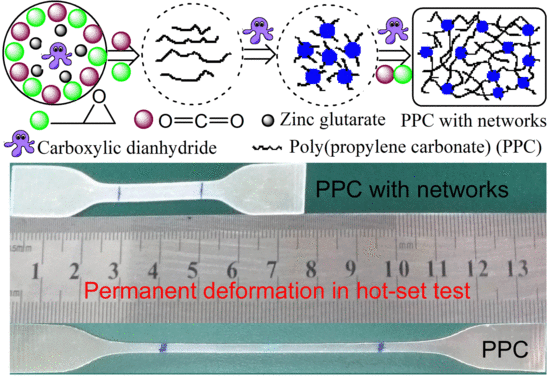
Enhanced Poly(Propylene Carbonate) with Thermoplastic Networks: A One-Pot Synthesis from Carbon Dioxide, Propylene Oxide, and a Carboxylic Dianhydride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Copolymerization Procedure
2.3. Characterization and Measurements
3. Results and Discussions
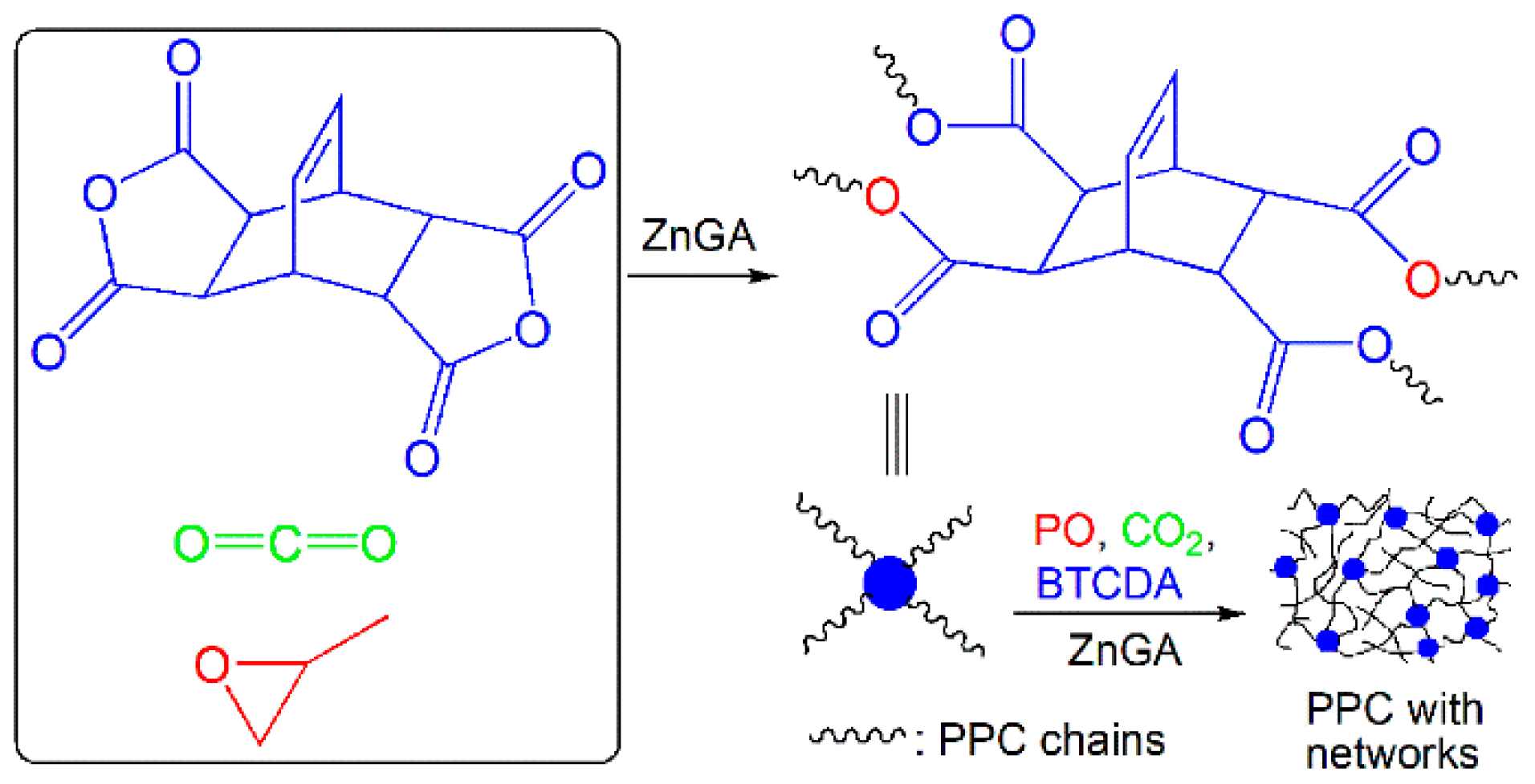
3.1. Synthesis
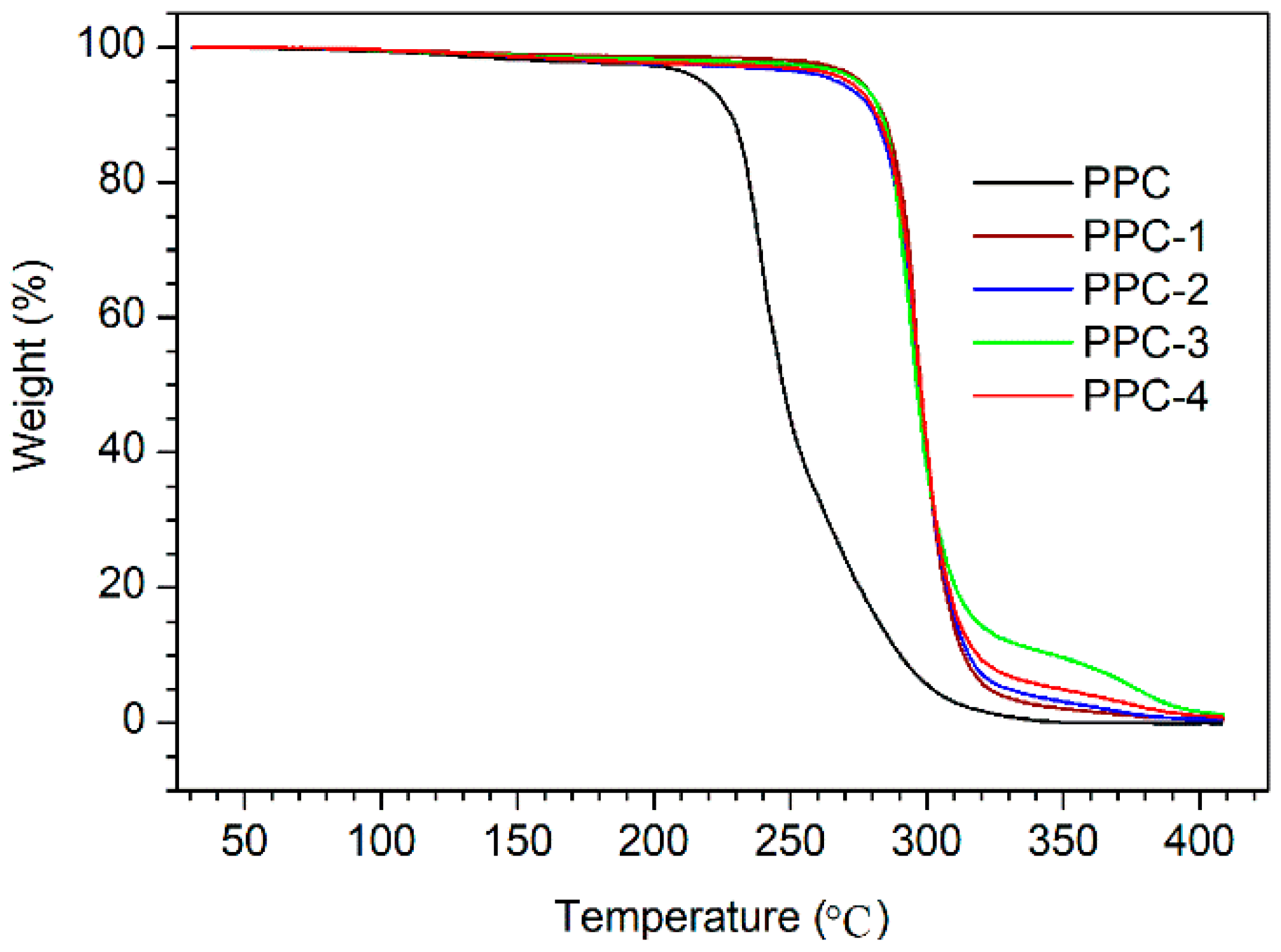
3.2. Thermal Properties
3.3. Mechanical Properties
3.4. Hot-Set Elongation and Permanent Deformation
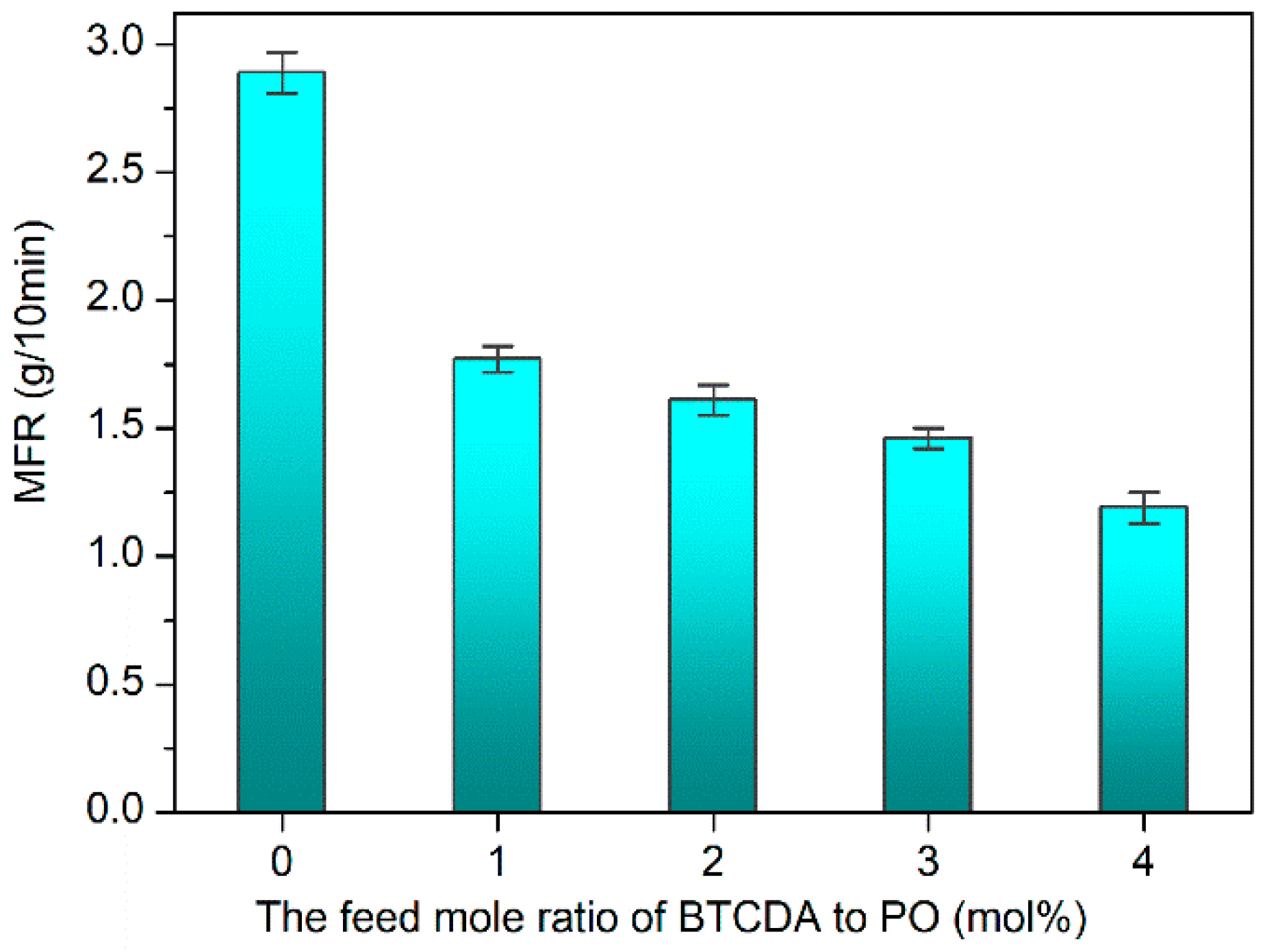
3.5. Rheological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.H.; Lee, J.H.; Lee, S.H.; Heo, S.B.; Pittman, C.U., Jr. Morphology, thermal stability and rheology of poly(propylene carbonate)/organoclay nanocomposites with different pillaring agents. Polymer 2008, 49, 2947–2956. [Google Scholar] [CrossRef]
- Ree, M.; Bae, J.Y.; Jung, J.H.; Shin, T.J. A new copolymerization process leading to poly(propylene carbonate) with a highly enhanced yield from carbon dioxide and propylene oxide. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1863–1876. [Google Scholar] [CrossRef]
- Sheng, X.F.; Wu, W.; Qin, Y.S.; Wang, X.H.; Wang, F.S. Efficient synthesis and stabilization of poly(propylene carbonate) from delicately designed bifunctional aluminum porphyrin complexes. Polym. Chem. 2015, 6, 4719–4724. [Google Scholar] [CrossRef]
- Klaus, S.; Lehenmeier, M.W.; Anderson, C.E.; Rieger, B. Recent advances in CO2/epoxide copolymerization-New strategies and cooperative mechanisms. Coord. Chem. Rev. 2011, 255, 1460–1479. [Google Scholar] [CrossRef]
- Coates, G.W.; Moore, D.R. Discrete metal-based catalysts for the copolymerization of CO2 and epoxides: Discovery, reactivity, optimization, and mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar] [CrossRef] [PubMed]
