Trithiocarbonate-Functionalized PNiPAAm-Based Nanocomposites for Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Trithiocarbonate-Functionalized Poly(N-Isopropylacrylamide) (TTC-PNiPAAm) by RAFT Polymerization
2.3. Synthesis of the Nanocomposites
2.4. Characterization
2.5. Silver Loading
2.6. Lower Critical Solubility Temperature (LCST) Determination
2.7. Stability
2.8. Release Kinetics
2.9. Antimicrobial Tests
2.10. Biocompatibility
3. Results and Discussion
3.1. Characterization
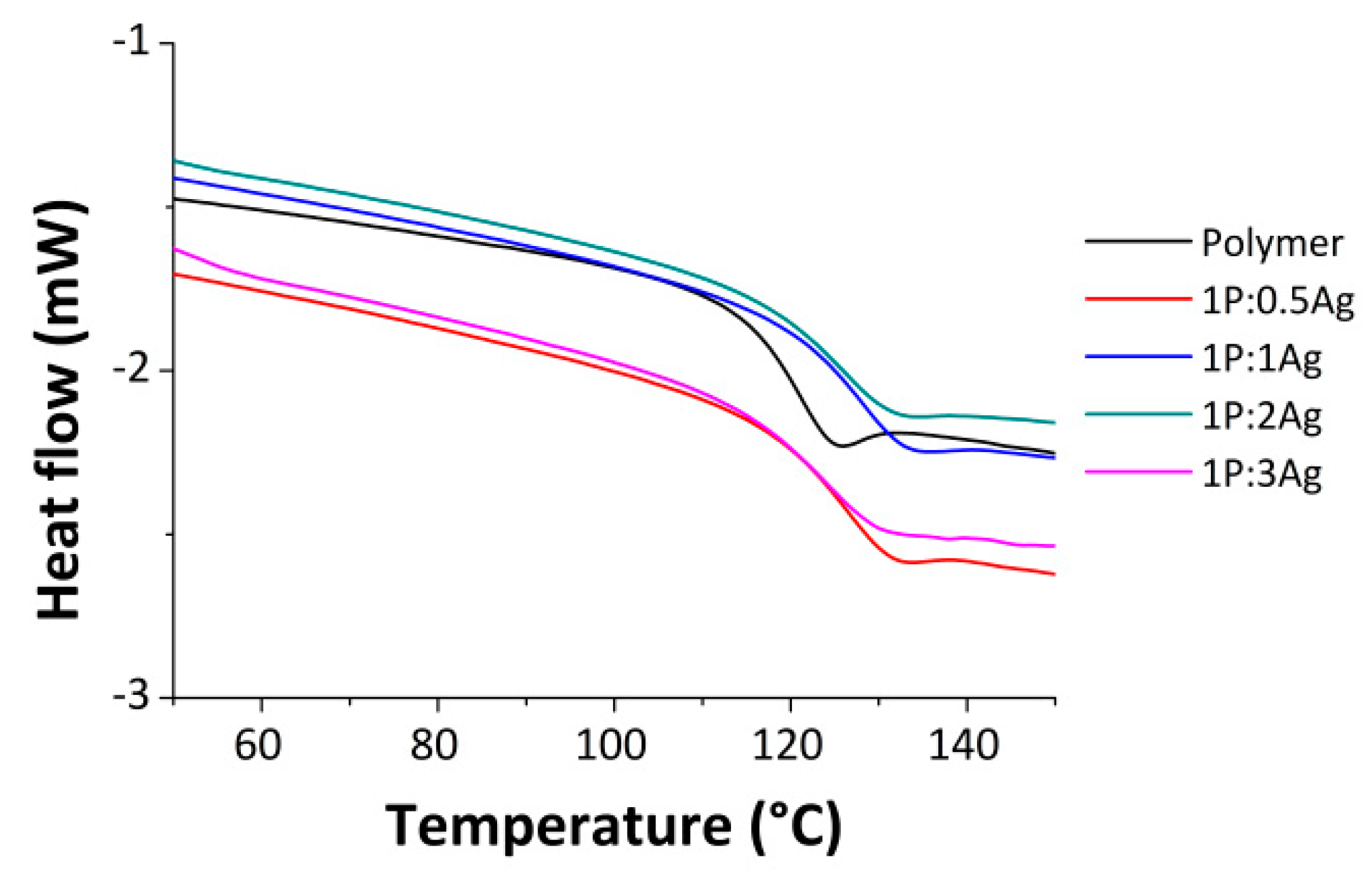
3.2. Thermal Properties
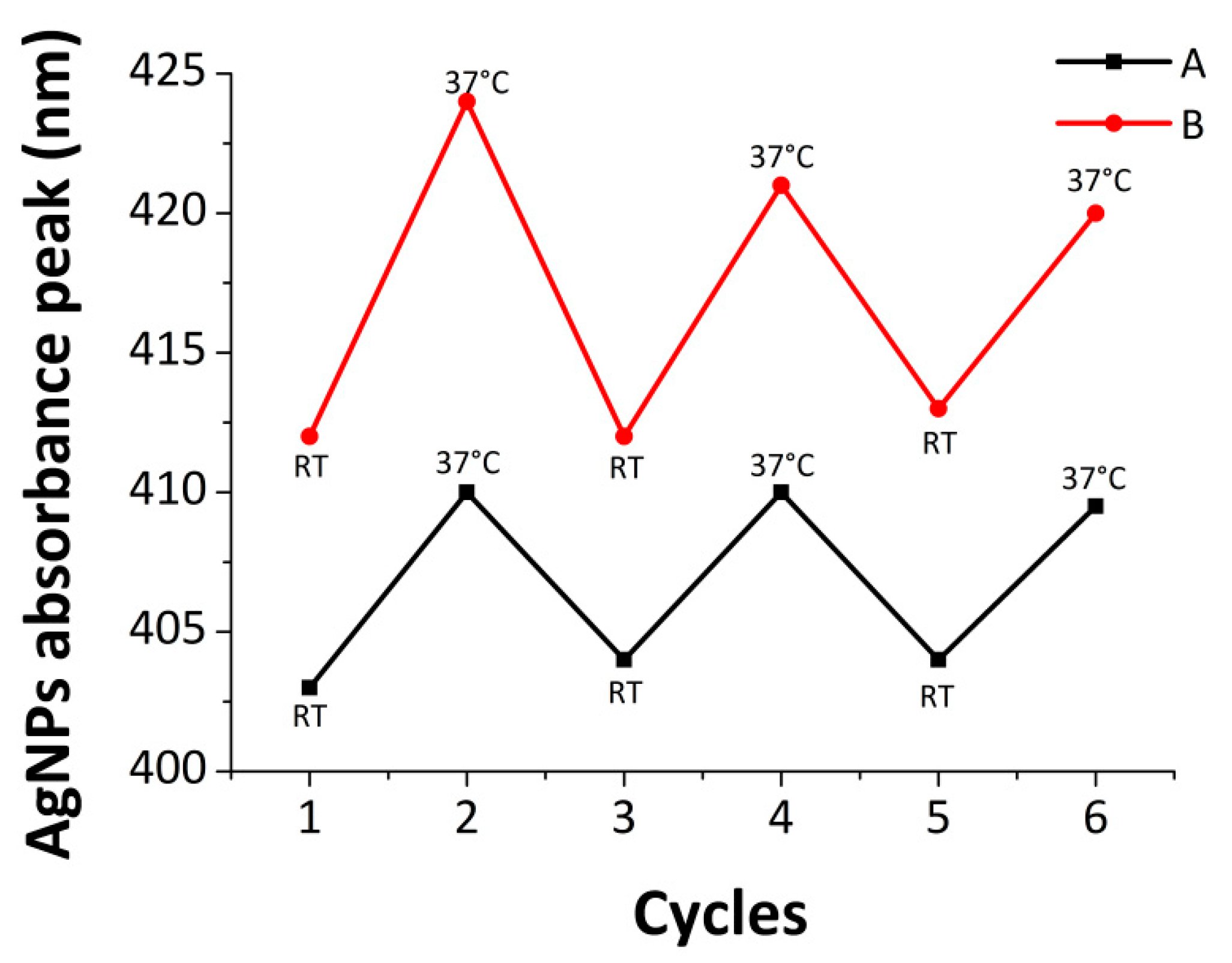
3.3. Stability of the Nanocomposite
3.4. Silver Release Profile
3.5. Antimicrobial Properties
3.5.1. E. coli
3.5.2. S. aureus
3.6. Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, G.M.; Bisno, A.L. Infections associated with indwelling devices: Infections related to extravascular devices. Antimicrob. Agents Chemother. 1989, 33, 602. [Google Scholar] [CrossRef] [PubMed]
- Slenters, T.V.; Hauser-Gerspach, I.; Daniels, A.U.; Fromm, K.M. Silver coordination compounds as light-stable, nano-structured and anti-bacterial coatings for dental implant and restorative materials. J. Mater. Chem. 2008, 18, 5359–5362. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Lisowska, K.; Ochocki, J. Synthesis, characterization and antimicrobial activity of silver(I) complexes of hydroxymethyl derivatives of pyridine and benzimidazole. J. Organomet. Chem. 2014, 749, 394–399. [Google Scholar] [CrossRef]
- Sague, J.L.; Meuwly, M.; Fromm, K.M. Counterion effect on the formation of coordination polymer networks between AgNO3 and L (2,2[prime or minute]-oxybis(ethane-2,1-diyl) diisonicotinate). Part 2. CrystEngComm 2008, 10, 1542–1549. [Google Scholar] [CrossRef]
- Gordon, O.; Vig Slenters, T.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver coordination polymers for prevention of implant infection: Thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob. Agents Chemother. 2010, 54, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Priebe, M.; Widmer, J.; Suhartha Löwa, N.; Abram, S.-L.; Mottas, I.; Woischnig, A.-K.; Brunetto, P.S.; Khanna, N.; Bourquin, C.; Fromm, K.M. Antimicrobial silver-filled silica nanorattles with low immunotoxicity in dendritic cells. Nanomedicine 2017, 13, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, P.; Patil, S.D.; Jeevanandam, P.; Navani, N.K.; Singla, M.L. Synthesis, characterization and bactericidal activity of silica/silver core–shell nanoparticles. J. Mater. Sci. Med. 2014, 25, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.; Joset, N.; Crochet, A.; Tan, M.; Holzheu, A.; Brunetto, P.; Fromm, K. Synthesis of New Polyether Ether Ketone Derivatives with Silver Binding Site and Coordination Compounds of Their Monomers with Different Silver Salts. Polymers 2016, 8, 208. [Google Scholar] [CrossRef]
