Reactive Pad-Steam Dyeing of Cotton Fabric Modified with Cationic P(St-BA-VBT) Nanospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
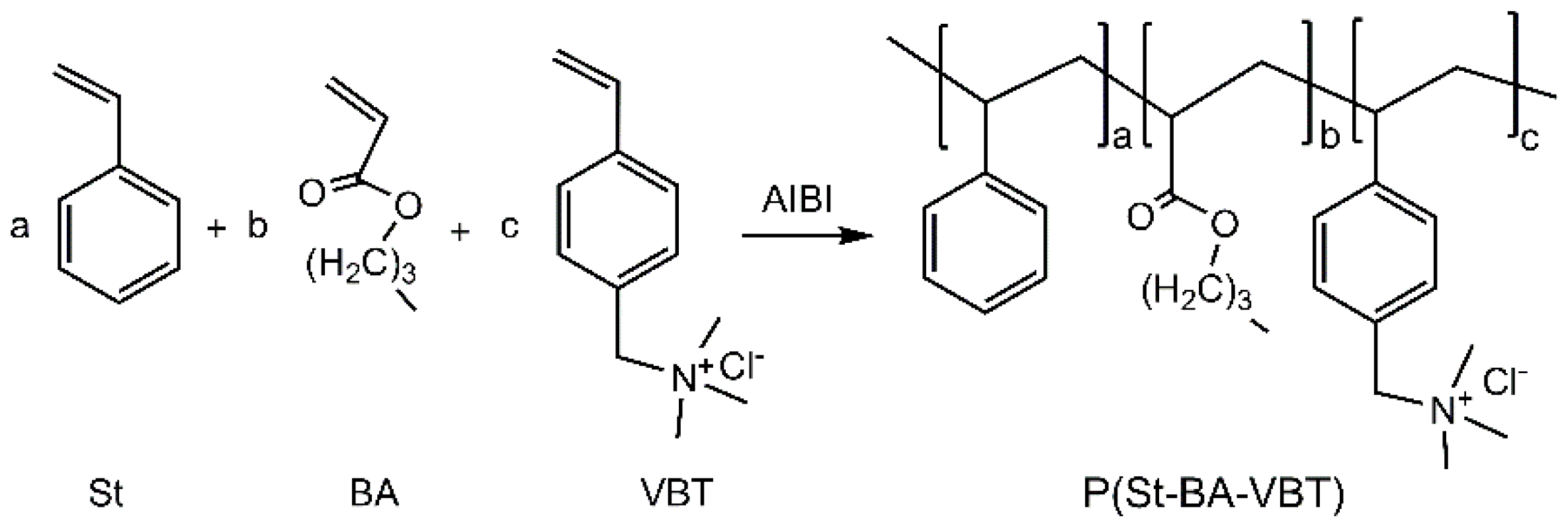
2.2. Grafting Cationic Nanospheres on Cotton Fabrics
2.3. Reactive Pad-Steam Dyeing
2.4. Characterization
2.4.1. Nanospheres Properties
2.4.2. Colorimetric Data
2.4.3. Dye Fixation Rate
2.4.4. Surface Morphology Observation by SEM
2.4.5. XPS Analysis
2.4.6. Rubbing and Washing Fastness
2.4.7. Breaking Strength
3. Results and Discussion
3.1. Properties of Cationic P(St-BA-VBT) Nanospheres
3.2. Effect of the Nanospheres on Pad-Steam Dyeing of Cotton
3.2.1. Nanosphere Concentrations
3.2.2. Alkali Amounts
3.2.3. Steaming Times
3.2.4. Dye Concentration
3.3. Performance Analysis of Cationic and Untreated Fabrics
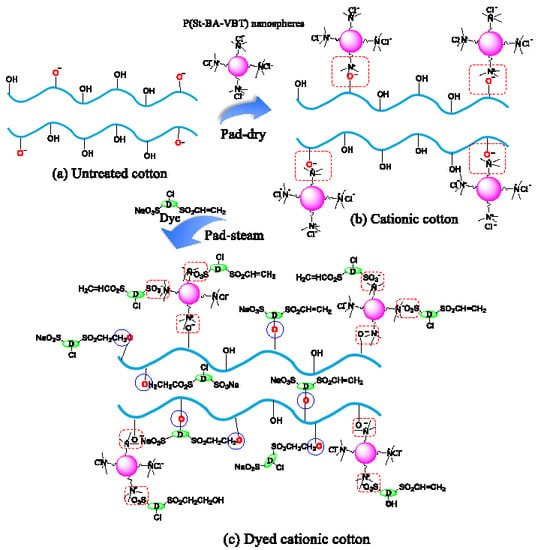
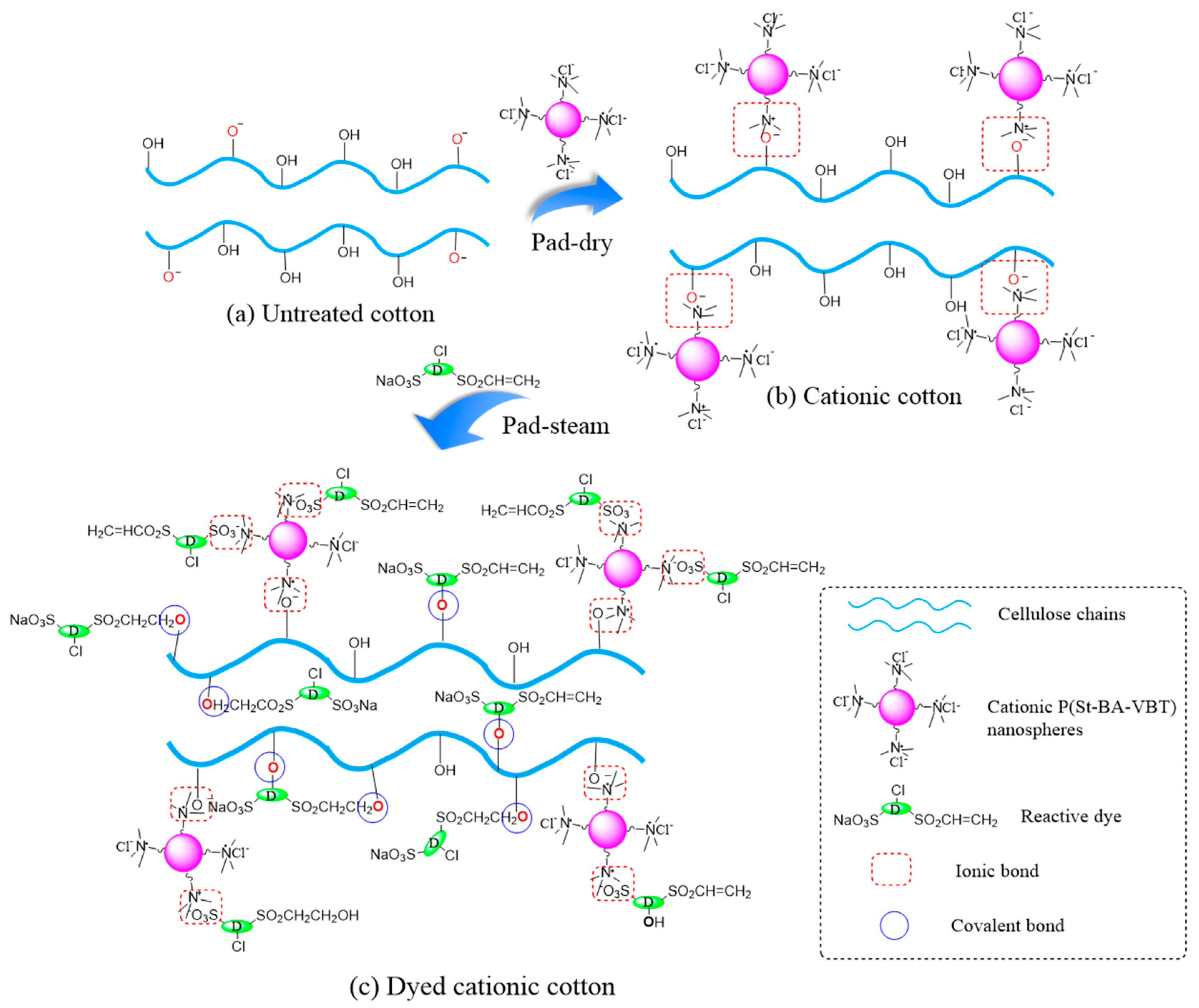
3.4. Mechanism of Nanospheres Modification and Reactive Dyeing
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jiang, S.; Wang, Y.; Sheng, D.; Xu, W.; Cao, G. Examination of the dyeing properties of pigment printing fabrics in a water-ethanol mixed solvent. Carbohydr. Polym. 2016, 153, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, K.; Zhang, J.; Shu, D.; Gong, J.; Liu, X. Padding vacuum-dehydration wet-steaming dyeing of cotton fabric using reactive golden yellow SRE. J. Text. Res. 2017, 38, 80–85. [Google Scholar]
- Biolchi, F.; Kawabata, A.; Taylor, J.A. Effect of sulphonation level upon the fixation and build up properties of reactive dyes. Color. Technol. 2006, 122, 153–156. [Google Scholar] [CrossRef]
- Shu, D.; Fang, K.; Liu, X.; Liu, Y.; Cai, Y.; Men, Y.; Li, F. Comparison on dyeing effect of reactive dyes by salt-free continuous pad-steam dyeing and cold pad-batch dyeing. J. Text. Res. 2018, 39, 77–81. [Google Scholar]