- Poland, S.J.; Darensbourg, D.J. A quest for polycarbonates provided via sustainable epoxide/CO2 copolymerization processes. Green Chem. 2017, 19, 4990–5011. [Google Scholar] [CrossRef]
- Lu, X.B.; Ren, W.M.; Wu, G.P. CO2 copolymers from epoxides: Catalyst activity, product selectivity, and stereochemistry control. Acc. Chem. Res. 2012, 45, 1721–1735. [Google Scholar] [CrossRef] [PubMed]
- Sujith, S.; Min, J.K.; Seong, J.E.; Na, S.J.; Lee, B.Y. A highly active and recyclable catalytic system for CO2/propylene oxide copolymerization. Angew. Chem. Int. Ed. 2008, 120, 7416–7419. [Google Scholar] [CrossRef]
- Vagin, S.I.; Reichardt, R.; Klaus, S.; Rieger, B. Conformationally flexible dimeric salphen complexes for bifunctional catalysis. J. Am. Chem. Soc. 2010, 132, 14367–14369. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Hashimoto, S.i.; Nakamura, M.; Kamada, T.; Nozaki, K. Stereocomplex of poly(propylene carbonate): Synthesis of stereogradient poly(propylene carbonate) by regio-and enantioselective copolymerization of propylene oxide with carbon dioxide. Angew. Chem. Int. Ed. 2011, 150, 4868–4871. [Google Scholar] [CrossRef] [PubMed]
- Luinstra, G.A.; Borchardt, E. Material properties of poly(propylene carbonates). Adv. Polym. Sci. 2011, 245, 29–48. [Google Scholar] [CrossRef]
- Chen, L.J.; Qin, Y.S.; Wang, X.H.; Li, Y.S.; Zhao, X.J.; Wang, F.S. Toughening of poly(propylene carbonate) by hyperbranched poly(ester-amide) via hydrogen bonding interaction. Polym. Int. 2011, 60, 1697–1704. [Google Scholar] [CrossRef]
- Li, B.; Wu, G.P.; Ren, W.M.; Wang, Y.M.; Rao, D.Y.; Lu, X.B. Asymmetric, region-and stereo-selective alternating copolymerization of CO2 and propylene oxide catalyzed by chiral chromium Salan complexes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6102–6113. [Google Scholar] [CrossRef]