- De Mel, A.; Chaloupka, K.; Malam, Y.; Darbyshire, A.; Cousins, B.; Seifalian, A.M. A silver nanocomposite biomaterial for blood-contacting implants. J. Biomed. Mater. Res. A 2012, 100, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Dehnavi, A.S.; Aroujalian, A.; Raisi, A.; Fazel, S. Preparation and characterization of polyethylene/silver nanocomposite films with antibacterial activity. J. Appl. Polym. Sci. 2013, 127, 1180–1190. [Google Scholar] [CrossRef]
- Silver, S.; Phung le, T.; Silver, G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol. 2006, 33, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Damm, C.; Münstedt, H. Kinetic aspects of the silver ion release from antimicrobial polyamide/silver nanocomposites. Appl. Phys. A 2008, 91, 479–486. [Google Scholar] [CrossRef]
- Damm, C.; Münstedt, H.; Rösch, A. Long-term antimicrobial polyamide 6/silver-nanocomposites. J. Mater. Sci. 2007, 42, 6067–6073. [Google Scholar] [CrossRef]
- Sintubin, L.; De Gusseme, B.; Van der Meeren, P.; Pycke, B.F.G.; Verstraete, W.; Boon, N. The antibacterial activity of biogenic silver and its mode of action. Appl. Microbiol. Biotechnol. 2011, 91, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. Engl. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Misra, R.D.K. Polymer nanocomposites: Current understanding and issues. Mater. Sci. Technol. 2013, 22, 742–755. [Google Scholar] [CrossRef]
- Yuan, W.; Fu, J.; Su, K.; Ji, J. Self-assembled chitosan/heparin multilayer film as a novel template for in situ synthesis of silver nanoparticles. Colloids Surf. Biointerfaces 2010, 76, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, U.; Jewrajka, S.K.; Guha, S. Dispersion of functionalized silver nanoparticles in polymer matrices: Stability, characterization, and physical properties. Polym. Compos. 2009, 30, 827–834. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Park, H.J.; Lee, J.; Kim, Y.; Yoon, J.; Park, K.; Choi, K.; Yi, J. Bacterial cytotoxicity of the silver nanoparticle related to physicochemical metrics and agglomeration properties. Environ. Toxicol. Chem. 2010, 29, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.-S.; Chen, W.-H. Synthesis of amphiphilic temperature-sensitive poly(N-isopropylacrylamide)-block-poly(tetramethylene carbonate) block copolymers and micellar characterization. Polym. Int. 2011, 60, 255–263. [Google Scholar] [CrossRef]
- Lue, S.J.; Hsu, J.-J.; Chen, C.-H.; Chen, B.-C. Thermally on–off switching membranes of poly(N-isopropylacrylamide) immobilized in track-etched polycarbonate films. J. Membr. Sci. 2007, 301, 142–150. [Google Scholar] [CrossRef]