- Khatri, A.; Peerzada, M.H.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Shu, D.; Fang, K.; Liu, X.; Li, X.; Liu, Y.; Zhang, J.; Zhang, X. Influence of fabric heating rate on salt-free pad-steam dyeing of reactive dye. J. Text. Res. 2018, 39, 106–111. [Google Scholar]
- Zhang, F.; Chen, Y.; Lin, H.; Lu, Y. Influence of fabric heating rate on salt-free pad-steam dyeing of reactive dye. Color. Technol. 2007, 123, 351–357. [Google Scholar] [CrossRef]
- Zolriasatein, A.A.; Yazdanshenas, M.E.; Khajavi, R.; Rashidi, A.; Najafi, F. The use of poly(amidoamine) dendrimer in modification of jute for improving dyeing properties of reactive dyes. J. Appl. Polym. Sci. 2013, 127, 4203–4210. [Google Scholar] [CrossRef]
- Singha, K.; Maity, S.; Singha, M. The salt-free dyeing on cotton: An approach to effluent free mechanism; can chitosan be a potential option? Int. J. Text. Sci. 2013, 1, 69–77. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Zhang, S.; Teng, X.; Yang, J. Preparation of cationic cotton with two-bath pad-bake process and its application in salt-free dyeing. Carbohydr. Polym. 2009, 78, 602–608. [Google Scholar] [CrossRef]
- Salimpour Abkenar, S.; Malek, R.M.A.; Mazaheri, F. Salt-free dyeing isotherms of cotton fabric grafted with PPI dendrimers. Cellulose 2015, 22, 897–910. [Google Scholar] [CrossRef]
- Arivithamani, N.; Dev, V.R.G. Sustainable bulk scale cationization of cotton hosiery fabrics for salt-free reactive dyeing process. J. Clean. Prod. 2017, 149, 1188–1199. [Google Scholar] [CrossRef]
- Fu, S.; Hinks, D.; Hauser, P.; Ankeny, M. High efficiency ultra-deep dyeing of cotton via mercerization and cationization. Cellulose 2013, 20, 3101–3110. [Google Scholar] [CrossRef]
- Lewis, D.M.; McIlroy, K.A. Modification of cotton with nicotinoyl thioglycollate to improve its dyeability. Dyes Pigm. 1997, 35, 69–86. [Google Scholar] [CrossRef]
- Xie, K.; Hou, A.; Sun, Y. Chemical graft of cellulose with the ion-pair emulsion containing the reactive groups and its dyeing properties. J. Disper. Sci. Technol. 2008, 29, 1385–1390. [Google Scholar] [CrossRef]