- Wu, G.P.; Jiang, S.D.; Lu, X.B.; Ren, W.M.; Yan, S.K. Stereoregular poly(cyclohexene carbonate)s: Unique crystallization behavior. Chin. J. Polym. Sci. 2012, 30, 487–492. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kojima, R. Crystal structure of poly(trimethylene carbonate). Macromolecules 2003, 36, 5139–5143. [Google Scholar] [CrossRef]
- Shi, L.; Lu, X.B.; Zhang, R.; Peng, X.J.; Zhang, C.Q.; Li, J.F.; Peng, X.M. Asymmetric alternating copolymerization and terpolymerization of epoxides with carbon dioxide at mild conditions. Macromolecules 2006, 39, 5679–5685. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xiao, M.; Wang, S.J.; Han, D.M.; Meng, Y.Z. Novel ternary block copolymerization of carbon dioxide with cyclohexene oxide and propylene oxide using zinc complex catalyst. J. Polym. Res. 2012, 19, 9800. [Google Scholar] [CrossRef]
- Seong, J.E.; Na, S.J.; Cyriac, A.; Kim, B.W.; Lee, B.Y. Terpolymerizations of CO2, propylene oxide, and various epoxides using a Cobalt(III) complex of salen-type ligand tethered by four quaternary ammonium salts. Macromolecules 2010, 43, 903–908. [Google Scholar] [CrossRef]
- Song, P.F.; Xu, H.D.; Mao, X.D.; Liu, X.J.; Wang, L. A one-step strategy for aliphatic poly(carbonate-ester)s with high performance derived from CO2, propylene oxide and l-lactide. Polym. Adv. Technol. 2017, 28, 736–741. [Google Scholar] [CrossRef]
- Tao, Y.H.; Wang, X.H.; Zhao, X.J.; Li, J.; Wang, F.S. Crosslinkable poly(propylene carbonate): High-yield synthesis and performance improvement. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5329–5336. [Google Scholar] [CrossRef]
- Song, P.F.; Wang, S.J.; Xiao, M.; Du, F.G.; Gan, L.Q.; Liu, G.Q.; Meng, Y.Z. Cross-linkable and thermally stable aliphatic polycarbonates derived from CO2, propylene oxide and maleic anhydride. J. Polym. Res. 2009, 16, 91–97. [Google Scholar] [CrossRef]