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002, 20, 305–311. [Google Scholar] [CrossRef]
- Tekin, H.; Sanchez, J.G.; Tsinman, T.; Langer, R.; Khademhosseini, A. Thermoresponsive Platforms for Tissue Engineering and Regenerative Medicine. AIChE J. 2011, 57, 3249–3258. [Google Scholar] [CrossRef] [PubMed]
- Elashnikov, R.; Lyutakov, O.; Kalachyova, Y.; Solovyev, A.; Svorcik, V. Tunable release of silver nanoparticles from temperature-responsive polymer blends. React. Funct. Polym. 2015, 93, 163–169. [Google Scholar] [CrossRef]
- Turan, E.; Demirci, S.; Caykara, T. Thermo- and pH-induced phase transitions and network parameters of poly(N-isopropylacrylamide-co-2-acrylamido-2-methyl-propanosulfonic acid) hydrogels. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1713–1724. [Google Scholar] [CrossRef]
- Xia, L.W.; Ju, X.J.; Liu, J.J.; Xie, R.; Chu, L.Y. Responsive hydrogels with poly(N-isopropylacrylamide-co-acrylic acid) colloidal spheres as building blocks. J. Colloid Interface Sci. 2010, 349, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-S.; Jeong, Y.-I.; Cho, C.-S.; Kim, S.-H. Thermo-responsive self-assembled polymeric micelles for drug delivery in vitro. Int. J. Pharm. 2000, 205, 165–172. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.M.K.; Chellamuthu, P.; Tang, L.; Nguyen, K.T. Development of a Temperature-Sensitive Composite Hydrogel for Drug Delivery Applications. Biotechnol. Prog. 2006, 22, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Spasojević, J.; Radosavljević, A.; Krstić, J.; Mitrić, M.; Popović, M.; Rakočević, Z.; Kalagasidis-Krušić, M.; Kačarević-Popović, Z. Structural characteristics and bonding environment of Ag nanoparticles synthesized by gamma irradiation within thermo-responsive poly(N-isopropylacrylamide) hydrogel. Polym. Compos. 2017, 38, 1014–1026. [Google Scholar] [CrossRef]
- He, M.; Wang, Q.; Zhang, J.; Zhao, W.; Zhao, C. Substrate-Independent Ag-Nanoparticle-Loaded Hydrogel Coating with Regenerable Bactericidal and Thermoresponsive Antibacterial Properties. ACS Appl. Mater. Interfaces 2017, 9, 44782–44791. [Google Scholar] [CrossRef] [PubMed]
- Niitsoo, O.; Couzis, A. Facile synthesis of silver core—Silica shell composite nanoparticles. J. Colloid Interface Sci. 2011, 354, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D. Physical Biochemistry: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Tenover, F.C. Antimicrobial Susceptibility Testing Methods for Bacterial Pathogens. In Antimicrobial Drug Resistance: Clinical and Epidemiological Aspects; Mayers, D.L., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 1151–1159. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Kyrychenko, A.; Pasko, D.A.; Kalugin, O.N. Poly(vinyl alcohol) as a water protecting agent for silver nanoparticles: The role of polymer size and structure. Phys. Chem. Chem. Phys. 2017, 19, 8742–8756. [Google Scholar] [CrossRef] [PubMed]
