Thermal and Mechanical Behavior of New Transparent Thermoplastic Polyurethane Elastomers Derived from Cycloaliphatic Diisocyanate
Abstract
:1. Introduction
2. Materials and Methods
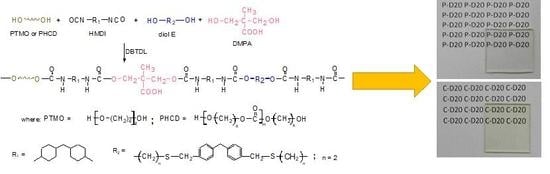
2.1. Materials and Synthesis
2.2. Methods
2.2.1. Attenuated Total Reflectance - Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.2.2. Thermogravimetric Analysis (TGA)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Optical Properties
2.2.5. Dynamical Mechanical Thermal Analysis (DMTA)
2.2.6. Mechanical Properties
3. Results and Discussion
3.1. TGA
3.2. DSC
3.3. Optical Properties
3.4. DMTA
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wirpsza, Z. Polyurethanes: Chemistry, Technology and Applications; Ellis Horwood: New York, NY, USA, 1993. [Google Scholar]
- Ulrich, H. Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; Wiley: New Jersey, NJ, USA, 2003. [Google Scholar]
- Gogolewski, S. Selected topics in biomedical polyurethanes. A review. Colloid. Polym. Sci. 1989, 267, 757–785. [Google Scholar] [CrossRef]
- Puszka, A.; Kultys, A.; Rogulska, M. Influence of DMPA content on the properties of new thermoplastic poly(ether-urethane) elastomers. J. Elastom. Plast. 2018, 50, 140–150. [Google Scholar] [CrossRef]
- Puszka, A.; Kultys, A. The influence of soft segments on some properties of new transparent segmented polyurethanes. Polym. Adv. Technol. 2017, 28, 1937–1944. [Google Scholar] [CrossRef]
- Govorčin, B.E.; Rek, V.; Agić, A. Thermal Degradation of Polyurethane Elastomers: Determination of Kinetic Parameters. J. Elastom. Plast. 2003, 35, 311–323. [Google Scholar]
- Gomez, C.M.; Gutierrez, D.; Asensio, M.; Costa, V.; Nohales, A. Transparent thermoplastic polyurethanes based on aliphatic diisocyanate and polycarbonate diol. J. Elastom. Plast. 2017, 49, 77–95. [Google Scholar] [CrossRef]
- Oprea, S. Influence of Hard Segment Structure on Degradation of Cross-linked Poly(ether urethanes) Elastomers. J. Elastom. Plast. 2010, 42, 163–179. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Nachman, M. The Abrasive Wear Resistance of Segmented Linear Polyurethane Elastomers Based on Variety of Polyols as Soft Segments. Polymers 2017, 9, 705. [Google Scholar] [CrossRef]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends, 1st ed.; Wiley: New Jersey, NJ, USA, 2014. [Google Scholar]
- Resiak, I.; Rokicki, G. Modified polyurethanes for biomedical applications. Polimery/Polymers 2000, 45, 592–602. [Google Scholar]
- Kojio, K.; Furukawa, M.; Motokucho, S.; Shimada, M.; Sakai, M. Structure-mechanical property relationships for poly(carbonate urethane) elastomers with novel soft segments. Macromolecules 2009, 42, 8322–8327. [Google Scholar] [CrossRef]
- Špirková, M.; Poręba, R.; Pavličević, J.; Kobera, L.; Baldrian, J.; Pekárek, M. Aliphatic polycarbonate-based polyurethane elastomers and nanocomposites. I. The influence of hard-segment content and macrodiol-constitution on bottom-up self-assembly. J. Appl. Polym. Sci. 2012, 126, 1016–1030. [Google Scholar] [CrossRef]
- Hrdlička, Z.; Kuta, A.; Poręba, R.; Špirková, M. Polycarbonate based polyurethane elastomers: Temperature-dependence of tensile properties. Chem. Pap. 2014, 68, 233–238. [Google Scholar] [CrossRef]
- Das, S.; Yilgor, I.; Yilgor, E.; Wilkes, G.L. Probing the urea hard domain connectivity in segmented, non-chain extended polyureas using hydrogen-bond screening agents. Polymer 2008, 49, 174–179. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E. Structure-Morphology-Property Behavior of Segmented Thermoplastic Polyurethanes and Polyureas Prepared without Chain Extenders. Polym. Rev. 2007, 47, 487–510. [Google Scholar]
- Holden, G. Encyclopedia of Chemical Technology, 5th ed.; Wiley Blackwell: New York, NY, USA, 2007. [Google Scholar]