- Cyriac, A.; Lee, S.H.; Lee, B.Y. Connection of polymer chains using diepoxide in CO2/propylene oxide copolymerizations. Polym. Chem. 2011, 2, 950–956. [Google Scholar] [CrossRef]
- Zeng, S.S.; Wang, S.J.; Xiao, M.; Han, D.M.; Meng, Y.Z. Preparation and properties of biodegradable blend containing poly(propylene carbonate) and starch acetate with different degrees of substitution. Carbohydr. Polym. 2011, 86, 1260–1265. [Google Scholar] [CrossRef]
- Kuang, T.R.; Li, K.C.; Chen, B.Y.; Peng, X.F. Poly(propylene carbonate)-based in situ nanofibrillar biocomposites with enhanced miscibility, dynamic mechanical properties, rheological behavior and extrusion foaming ability. Compos. Part B Eng. 2017, 123, 112–123. [Google Scholar] [CrossRef]
- Enriquez, E.; Mohanty, A.K.; Misra, M. Biobased blends of poly(propylene carbonate) and poly(hydroxybutyrate-co-hydroxyvalerate): Fabrication and characterization. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Chen, S.S.; Chen, B.; Fan, J.S.; Feng, J.C. Exploring the application of sustainable poly(propylene carbonate) copolymer in toughening epoxy thermosets. ACS Sustain. Chem. Eng. 2015, 3, 2077–2083. [Google Scholar] [CrossRef]
- Yang, J.; Pan, H.W.; Li, X.; Sun, S.L.; Zhang, H.L.; Dong, L.S. A study on the mechanical, thermal properties and crystallization behavior of poly(lactic acid)/thermoplastic poly(propylene carbonate) polyurethane blends. RSC Adv. 2017, 7, 46183–46194. [Google Scholar] [CrossRef]
- Gao, J.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Bai, H.W.; Fu, Q. A promising alternative to conventional polyethylene with poly(propylene carbonate) reinforced by graphene oxide nanosheets. J. Mater. Chem. 2011, 21, 17627–17630. [Google Scholar] [CrossRef]