- Slistan-Grijalva, A.; Herrera-Urbina, R.; Rivas-Silva, J.F.; Ávalos-Borja, M.; Castillón-Barraza, F.F.; Posada-Amarillas, A. Assessment of growth of silver nanoparticles synthesized from an ethylene glycol–silver nitrate–polyvinylpyrrolidone solution. Phys. E Low-Dimens. Syst. Nanostruct. 2005, 25, 438–448. [Google Scholar] [CrossRef]
- Alarcon, E.I.; Udekwu, K.; Skog, M.; Pacioni, N.L.; Stamplecoskie, K.G.; González-Béjar, M.; Polisetti, N.; Wickham, A.; Richter-Dahlfors, A.; Griffith, M.; et al. The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomaterials 2012, 33, 4947–4956. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Willcock, H.; O’Reilly, R.K. End group removal and modification of RAFT polymers. Polym. Chem. 2010, 1, 149–157. [Google Scholar] [CrossRef]
- Beattie, D.A.; Addai-Mensah, J.; Beaussart, A.; Franks, G.V.; Yeap, K.-Y. In situ particle film ATR FTIR spectroscopy of poly(N-isopropyl acrylamide) (PNIPAM) adsorption onto talc. Phys. Chem. Chem. Phys. 2014, 16, 25143–25151. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.J.; Chang, S.-H.; Schatz, G.C.; Van Duyne, R.P.; Wiley, B.J.; Xia, Y. Localized Surface Plasmon Resonance Spectroscopy of Single Silver Nanocubes. Nano Lett. 2005, 5, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Nie, J.; Du, B.; Peng, Z.; Tesche, B.; Kleinermanns, K. Thermoresponsive polymer-stabilized silver nanoparticles. J. Colloid Interface Sci. 2008, 319, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, J.; Zhu, Z.; Liu, H.; Liu, S. In-Situ Formation of Silver Nanoparticles with Tunable Spatial Distribution at the Poly(N-isopropylacrylamide) Corona of Unimolecular Micelles. Macromolecules 2006, 39, 8451–8455. [Google Scholar] [CrossRef]
- Lin, H.C.; Su, Y.A.; Liu, T.Y.; Sheng, Y.J.; Lin, J.J. Thermo-responsive nanoarrays of silver nanoparticle, silicate nanoplatelet and PNiPAAm for the antimicrobial applications. Colloids Surf. B 2017, 152, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Palomba, M.; Carotenuto, G.; Cristino, L.; Di Grazia, M.A.; Nicolais, F.; De Nicola, S. Activity of Antimicrobial Silver Polystyrene Nanocomposites. J. Nanomater. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Yang, H.-W.; Lee, A.-W.; Huang, C.-H.; Chen, J.-K. Characterization of poly(N-isopropylacrylamide)-nucleobase supramolecular complexes featuring bio-multiple hydrogen bonds. Soft Matter. 2014, 10, 8330–8340. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.E.S.R.E.; Dutra, E.R.; Mano, V.; Machado, J.C. Preparation and thermal study of polymers derived from acrylamide. Polym. Degrad. Stab. 2000, 67, 491–495. [Google Scholar] [CrossRef]
