High Performance Soluble Polyimides from Ladder-Type Fluorinated Dianhydride with Polymorphism
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Instrumentation
2.3. Monomer Synthesis of 8FDA
2.4. Polymer Preparation
3. Results and Discussion
3.1. Monomer Synthesis
3.2. Description of Crystal Structure of Compounds 2–4, and 8FDA (6a, 6b, and 6c)
3.3. The Solubility and WAXD of the Polyimide Films
3.4. Optical Properties of PIs
3.5. Thermal Properties of PIs
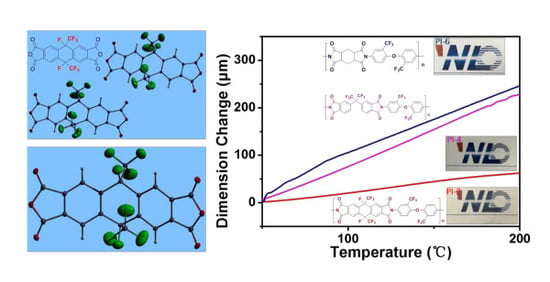
3.6. Mechanical Properties
3.7. Surface Properties of the PIs
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Spechler, J.A.; Koh, T.-W.; Herb, J.T.; Rand, B.P.; Arnold, C.B. A transparent, smooth, thermally robust, conductive polyimide for flexible electronics. Adv. Funct. Mater. 2015, 25, 7428–7434. [Google Scholar] [CrossRef]
- Ling, Q.-D.; Chang, F.-C.; Song, Y.; Zhu, C.-X.; Liaw, D.-J.; Chan, D.S.-H.; Kang, E.-T.; Neoh, K.-G. Synthesis and dynamic random access memory behavior of a functional polyimide. J. Am. Chem. Soc. 2006, 128, 8732–8733. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Nehm, F.; Muller-Meskamp, L.; Vandewal, K.; Leo, K. Optical display film as flexible and light trapping substrate for organic photovoltaics. Opt. Express 2016, 24, A974–A980. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lin, L.; Liu, Y.; Huang, X. LTPS TFT process on polyimide substrate for flexible amoled. J. Disp. Technol. 2015, 11, 666–669. [Google Scholar] [CrossRef]
- Fang, Y.; Ding, K.; Wu, Z.; Chen, H.; Li, W.; Zhao, S.; Zhang, Y.; Wang, L.; Zhou, J.; Hu, B. Architectural engineering of nanowire network fine pattern for 30 μm wide flexible quantum dot light-emitting diode application. ACS Nano 2016, 10, 10023–10030. [Google Scholar] [CrossRef] [PubMed]
- Matthias, T.; Neville, V.R.; Roman, F. Fabrication of surface-supported low-dimensional polyimide networks. J. Am. Chem. Soc. 2008, 130, 14054–14055. [Google Scholar]
- Park, Y.; Berger, J.; Tang, Z.; Müller-Meskamp, L.; Lasagni, A.F.; Vandewal, K.; Leo, K. Flexible, light trapping substrates for organic photovoltaics. Appl. Phys. Lett. 2016, 109, 093301. [Google Scholar] [CrossRef]
- Park, J.; Hyun, B.G.; An, B.W.; Im, H.G.; Park, Y.G.; Jang, J.; Park, J.U.; Bae, B.S. Flexible transparent conductive films with high performance and reliability using hybrid structures of continuous metal nanofiber networks for flexible optoelectronics. ACS Appl. Mater. Interfaces 2017, 9, 20299–20305. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Huang, H.; Jing, Y.; Fu, H.; Chang, P.; Li, D.; Yao, Y.; Fan, Z. Flexible photovoltaic technologies. J. Mater. Chem. C 2014, 2, 1233–1247. [Google Scholar] [CrossRef]
- Yu, H.-C.; Kumar, S.V.; Lee, J.H.; Oh, S.Y.; Chung, C.-M. Preparation of robust, flexible, transparent films from partially aliphatic copolyimides. Macromol. Res. 2015, 23, 566–573. [Google Scholar] [CrossRef]
- Bae, W.J.; Kovalev, M.K.; Kalinina, F.; Kim, M.; Cho, C. Towards colorless polyimide/silica hybrids for flexible substrates. Polymer 2016, 105, 124–132. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kaneki, T.; Tsukui, M.; Okubo, N.; Ishii, J. High-temperature polymers overcoming the trade-off between excellent thermoplasticity and low thermal expansion properties. Polymer 2016, 99, 292–306. [Google Scholar] [CrossRef]
- Shen, C.; Bao, Y.; Wang, Z. Tetraphenyladamantane-based microporous polyimide for adsorption of carbon dioxide, hydrogen, organic and water vapors. Chem. Commun. 2013, 49, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, Y.; Liu, S.; Chi, Z.; Chen, X.; Xu, J. Flexible and highly fluorescent aromatic polyimide: Design, synthesis, properties, and mechanism. J. Mater. Chem. C 2016, 4, 10509–10517. [Google Scholar] [CrossRef]