- Bian, J.; Wei, X.W.; Lin, H.L.; Gong, S.J.; Zhang, H.; Guan, Z.P. Preparation and characterization of modified graphite oxide/poly(propylene carbonate) composites by solution intercalation. Polym. Degrad. Stab. 2011, 96, 1833–1840. [Google Scholar] [CrossRef]
- Shi, X.D.; Gan, Z.H. Preparation and characterization of poly(propylene carbonate)/montmorillonite nanocomposites by solution intercalation. Eur. Polym. J. 2007, 43, 4852–4858. [Google Scholar] [CrossRef]
- Zhai, L.P.; Li, G.F.; Xu, Y.; Xiao, M.; Wang, S.J.; Meng, Y.Z. Poly(propylene carbonate)/aluminum flake composite films with enhanced gas barrier properties. J. Appl. Polym. Sci. 2015, 132, 13–22. [Google Scholar] [CrossRef]
- Liao, J.G.; Li, Y.Q.; Zou, Q.; Duan, X.Z.; Yang, Z.P.; Xie, Y.F.; Liu, H.H. Preparation, characterization and properties of nano-hydroxyapatite/polypropylene carbonate biocomposite. Mat. Sci. Eng. C 2016, 63, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Qin, Y.S.; Wang, X.H.; Zhao, X.J.; Wang, F.S. Plasticizing while toughening and reinforcing poly(propylene carbonate) using low molecular weight urethane: Role of hydrogen-bonding interaction. Polymer 2011, 52, 4873–4880. [Google Scholar] [CrossRef]
- Qin, Y.S.; Chen, L.J.; Wang, X.H.; Zhao, X.J.; Wang, F.S. Enhanced mechanical performance of poly(propylene carbonate) via hydrogen bonding interaction with o-lauroyl chitosan. Carbohydr. Polym. 2011, 84, 329–334. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, R.K.Y.; Cao, Y.X.; Meng, Y.Z. Essential work of fracture analysis for starch filled poly(propylene carbonate) composites. Mater. Des. 2007, 28, 1934–1939. [Google Scholar] [CrossRef]
- Qi, X.D.; Jing, M.F.; Liu, Z.W.; Dong, P.; Liu, T.Y.; Fu, Q. Microfibrillated cellulose reinforced bio-based poly(propylene carbonate) with dual-responsive shape memory properties. RSC Adv. 2016, 6, 7560–7567. [Google Scholar] [CrossRef]