- An, J.; Yuan, X.; Luo, Q.; Wang, D. Preparation of chitosan-graft-(methyl methacrylate)/Ag nanocomposite with antimicrobial activity. Polym. Int. 2010, 59, 62–70. [Google Scholar] [CrossRef]
- Strausser, Y. Preface. In Characterization in Silicon Processing; Newnes: Boston, MA, USA, 1993; pp. x–xi. [Google Scholar]
- Grubbs, R.B. Roles of Polymer Ligands in Nanoparticle Stabilization. Polym. Rev. 2007, 47, 197–215. [Google Scholar] [CrossRef]
- Kracht, S.; Messerer, M.; Lang, M.; Eckhardt, S.; Lauz, M.; Grobety, B.; Fromm, K.M.; Giese, B. Electron transfer in peptides: On the formation of silver nanoparticles. Angew. Chem. Int. Ed. Engl. 2015, 54, 2912–2916. [Google Scholar] [CrossRef] [PubMed]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Erichsen, J.; Dolgner, K.; Thran, A.; Kiene, M. Fundamental Aspects of Polymer Metallization. In Metallization of Polymers 2; Sacher, E., Ed.; Springer: Boston, MA, USA, 2002; pp. 73–96. [Google Scholar]
- Xu, G.; Gao, S.; Ji, X.; Zhang, X. Characterization and Synthesis Mechanism of Nanosilver/PAMPS Composites by Microwave. Soft Nanosci. Lett. 2014, 4, 15–23. [Google Scholar] [CrossRef]
- Lee, K.H.; Rah, S.C.; Kim, S.G. Formation of monodisperse silver nanoparticles in poly(vinylpyrrollidone) matrix using spray pyrolysis. J. Sol-Gel Sci. Technol. 2008, 45, 187–193. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Stansbury, J.W.; Zhu, X.; Wang, X.; Nie, J. Smart Antibacterial Surface Made by Photopolymerization. ACS Appl. Mater. Interfaces 2016, 8, 28047–28054. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, K.; Hajibeygi, M. Synthesis and properties of polyimide/silver nanocomposite containing dibenzalacetone moiety in the main chain. J. Saudi Chem. Soc. 2013, 17, 419–423. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Pizarro, G.C.; Sarabia-Vallejos, M.A.; Terraza, C.A.; López-Cabaña, Z.E. In situ-preparation and characterization of silver-HEMA/PEGDA hydrogel matrix nanocomposites: Silver inclusion studies into hydrogel matrix. Arabian J. Chem. 2014, in press. [Google Scholar] [CrossRef]
- Liao, K.-H.; Aoyama, S.; Abdala, A.A.; Macosko, C. Does Graphene Change Tg of Nanocomposites? Macromolecules 2014, 47, 8311–8319. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Samantaray, B.K.; Choudhary, R.N.P.; Karan, N.K.; Thomas, R.; Katiyar, R.S. Effect of plasticizer on structural and electrical properties of nanocomposite solid polymer electrolytes. Ionics 2011, 17, 127–134. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Licea-Claverie, A.; Cornejo-Bravo, J.M.; Schwarz, S.; Arndt, K.-F. Well-defined N-Isopropylacrylamide Dual-Sensitive Copolymers with LCST ≈ 38 °C in Different Architectures: Linear, Block and Star Polymers. Macromol. Chem. Phys. 2012, 213, 301–314. [Google Scholar] [CrossRef]