- Fang, K.; Zhao, H.; Li, J.; Chen, W.; Cai, Y.; Hao, L. Salt-free dyeing of cotton fabrics modified with cationic copolymer nanospheres using an acid dye. Fibers Polym. 2017, 18, 400–406. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Mignanelli, M.; Froehling, P.E.; Bide, M.J. The use of dendrimers to modify the dyeing behaviour of reactive dyes on cotton. Dyes Pigm. 2000, 47, 259–267. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, D.; Chen, Y.; Lin, H. The antimicrobial activity of the cotton fabric grafted with an amino-terminated hyperbranched polymer. Cellulose 2008, 16, 281–288. [Google Scholar] [CrossRef]
- Janhom, S.; Watanesk, R.; Watanesk, S.; Griffiths, P.; Arquero, O.; Naksata, W. Comparative study of lac dye adsorption on cotton fibre surface modified by synthetic and natural polymers. Dyes Pigm. 2006, 71, 188–193. [Google Scholar] [CrossRef]
- Zhang, M.; Ju, B.; Zhang, S.; Ma, W.; Yang, J. Synthesis of cationic hydrolyzed starch with high DS by dry process and use in salt-free dyeing. Carbohydr. Polym. 2007, 69, 123–129. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Safapour, S. Salt-free reactive dyeing of the cotton fabric modified with chitosan-poly(propylene imine) dendrimer hybrid. Fibers Polym. 2015, 16, 1075–1081. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Fang, K. Adsorption of cationic copolymer nanoparticles onto bamboo fiber surfaces measured by conductometric titration. Chin. Chem. Lett. 2015, 26, 1174–1178. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Fang, K.; Cai, Y. Preparation of reactive dye/polymer composite copolymer microspheres. J. Text. Res. 2017, 38, 80–84. [Google Scholar]
- Fang, K.; Song, T.; Zhang, K.; Chen, W.; Cai, Y.; Hao, L. Fixation of cationic P (st-BA-AA-GMA) emulsion on pigment particles in dyeing of cotton fabrics. J. Appl. Polym. Sci. 2017, 134, 44987. [Google Scholar] [CrossRef]