- Rogulska, M.; Podkoscielny, W.; Kultys, A.; Pikus, S.; Pozdzik, E. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. I. Synthesis and characterization of nonsegmented polyurethanes from HDI and MDI. Eur. Polym. J. 2006, 42, 1786–1797. [Google Scholar] [CrossRef]
- Zia, K.M.; Zuber, M.; Barikani, M.; Bhatti, I.A.; Sheikh, M.A. Structural characteristics of UV-irradiated polyurethane elastomers extended with α,ω-alkane diols. J. Appl. Polym. Sci. 2009, 113, 2843–2850. [Google Scholar] [CrossRef]
- Zia, K.M.; Barikani, M.; Zuber, M.; Bhatti, I.A.; Bhatti, H.N. Morphological studies of polyurethane elastomers extended with α,ω alkane diols. Iran. Polym. J. 2008, 17, 61–72. [Google Scholar]
- Kultys, A.; Rogulska, M.; Pikus, S. New thermoplastic segmented polyurethanes with hard segment derived from 4,4’-diphenylmethane diisocyanate and methylenebis(1,4-phenylenemethylenethio)dialcanols. J. Appl. Polym. Sci. 2012, 123, 331–346. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Gluchowska, H. The effect of soft segment structure on the properties of novel thermoplastic polyurethane elastomers based on an unconventional chain extender. Polym. Int. 2011, 60, 652–659. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M. New thermoplastic poly(carbonate-urethane) elastomers. Pol. J. Chem. Technol. 2011, 13, 23–30. [Google Scholar] [CrossRef]
- Kultys, A.; Puszka, A. New thermoplastic polyurethane elastomers based on sulfur-containing chain extenders. Pol. J. Chem. Technol. 2013, 15, 65–70. [Google Scholar] [CrossRef]
- Kultys, A.; Puszka, A. Transparent poly(thiourethane-urethane)s based on dithiol chain extender. J. Therm. Anal. Calorim. 2014, 117, 1427–1439. [Google Scholar] [CrossRef]
- Puszka, A.; Kultys, A. New thermoplastic polyurethane elastomers based on aliphatic diisocyanate: Synthesis and characterization. J. Therm. Anal. Calorim. 2017, 128, 407–416. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Pikus, S. The effect of chain extender structure on the properties of new thermoplastic poly(carbonate–urethane)s derived from MDI. J. Therm. Anal. Calorim. 2017, 127, 2325–2339. [Google Scholar] [CrossRef]
- Čulin, J.; Šmit, I.; Veksli, Z.; Anžlovar, A.; Žigon, M. Phase morphology of functionalized polyester polyurethanes. Effect of functional group concentration. Polym. Int. 2006, 55, 285–291. [Google Scholar] [CrossRef]
- KosheelaDevi, P.P.; Tuan Noor Maznee, T.I.; Hoong, S.S.; Nurul ‘Ain, H.; Mohd Norhisham, S.; Norhayati, M.N.; Srihanum, A.; Yeong, S.K.; Hazimah, A.H.; Sadijarevic, V.; et al. Performance of palm oil-based dihydroxystearic acid as ionizable molecule in waterborne polyurethane dispersions. J. Appl. Polym. Sci. 2016, 133, 43614. [Google Scholar] [CrossRef]
- Žagar, E.; Žigon, E. Dilute solution behaviour of hexamethylene diisocyanate-based carboxylated polyurethanes and related ionomers in tetrahydrofuran. Polymer 1999, 40, 2727–2735. [Google Scholar] [CrossRef]
- Žagar, E.; Žigon, E. Solution properties of carboxylated polyurethanes nad related ionomers in polar solvents (DMF and LiBr/DMF). Polymer 2000, 41, 3513–3521. [Google Scholar] [CrossRef]
- Zhang, S.; Miao, W.; Zhou, Y. Reaction study of water-borne polyurethanes based on isophorone diisocyanate, dimethylol propionic acid, and poly(hexane neopentyl adipate glycol). J. Appl. Polym. Sci. 2004, 92, 161–164. [Google Scholar] [CrossRef]
- Matsunaga, K.; Nakagawa, K.; Sawai, S.; Sonoda, O.; Tajima, M.; Yoshida, Y. Synthesis and characterization of polyurethane anionomers. J. Appl. Polym. Sci. 2005, 98, 2144–2148. [Google Scholar] [CrossRef]
- Barikani, M.; Valipour Ebrahimi, M.; Seyed Mohaghegh, S.M. Preparation and characterization of aqueous polyurethane dispersions containing ionic centers. J. Appl. Polym. Sci. 2007, 104, 3931–3937. [Google Scholar] [CrossRef]