- Yu, X.; Liang, W.; Cao, J.; Wu, D. Mixed rigid and flexible component design for high-performance polyimide films. Polymers 2017, 9, 451. [Google Scholar] [CrossRef]
- Yu, H.-C.; Jung, J.-W.; Choi, J.-Y.; Oh, S.Y.; Chung, C.-M. Structure-property relationship study of partially aliphatic copolyimides for preparation of flexible and transparent polyimide films. J. Membr. Sci. Part A 2017, 54, 97–104. [Google Scholar] [CrossRef]
- Park, J.; Enomoto, K.; Yamashita, T.; Takagi, Y.; Todaka, K.; Maekawa, Y. Polymerization mechanism for radiation-induced grafting of styrene into alicyclic polyimide films for preparation of polymer electrolyte membranes. J. Membr. Sci. 2013, 438, 1–7. [Google Scholar] [CrossRef]
- Fukukawa, K.-I.; Okazaki, M.; Sakata, Y.; Urakami, T.; Yamashita, W.; Tamai, S. Synthesis and properties of multi-block semi-alicyclic polyimides for thermally stable transparent and low CTE film. Polymer 2013, 54, 1053–1063. [Google Scholar] [CrossRef]
- Luo, Y.; Li, B.; Liang, L.; Tan, B. Synthesis of cost-effective porous polyimides and their gas storage properties. Chem. Commun. 2011, 47, 7704–7706. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Yen, H.-J.; Hu, Y.-C.; Liou, G.-S. Novel programmable functional polyimides: Preparation, mechanism of CT induced memory, and ambipolar electrochromic behavior. J. Mater. Chem. C 2013, 1, 7623–7634. [Google Scholar] [CrossRef]
- Xu, Z.; Li, M.; Xu, M.; Zou, J.; Tao, H.; Wang, L.; Peng, J. Light extraction of flexible oleds based on transparent polyimide substrates with 3-D photonic structure. Org. Electron. 2017, 44, 225–231. [Google Scholar] [CrossRef]
- Williams, J.C.; Nguyen, B.N.; McCorkle, L.; Scheiman, D.; Griffin, J.S.; Steiner, S.A., 3rd; Meador, M.A. Highly porous, rigid-rod polyamide aerogels with superior mechanical properties and unusually high thermal conductivity. ACS Appl. Mater. Interfaces 2017, 9, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.J.; Zhu, L.; Yu, Q. Design and development of plasmonic nanostructured electrodes for ito-free organic photovoltaic cells on rigid and highly flexible substrates. Nanotechnology 2017, 28, 165401. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, F.; Cao, Z.; Barat, D.; Tu, G. Light scattering in nanoparticle doped transparent polyimide substrates. ACS Appl. Mater. Interfaces 2017, 9, 14990–14997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, B.; Yu, H.; Sun, L.; Jiao, C.; Liu, W. Microporous polyimide networks with large surface areas and their hydrogen storage properties. Chem. Commun. 2010, 46, 7730–7732. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Seong, J.G.; Do, Y.S.; Jo, H.J.; Lee, M.J.; Wang, G.; Guiver, M.D.; Lee, Y.M. Effect of isomerism on molecular packing and gas transport properties of poly(benzoxazole-co-imide)s. Macromolecules 2014, 47, 7947–7957. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Hu, X.; Yan, J.; Wang, Y.; Mu, H.; Wang, Z.; Cheng, H.; Zhao, F.; Wang, Z. Colorless polyimides derived from 2R,5R,7S,10S-naphthanetetracarboxylic dianhydride. Polym. Chem. UK 2017, 8, 6165–6172. [Google Scholar] [CrossRef]
- Yu, H.-C.; Jung, J.-W.; Choi, J.-Y.; Chung, C.-M. Kinetic study of low-temperature imidization of poly(amic acid)s and preparation of colorless, transparent polyimide films. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1593–1602. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride with controlled steric structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Yoshiaki, E.; Norihiko, M.; Isao, T. Preparation of poly(bis(trialkylammonium) 4,4-oxydiphenylenepyromellitamate) films: A useful polyimide precursor film. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 2493–2499. [Google Scholar]