- Gong, J.; Ren, Y.; Fu, R.; Li, Z.; Zhang, J. pH-mediated antibacterial dyeing of cotton with prodigiosins nanomicelles produced by microbial fermentation. Polymers 2017, 9, 468. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, K.; Zhang, J.; Shu, D.; Gong, J.; Liu, X. A vacuum-dehydration aided pad-steam process for improving reactive dyeing of cotton fabric. J. Clean. Prod. 2017, 168, 1193–1200. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Fang, K.; Shu, D.; Guo, Z. Coloration of Apocynum venetum/cotton blends with an acid dye through combined pretreatment using cationic nanoparticles. Color. Technol. 2017, 133, 293–299. [Google Scholar] [CrossRef]
- Khatri, A.; White, M.; Padhye, R.; Momin, N.H. The use of reflectance measurements in the determination of diffusion of reactive dyes into cellulosic fiber. Color. Res. Appl. 2014, 39, 63–69. [Google Scholar] [CrossRef]
- Bredereck, K.; Schumacher, C. Structure reactivity correlations of azo reactive dyes based on H-acid: I. NMR chemical shift values, pKa values, dyestuff aggregation and dyeing behavior. Dyes Pigm. 1993, 21, 23–43. [Google Scholar] [CrossRef]
- Lewis, D.M. Covalent fixation of reactive dyes on cotton under neutral conditions. AATCC Rev. 2008, 8, 35–41. [Google Scholar]
- Lewis, D.M. Developments in the chemistry of reactive dyes and their application processes. Color. Technol. 2014, 130, 382–412. [Google Scholar] [CrossRef]
- Hou, X.; Chen, X.; Cheng, Y.; Xu, H.; Chen, L.; Yang, Y. Dyeing and UV-protection properties of water extracts from orange peel. J. Clean. Prod. 2013, 52, 410–419. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry: Synthesis, Properties and Applications of Organic Dyes and Pigments, 3rd ed.; Wiley-VCH Publishers: Weinheim, Germany, 2003; p. 57. [Google Scholar]
- Wang, L.; Yan, K.; Hu, C. Cleaner production of inkjet printed cotton fabrics using a urea-free ecosteam process. J. Clean. Prod. 2017, 143, 1215–1220. [Google Scholar] [CrossRef]