- Dimitrov, I.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Prog. Polym. Sci. 2007, 32, 1275–1343. [Google Scholar] [CrossRef]
- Chang, K.; Dicke, Z.T.; Taite, L.J. Engineering a sharp physiological transition state for poly(N-isopropylacrylamide) through structural control. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 976–985. [Google Scholar] [CrossRef]
- Furyk, S.; Zhang, Y.; Ortiz-Acosta, D.; Cremer, P.S.; Bergbreiter, D.E. Effects of end group polarity and molecular weight on the lower critical solution temperature of poly(N-isopropylacrylamide). J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1492–1501. [Google Scholar] [CrossRef]
- Qiu, X.-P.; Tanaka, F.; Winnik, F.M. Temperature-Induced Phase Transition of Well-Defined Cyclic Poly(N-isopropylacrylamide)s in Aqueous Solution. Macromolecules 2007, 40, 7069–7071. [Google Scholar] [CrossRef]
- Van Durme, K.; Rahier, H.; Van Mele, B. Influence of Additives on the Thermoresponsive Behavior of Polymers in Aqueous Solution. Macromolecules 2005, 38, 10155–10163. [Google Scholar] [CrossRef]
- Jain, K.; Vedarajan, R.; Watanabe, M.; Ishikiriyama, M.; Matsumi, N. Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers. Polym. Chem. 2015, 6, 6819–6825. [Google Scholar] [CrossRef]
- Hoskins, J.S.; Karanfil, T.; Serkiz, S.M. Removal and Sequestration of Iodide Using Silver-Impregnated Activated Carbon. Environ. Sci. Technol. 2002, 36, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Tilley, R.D. Preparation, Self-Assembly, and Mechanistic Study of Highly Monodispersed Nanocubes. J. Am. Chem. Soc. 2007, 129, 3287–3291. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.P.; Wang, Y.X.; Wei, Y.; Li, G. Preparation of Uniform-Sized and Dual Stimuli-Responsive Microspheres of Poly(N-Isopropylacrylamide)/Poly(Acrylic acid) with Semi-IPN Structure by One-Step Method. Polymers 2016, 8, 90. [Google Scholar] [CrossRef]
- Park, T.G.; Hoffman, A.S. Sodium chloride-induced phase transition in nonionic poly(N-isopropylacrylamide) gel. Macromolecules 1993, 26, 5045–5048. [Google Scholar] [CrossRef]