- Xu, S.; Wang, Y. Novel thermally cross-linked polyimide membranes for ethanol dehydration via pervaporation. J. Membr. Sci. 2015, 496, 142–155. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Lan, Q.; Qin, Z.; Liu, S.; Zhao, C.; Chi, Z.; Xu, J. Synthesis and properties of high-performance functional polyimides containing rigid nonplanar conjugated tetraphenylethylene moieties. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1302–1314. [Google Scholar] [CrossRef]
- Das, D.; Barbour, L.J. Polymorphism of a hexa-host: Isolation of four different single-crystal phases by melt crystallization. J. Am. Chem. Soc. 2008, 130, 14032–14033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, L.; Han, S.; Qi, H.; Cheng, Y.; Liu, F. Polyimides from an asymmetric hydroxyl-containing aliphatic-aromatic diamine synthesized via henry reaction. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3413–3423. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.H.; Ahn, S.K.; Kwon, S.K. Synthesis and characterization of highly soluble and oxygen permeable new polyimides bearing a noncoplanar twisted biphenyl unit containing tert-butylphenyl or trimethylsilyl phenyl groups. Macromolecules 2003, 36, 2327–2332. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Liu, F.; Lai, J.; Qi, H.; You, X. A new series of fluorinated alicyclic-functionalized polyimides derivated from natural-(D)-camphor: Synthesis, structure–properties relationships and dynamic dielectric analyses. Polymer 2013, 54, 5673–5683. [Google Scholar] [CrossRef]
- Li, F.; Shen, J.; Liu, X.; Cao, Z.; Cai, X.; Li, J.; Ding, K.; Liu, J.; Tu, G. Flexible qled and opv based on transparent polyimide substrate with rigid alicyclic asymmetric isomer. Org. Electron. 2017, 51, 54–61. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Tan, J.; Zeng, Y.; Liu, J.; Zhang, H.; Pei, Y.; Xiang, X.; Liu, Y. Intrinsic high-barrier polyimide with low free volume derived from a novel diamine monomer containing rigid planar moiety. Polymer 2017, 114, 289–297. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Lan, Q.; Liu, S.; Qin, Z.; Chen, L.; Zhao, C.; Chi, Z.; Xu, J.; Economy, J. High-performance functional polyimides containing rigid nonplanar conjugated triphenylethylene moieties. Chem. Mater. 2012, 24, 1212–1222. [Google Scholar] [CrossRef]
- Wu, S.; Hayakawa, T.; Kikuchi, R.; Grunzinger, S.J.; Kakimoto, M. Synthesis and ccharacterization of semiaromatic polyimides containing poss in main chain derived from double-decker-shaped silsesquioxane. Macromoleclars 2007, 40, 5698–5705. [Google Scholar] [CrossRef]
- Eastmond, G.C.; Paprotny, J.; Pethrick, R.A.; Santamaria-Mendia, F. A comparison of poly(ether imide)s with 3-phthalimide and 4-phthalimide units: Synthesis, characterization, and physical properties. Macromoleculars 2006, 39, 7534–7548. [Google Scholar] [CrossRef]
- Lioua, H.-C.; Hoa, P.S.; Stierman, R. Thickness dependence of the anisotropy in thermal expansion of PMDA-ODA and BPDA-PDA thin films. Thin Solid Films 1999, 338, 68–73. [Google Scholar] [CrossRef]
- Ando, S.; Sekiguchi, K.; Mizoroki, M.; Okada, T.; Ishige, R. Anisotropic linear and volumetric thermal-expansion behaviors of self-standing polyimide films analyzed by thermomechanical analysis (TMA) and optical interferometry. Macromol. Chem. Phys. 2018, 219, 1700354. [Google Scholar] [CrossRef]
- Bronnikow, S.V.; Sukhanova, T.E.; Goikhman, M.Y. Evolution of statistical ensemble of mierodomains on the surface of films of rigid-chain polymide during thermal imidezation. Russian J. Appl. Chem. 2003, 76, 967–971. [Google Scholar] [CrossRef]
- Ando, S.; Matsuura, T.; Sasaki, S. Coloration of aromatic polyimides and electronic properties of their source materials. Polym. J. 1997, 29, 69–76. [Google Scholar] [CrossRef]
- Kotov, B.V.; Gordina, T.A.; Voishchev, V.S.; Kolninov, O.V.; Pravednikov, A.N. Aromatic polyimides as charge transfer complexes. Polym. Sci. USSR 1977, 19, 711–716. [Google Scholar] [CrossRef]