- Abdali, H.; Ajji, A. Preparation of electrospun nanocomposite nanofibers of polyaniline/poly(methyl methacrylate) with amino-functionalized graphene. Polymers 2017, 9, 453. [Google Scholar] [CrossRef]


| Fabrics | Dye Concentration (g/L) | Color Characteristic Values | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° | ||
| Cationic | 15 | 31.3 | −9.6 | −18.7 | 21.0 | 242.9 |
| 35 | 25.0 | −7.1 | −16.8 | 18.2 | 247.3 | |
| 65 | 20.3 | −4.3 | −13.2 | 13.9 | 252.2 | |
| Untreated | 15 | 38.8 | −9.9 | −19.3 | 21.7 | 242.8 |
| 35 | 28.7 | −7.2 | −17.5 | 18.9 | 247.8 | |
| 65 | 23.4 | −5.0 | −14.8 | 15.6 | 259.3 | |
| Fabrics | Dye Concentration (g/L) | Rubbing Fastness | Washing Fastness | |||
|---|---|---|---|---|---|---|
| Dry | Wet | SC | SW | CC | ||
| Cationic | 15 | 4–5 | 4 | 5 | 5 | 5 |
| 35 | 4–5 | 3–4 | 4–5 | 4–5 | 4–5 | |
| 65 | 4–5 | 3 | 4–5 | 4–5 | 4–5 | |
| Untreated | 15 | 4–5 | 4 | 5 | 5 | 5 |
| 35 | 4–5 | 3–4 | 4–5 | 4–5 | 4–5 | |
| 65 | 4–5 | 3–4 | 4–5 | 4–5 | 4–5 | |
| Cotton Fabric | Chemical Composition | Atomic Ratio O/C | ||
|---|---|---|---|---|
| C (%) | O (%) | N (%) | ||
| Cationic | 74.32 | 22.58 | 1.57 | 0.30 |
| Untreated | 57.53 | 36.72 | 0 | 0.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, K.; Shu, D.; Liu, X.; Cai, Y.; An, F.; Zhang, X. Reactive Pad-Steam Dyeing of Cotton Fabric Modified with Cationic P(St-BA-VBT) Nanospheres. Polymers 2018, 10, 564. https://doi.org/10.3390/polym10060564
Fang K, Shu D, Liu X, Cai Y, An F, Zhang X. Reactive Pad-Steam Dyeing of Cotton Fabric Modified with Cationic P(St-BA-VBT) Nanospheres. Polymers. 2018; 10(6):564. https://doi.org/10.3390/polym10060564
Chicago/Turabian StyleFang, Kuanjun, Dawu Shu, Xiuming Liu, Yuqing Cai, Fangfang An, and Xinqing Zhang. 2018. "Reactive Pad-Steam Dyeing of Cotton Fabric Modified with Cationic P(St-BA-VBT) Nanospheres" Polymers 10, no. 6: 564. https://doi.org/10.3390/polym10060564