- Wang, Y.M.; Song, X.Y.; Shao, S.H.; Xu, P.X.; Ren, W.M.; Lu, X.B. Functionalization of carbon nanotubes by surface-initiated immortal alternating polymerization of CO2 and epoxides. Polym. Chem. 2013, 4, 629–636. [Google Scholar] [CrossRef]
- Okada, A.; Kikuchi, S.; Yamada, T. Alternating copolymerization of propylene oxide/alkylene oxide and carbon dioxide: Tuning thermal properties of polycarbonates. Chem. Lett. 2011, 40, 209–211. [Google Scholar] [CrossRef]
- Tao, Y.H.; Wang, X.H.; Zhao, X.J.; Li, J.; Wang, F.S. Double propagation based on diepoxide, a facile route to high molecular weight poly(propylene carbonate). Polymer 2006, 47, 7368–7373. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Zhang, H.Y.; Ding, H.N.; Liu, B.Y.; Wang, X.H. One-pot synthesis and postpolymerization functionalization of cyclic carbonate/epoxide-difunctional polycarbonates prepared by regioselective diepoxide/CO2 copolymerization. Polym. Chem. 2016, 7, 4453–4457. [Google Scholar] [CrossRef]
- Song, P.F.; Xiao, M.; Du, F.G.; Wang, S.J.; Gan, L.Q.; Liu, G.Q.; Meng, Y.Z. Synthesis and properties of aliphatic polycarbonates derived from carbon dioxide, propylene oxide and maleic anhydride. J. Appl. Polym. Sci. 2008, 109, 4121–4129. [Google Scholar] [CrossRef]
- Liu, Y.F.; Huang, K.L.; Peng, D.M.; Wu, H. Synthesis, characterization and hydrolysis of an aliphatic polycarbonate by terpolymerization of carbon dioxide, propylene oxide and maleic anhydride. Polymer 2006, 47, 8453–8461. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Eo, S.C.; Varghese, J.K.; Lee, B.Y. Copolymerization and terpolymerization of carbon dioxide/propylene oxide/phthalic anhydride using a (salen)Co(III) complex tethering four quaternary ammonium salts. J. Org. Chem. 2014, 10, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Nörnberg, B.; Luinstra, G.A. Influence of norbornene dicarboxylic anhydride on the copolymerization of carbon dioxide and propylene oxide. Eur. Polym. J. 2015, 73, 297–307. [Google Scholar] [CrossRef]