- Ebisawa, S.; Ishii, J.; Sato, M.; Vladimirov, L.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (5). Effect of ordered structure formation in polyimide precursors on cte. Eur. Polym. 2010, 46, 283–297. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hoshino, Y.; Katsura, N.; Ishii, J. Superheat-resistant polymers with low coefficients of thermal expansion. Polymer 2017, 111, 91–102. [Google Scholar] [CrossRef]
- Lu, Y.; Hao, J.; Xiao, G.; Zhao, H.; Hu, Z.; Wang, T. In situ polymerization and performance of alicyclic polyimide/graphene oxide nanocomposites derived from 6FAPB and CBDA. Appl. Surf. Sci. 2017, 394, 78–86. [Google Scholar] [CrossRef]
- Adachi, S.; Arai, T.; Kobayashi, K. Chemical treatment effect of Si(111) surfaces in F-based aqueous solutions. J. Appl. Phys. 1996, 80, 5422–5426. [Google Scholar] [CrossRef]
- Meador, M.A.; Agnello, M.; McCorkle, L.; Vivod, S.L.; Wilmoth, N. Moisture-resistant polyimide aerogels containing propylene oxide links in the backbone. ACS Appl. Mater. Interfaces 2016, 8, 29073–29079. [Google Scholar] [CrossRef] [PubMed]
- Vora, R.H.; Krishnan, R.S.G.; Goh, S.H.; Chung, T.S. Synthesis and properties of designed low-k fluoro-copolyetherimides. Part 1. Adv. Funct. Mater. 2001, 11, 361–373. [Google Scholar] [CrossRef]
- Barzic, A.I.; Stoica, I.; Fifere, N.; Vlad, C.D.; Hulubei, C. Morphological effects on transparency and absorption edges of some semi-alicyclic polyimides. J. Polym. Res. 2013, 20, 130. [Google Scholar] [CrossRef]








| Polymer Code | Dianhydride | Diamine | Solubility a | WAXD b | Surface Properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THF | EA | Toluene | CH2Cl2 | m-Cresol | DMAc | NMP | DMF | 2θ (°) | d-Spacing (Å) | Water Absorbtion (%) c | Contact Angle (°) d | RMS (nm) | |||
| PI-1 | 8FDA | TFDB | +s | +s | - | +- | +s | +s | +s | +s | 14.01 | 6.31 | 0.85 | 95 | 1.79 |
| PI-2 | 8FDA | TFODA | +s | +s | - | + | + | + | + | + | 13.84 | 6.38 | 1.06 | 92 | 1.16 |
| PI-3 | 6FDA | TFDB | +- | +- | - | +- | + | +s | + | +s | 14.38 | 6.20 | 1.21 | 92 | 2.01 |
| PI-4 | 6FDA | TFODA | + | +- | - | +- | + | +s | + | +s | 14.28 | 6.26 | 1.64 | 90 | 2.23 |
| PI-5 | HPMDA | TFDB | - | - | - | - | + | +s | + | +s | 15.10 | 5.79 | 1.21 | 84 | 1.93 |
| PI-6 | HPMDA | TFODA | - | - | - | - | + | + | + | + | 15.28 | 5.86 | 1.33 | 82 | 1.91 |
| Polymer | Dianhydride | Dianmine | Thermal Properties | Mechanical Properties a | Optical Properties | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg (°C) DMA | Td1 (°C) | Td5 (°C) | Char Yield (%) b | CTE (ppm K−1) c | Ts (MPa) | Eb (%) | Tm (GPa) | T400d (%) | λ0 e (nm) | |||
| PI-1 | 8FDA | TFDB | 401 | 518 | 557 | 64 | 14 | 113 | 7.0 | 1.2 | 46 | 358 |
| PI-2 | 8FDA | TFODA | 369 | 492 | 550 | 60 | 18 | 96 | 2.1 | 4.5 | 64 | 344 |
| PI-3 | 6FDA | TFDB | 315 | 488 | 521 | 53 | 49 | 84 | 3.3 | 2.6 | 43 | 370 |
| PI-4 | 6FDA | TFODA | 300 | 490 | 520 | 50 | 63 | 65 | 5.8 | 1.1 | 71 | 353 |
| PI-5 | HPMDA | TFDB | 292 | 473 | 501 | 56 | 44 | 71 | 4.0 | 1.8 | 79 | 304 |
| PI-6 | HPMDA | TFODA | 348 | 462 | 497 | 57 | 64 | 39 | 2.7 | 1.8 | 83 | 296 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liu, J.; Liu, X.; Wang, Y.; Gao, X.; Meng, X.; Tu, G. High Performance Soluble Polyimides from Ladder-Type Fluorinated Dianhydride with Polymorphism. Polymers 2018, 10, 546. https://doi.org/10.3390/polym10050546
Li F, Liu J, Liu X, Wang Y, Gao X, Meng X, Tu G. High Performance Soluble Polyimides from Ladder-Type Fluorinated Dianhydride with Polymorphism. Polymers. 2018; 10(5):546. https://doi.org/10.3390/polym10050546
Chicago/Turabian StyleLi, Fu, Jikang Liu, Xiangfu Liu, Yao Wang, Xiang Gao, Xianggao Meng, and Guoli Tu. 2018. "High Performance Soluble Polyimides from Ladder-Type Fluorinated Dianhydride with Polymorphism" Polymers 10, no. 5: 546. https://doi.org/10.3390/polym10050546