- Rogulska, M. Transparent sulfur-containing thermoplastic polyurethanes with polyether and polycarbonate soft segments. Polym. Bull. 2018, 75, 1211–1235. [Google Scholar] [CrossRef]
- Jha, G.S.; Seshadri, G.; Mohan, A.; Khandal, R.K. Sulfur containing optical plastics and its ophthalmic lenses applications. E-Polymers 2008, 8. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Puszka, A. New thermoplastic poly(carbonate-urethane)s based on chain extenders with sulfur atoms. Chem. Pap. 2017, 71, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, C. Trends in polyurethane elastomer technology. Iran. J. Polym. Sci. Technol. 1992, 1, 84–110. [Google Scholar]
- Menard, P.K. Dynamic Mechanical Analysis: A Practical Introduction; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]









| TPUR | Soft Segment | Amount of HMDI/mol | Amount of Diol E/mol | Amount of DMPA/mol | Amount of Soft Segment/mol | 1 Hard-Segment content/mass % |
|---|---|---|---|---|---|---|
| P-D20 | PTMO | 0.0105 | 0.004 | 0.001 | 0.005 | 46.14 |
| P-D40 | 0.0105 | 0.003 | 0.002 | 0.005 | 44.86 | |
| P-D60 | 0.0105 | 0.002 | 0.003 | 0.005 | 43.53 | |
| C-D20 | PHCD | 0.0105 | 0.004 | 0.001 | 0.005 | 49.90 |
| C-D40 | 0.0105 | 0.003 | 0.002 | 0.005 | 48.62 | |
| C-D60 | 0.0105 | 0.002 | 0.003 | 0.005 | 47.27 |
| TPUR | T1 1/°C | T5 2/°C | T10 3/°C | T50 4/°C | Tmax 5/°C | Tg/°C | Tm/°C | ΔH/J/g | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I 6 | II 6 | I 6 | II 6 | I 6 | II 6 | ||||||
| P-D20 | 290 | 309 | 325 | 392 | 353; 401 | −21 | −19 | − | − | − | − |
| P-D40 | 279 | 306 | 323 | 383 | 308;360; 405 | −25 | −31 | − | − | − | − |
| P-D60 | 273 | 296 | 313 | 380 | 308; 358; 403 | −17 | −21 | 156 | − | 0.20 | − |
| C-D20 | 250 | 289 | 303 | 346 | 308; 349; 425 | 20 | 21 | − | − | − | − |
| C-D40 | 243 | 283 | 297 | 340 | 300; 344; 438 | 17 | 21 | 156 | − | 0.54 | − |
| C-D60 | 234 | 273 | 287 | 327 | 295; 328; 433 | 20 | 20 | 156 | − | 0.45 | − |
| TPUR | Refractive Index | Transmittance/% | |
|---|---|---|---|
| T500 1 | T800 2 | ||
| P-D20 | 1.510 | 85.0 | 89.9 |
| P-D40 | 1.504 | 82.3 | 87.8 |
| P-D60 | 1.501 | 78.5 | 84.0 |
| C-D20 | 1.520 | 77.9 | 85.4 |
| C-D40 | 1.514 | 75.4 | 84.2 |
| C-D60 | 1.507 | 72.7 | 82.1 |
| TPUR | 1 E′onset/°C | 2 E″max/°C | 3 tanδmax/°C | tanδmax3 |
|---|---|---|---|---|
| P-D20 | −12.7 | −15.1 | 12.1 | 0.66 |
| P-D40 | −17.7 | −17.5 | 15.6 | 0.55 |
| P-D60 | −13.7 | −16.0 | 17.6 | 0.61 |
| C-D20 | 23.5 | −57.7; 24.6 | −53.1; 40.4 | 0.89 |
| C-D40 | 25.8 | −59.7; 25.4 | −54.7; 43.7 | 0.82 |
| C-D60 | 24.4 | −61.2; 23.2 | −56.5; 47.8 | 0.66 |
| TPUR | Hardness/Shore A/D | Tensile Strength/MPa | Elongation at Break/% | Modulus of Elasticity/MPa |
|---|---|---|---|---|
| P-D20 | 60/24 | 10.1 ± 0.24 | 425 ± 25 | 1.93 ± 0.02 |
| P-D40 | 53/17 | 8.12 ± 0.27 | 700 ± 14 | 0.15 ± 0.01 |
| P-D60 | 53/17 | 4.24 ± 0.13 | 750 ± 25 | 0.09 ± 0.01 |
| C-D20 | 72/31 | 41.1 ± 0.27 | 300 ± 25 | 19.1 ± 0.04 |
| C-D40 | 77/35 | 38.2 ± 0.23 | 270 ± 14 | 34.8 ± 0.02 |
| C-D60 | 80/36 | 39.0 ± 0.35 | 250 ± 14 | 46.3 ± 0.09 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puszka, A. Thermal and Mechanical Behavior of New Transparent Thermoplastic Polyurethane Elastomers Derived from Cycloaliphatic Diisocyanate. Polymers 2018, 10, 537. https://doi.org/10.3390/polym10050537
Puszka A. Thermal and Mechanical Behavior of New Transparent Thermoplastic Polyurethane Elastomers Derived from Cycloaliphatic Diisocyanate. Polymers. 2018; 10(5):537. https://doi.org/10.3390/polym10050537
Chicago/Turabian StylePuszka, Andrzej. 2018. "Thermal and Mechanical Behavior of New Transparent Thermoplastic Polyurethane Elastomers Derived from Cycloaliphatic Diisocyanate" Polymers 10, no. 5: 537. https://doi.org/10.3390/polym10050537