- Duan, Z.Y.; Wang, X.Y.; Gao, Q.; Zhang, L.; Liu, B.Y.; Kim, I. Highly active bifunctional cobalt-salen complexes for the synthesis of poly(ester-block-carbonate) copolymer via terpolymerization of carbon dioxide, propylene oxide, and norbornene anhydride isomer: Roles of anhydride conformation consideration. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 789–795. [Google Scholar] [CrossRef]
- Liu, Y.L.; Xiao, M.; Wang, S.J.; Xia, L.; Han, D.M.; Cui, G.F.; Meng, Y.Z. Mechanism studies of terpolymerization of phthalic anhydride, propylene epoxide, and carbon dioxide catalyzed by ZnGA. RSC Adv. 2014, 4, 9503–9508. [Google Scholar] [CrossRef]
- Gao, L.J.; Feng, J.Y. A one-step strategy for thermally and mechanically reinforced pseudo-interpenetrating poly(propylene carbonate) networks by terpolymerization of CO2, propylene oxide and pyromellitic dianhydride. J. Mater. Chem. A. 2013, 1, 3556–3560. [Google Scholar] [CrossRef]
- Song, P.F.; Mao, X.D.; Zhang, X.F.; Zhu, X.G.; Wang, R.M. A one-step strategy for cross-linkable aliphatic polycarbonates with high degradability derived from CO2, propylene oxide and itaconic anhydride. RSC Adv. 2014, 4, 9503–9508. [Google Scholar] [CrossRef]
- Hilf, J.; Scharfenberg, M.; Poon, J.; Moers, C.; Frey, H. Aliphatic polycarbonates based on carbon dioxide, furfuryl glycidyl ether, and glycidyl methyl ether: Reversible functionalization and cross-linking. Macromol. Rapid. Commun. 2015, 36, 174–179. [Google Scholar] [CrossRef] [PubMed]
- SDBSWeb. Available online: http://sdbs.db.aist.go.jp (accessed on 7 May 2018).
