 41–57. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, J.W.; Chun, H.J.; Choi, K.S. Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polym. Bull. 1997, 38, 387–393. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Caramella, C. Chitosan and its salts for mucosal and transmucosal delivery. Expert Opin. Drug Deliv. 2009, 6, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Libio, I.C.; Demori, R.; Ferrão, M.F.; Lionzo, M.I.Z.; da Silveira, N.P. Films based on neutralized chitosan citrate as innovative composition for cosmetic application. Mater. Sci. Eng. C 2016, 67, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kam, H.M.; Khor, E.; Lim, L.Y. Storage of partially deacetylated chitosan films. J. Biomed. Mater. Res. 1999, 48, 881–888. [Google Scholar] [CrossRef]
- El-Barghouthi, M.; Eftaiha, A.; Rashid, I.; Al-Remawi, M.; Badwan, A. A novel superdisintegrating agent made from physically modified chitosan with silicon dioxide. Drug Dev. Ind. Pharm. 2008, 34, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.; Gregory, K.; Morgan, J. Tissue Dressing Assemblies, Systems, and Methods Formed from Hydrophilic Polymer Sponge Structures such as Chitosan. U.S. Patent 20050147656, 2005. [Google Scholar]
- Calamari, S.E.; Bojanich, M.A.; Barembaum, S.R.; Berdicevski, N.; Azcurra, A.I. Antifungal and post-antifungal effects of chlorhexidine, fluconazole, chitosan and its combinations on candida albicans. Med. Oral. Patol. Oral. Cir. Bucal. 2011, 16, e23–e28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Neau, S.H. In vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef]
- Hermann, C.; Mesquita, M.F.; Consani, R.L.; Henriques, G.E. The effect of aging by thermal cycling and mechanical brushing on resilient denture liner hardness and roughness. J. Prosthodont. 2008, 17, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Sexson, J.C.; Phillips, R.W. Studies on the effects of abrasives on acrylic resins. J. Prosthet. Dent. 1951, 1, 454–471. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection and sterilization: An overview. Am. J. Infect Control 2013, 41, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Crofton, A.; Chrisler, J.; Hudson, S.; Inceoglu, S.; Petersen, F.; Kirsch, W. Effect of plasma sterilization on the hemostatic efficacy of a chitosan hemostatic agent in a rat model. Adv. Ther. 2016, 33, 268–281. [Google Scholar] [CrossRef] [PubMed]







| Brand Name | ID | Manufacturer | Group of Active Agents | Active Agents | pH |
|---|---|---|---|---|---|
| Printosept- ID | L1 | Alpro Medical, St. Georgen, Germany | QUAT, alkyl amine | N,N-Didecyl-N-methylpoly(oxyethyl)- ammoniumpropionate, N-(3-Aminopropyl)-N-dodecylpropane-1,3- diamine | 10.5–11.5 |
| MD 520 | L2 | Dürr Dental, Bietigheim- Bissingen, Germany | GA, QUAT | Glutardialdehyde, Aalkyl-benzyl-dimethyl-ammonium- chloride | ~4.3 |
| Silosept | L3 | Kettenbach, Eschenbur, Germany | active oxygen | Pentapotassiumbis(peroxymono-sulphate)- bis(sulphate) (MPS) | 3.71 (1% solution) |
| Dentavon | L4 | Schülke, Norderstedt, Germany | active oxygen | Pentapotassiumbis(peroxymono-sulphate)- bis(sulphate) (MPS) | ~4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walczak, K.; Thiele, J.; Geisler, D.; Boening, K.; Wieckiewicz, M. Effect of Chemical Disinfection on Chitosan Coated PMMA and PETG Surfaces—An In Vitro Study. Polymers 2018, 10, 536. https://doi.org/10.3390/polym10050536
Walczak K, Thiele J, Geisler D, Boening K, Wieckiewicz M. Effect of Chemical Disinfection on Chitosan Coated PMMA and PETG Surfaces—An In Vitro Study. Polymers. 2018; 10(5):536. https://doi.org/10.3390/polym10050536
Chicago/Turabian StyleWalczak, Katarzyna, Jessica Thiele, Daniel Geisler, Klaus Boening, and Mieszko Wieckiewicz. 2018. "Effect of Chemical Disinfection on Chitosan Coated PMMA and PETG Surfaces—An In Vitro Study" Polymers 10, no. 5: 536. https://doi.org/10.3390/polym10050536