- Li, X.H.; Meng, Y.Z.; Zhu, Q.; Tjong, S.C. Thermal decomposition characteristics of poly(propylene carbonate) using TG/IR and Py-GC/MS techniques. Polym. Degrad. Stab. 2003, 81, 157–165. [Google Scholar] [CrossRef]
- Wu, J.S.; Xiao, M.; He, H.; Wang, S.J.; Han, D.M.; Meng, Y.Z. Copolymerization of propylene oxide and carbon dioxide in the presence of diphenylmethane diisocyanate. J. Polym. Res. 2011, 18, 1479–1486. [Google Scholar] [CrossRef]

| Sample | Feed Molar Ratio of BTCDA to PO (%) | Yield (g Copolymer/g ZnGA) | Gel (%) |
|---|---|---|---|
| PPC | 0 | 28.2 | 0 |
| PPC-1 | 1 | 25.4 | 11.4 ± 1.1 |
| PPC-2 | 2 | 23.0 | 14.6 ± 1.3 |
| PPC-3 | 3 | 22.5 | 30.3 ± 2.0 |
| PPC-4 | 4 | 22.8 | 38.2 ± 2.3 |
| Sample | Td,−5% (°C) | Td,max (°C) | Tg (°C) |
|---|---|---|---|
| PPC | 217.8 | 239.6, 255.4 | 25.7 |
| PPC-1 | 271.9 | 294.4 | 26.3 |
| PPC-2 | 274.4 | 293.5 | 26.3 |
| PPC-3 | 275.3 | 294.9 | 26.0 |
| PPC-4 | 276.4 | 294.7 | 30.3, 45.2 |
| Sample | Tensile Strength/MPa | Young’s Modulus/MPa | Elongation at Break/% |
|---|---|---|---|
| PPC | 12.9 ± 1.6 | 1233 ± 17 | 652.3 ± 16 |
| PPC-1 | 18.1 ± 1.3 | 1465.4 ± 21 | 413.8 ± 13 |
| PPC-2 | 29.5 ± 1.8 | 1932.2 ± 13 | 97.8 ± 5 |
| PPC-3 | 31.9 ± 1.5 | 2329.2 ± 18 | 60.9 ± 3 |
| PPC-4 | 37.4 ± 1.6 | 2491.5 ± 23 | 7.4 ± 1 |
| Sample | Hot-Set Elongation (%) | Permanent Deformation (%) |
|---|---|---|
| PPC | 337.5 | 176.3 |
| PPC-1 | 71.5 | 38.8 |
| PPC-2 | 55.0 | 16.3 |
| PPC-3 | 26.5 | 6.3 |
| PPC-4 | 19.0 | 3.8 |
| Crosslinking Method a | Feed Content b | Td,−5% (°C) | Td,max (°C) | Tensile Strength (MPa) | Elongation at Break (%) | Hot-Set Elongation (%) | Permanent Deformation (%) | MFR (g Copolymer/10 min) |
|---|---|---|---|---|---|---|---|---|
| CO2/PO/AGE terpolymerization followed crosslinked by UV-radiation [20] | 5:100 | 220.5 | 233.9 | - d | - | 17.3 | 0 | - |
| CO2/PO/MA terpolymerization followed crosslinked by DCP [21] | 1:30 | 261 | 300 | 45.6 | - | - | - | - |
| CO2/PO/MDI terpolymerization [52] | 1.5:100 c | 242 | 275 | 36.9 | 12 | - | - | - |
| CO2/PO/PMDA terpolymerization [47] | 3:100 | 281 | 306 | 41 | 406 | - | - | - |
| CO2/PO/IAn terpolymerization [48] | 5:100 | 251.3 | 284.7 | 27.5 | 554.6 | - | - | - |
| CO2/PO/BTCDA terpolymerization | 2:100 | 274.4 | 293.5 | 29.5 | 97.8 | 55 | 16.3 | 1.61 |
| 4:100 | 276.4 | 294.7 | 37.4 | 7.4 | 19 | 3.8 | 1.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, L.; Feng, J.; Huang, X.; Guo, X.; Chen, J.; Xiao, Z.; Liang, X.; Gao, L. Enhanced Poly(Propylene Carbonate) with Thermoplastic Networks: A One-Pot Synthesis from Carbon Dioxide, Propylene Oxide, and a Carboxylic Dianhydride. Polymers 2018, 10, 552. https://doi.org/10.3390/polym10050552
Chen X, Wang L, Feng J, Huang X, Guo X, Chen J, Xiao Z, Liang X, Gao L. Enhanced Poly(Propylene Carbonate) with Thermoplastic Networks: A One-Pot Synthesis from Carbon Dioxide, Propylene Oxide, and a Carboxylic Dianhydride. Polymers. 2018; 10(5):552. https://doi.org/10.3390/polym10050552
Chicago/Turabian StyleChen, Xianggen, Lingyun Wang, Jiuying Feng, Xianling Huang, Xiuzhi Guo, Jing Chen, Zhenyuan Xiao, Xiangjun Liang, and Lijun Gao. 2018. "Enhanced Poly(Propylene Carbonate) with Thermoplastic Networks: A One-Pot Synthesis from Carbon Dioxide, Propylene Oxide, and a Carboxylic Dianhydride" Polymers 10, no. 5: 552. https://doi.org/10.3390/polym10050552