- Modi, S.; Anderson, B.D. Determination of drug release kinetics from nanoparticles: Overcoming pitfalls of the dynamic dialysis method. Mol. Pharm. 2013, 10, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled Release of Biologically Active Silver from Nanosilver Surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-Z.; Wu, D.-Q.; Chu, C.-C. Synthesis, characterization and controlled drug release of thermosensitive IPN–PNIPAAm hydrogels. Biomaterials 2004, 25, 3793–3805. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xu, Y. Pirfenidone-loaded liposomes for lung targeting: Preparation and in vitro/in vivo evaluation. Drug Des. Dev. Ther. 2015, 9, 3369. [Google Scholar] [CrossRef]
- Agnihotri, S.; Pathak, R.; Jha, D.; Roy, I.; Gautam, H.K.; Sharma, A.K.; Kumar, P. Synthesis and antimicrobial activity of aminoglycoside-conjugated silica nanoparticles against clinical and resistant bacteria. New J. Chem. 2015, 39, 6746–6755. [Google Scholar] [CrossRef]
- Tripathi, K. Essentials of Medical Pharmacology; JP Medical Ltd.: London, UK, 2013. [Google Scholar]
- Helmlinger, J.; Sengstock, C.; Groβ-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Koller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Gao, S.; Ge, W.; Zhao, C.; Cheng, C.; Jiang, H.; Wang, X. Novel conjugated Ag@PNIPAM nanocomposites for an effective antibacterial wound dressing. RSC Adv. 2015, 5, 25870–25876. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. BioMetals 2011, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, Z.; Wu, H.; Pan, X.; Xie, X.; Wu, C. Antimicrobial activity and the mechanism of silver nanoparticle thermosensitive gel. Int. J. Nanomed. 2011, 6, 2873–2877. [Google Scholar] [CrossRef]
- Egger, S.; Lehmann, R.P.; Height, M.J.; Loessner, M.J.; Schuppler, M. Antimicrobial properties of a novel silver-silica nanocomposite material. Appl. Environ. Microbiol. 2009, 75, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- Składanowski, M.; Golinska, P.; Rudnicka, K.; Dahm, H.; Rai, M. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med. Microbiol. Immunol. 2016, 205, 603–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Díaz, E.; Pfeiffer, C.; Kastl, L.; Rivera-Gil, P.; Simonet, B.; Valcárcel, M.; Jiménez-Lamana, J.; Laborda, F.; Parak, W.J. The Toxicity of Silver Nanoparticles Depends on Their Uptake by Cells and Thus on Their Surface Chemistry. Part. Part. Syst. Charact. 2013, 30, 1079–1085. [Google Scholar] [CrossRef]




















| Targeted Ratio TTC-PNiPAAm:Ag+ | Abbreviation |
|---|---|
| 1P:0.5Ag+ | 1 |
| 1P:1Ag+ | 2 |
| 1P:2Ag+ | 3 |
| 1P:3Ag+ | 4 |
| Label | Sample | DP Targeted | DP Obtained | Conv (%) a | Mn NMR (g.mol−1) b | ĐM c |
|---|---|---|---|---|---|---|
| A | TTC-PNiPAAm 25 | 25 | 24 | 94 | 3080 | 1.1 |
| B | TTC-PNiPAAm 50 | 50 | 49 | 95 | 5350 | 1.1 |
| C | TTC-PNiPAAm 100 | 100 | 103 | 99 | 12,020 | 1.02 |
| D | TTC-PNiPAAm 200 | 200 | 167 | 96 | 18,470 | 1.2 |
| Nanocomposite | 1P:0.5Ag+ (1) | 1P:1Ag+ (2) | 1P:2Ag+ (3) | 1P:3Ag+ (4) |
|---|---|---|---|---|
| A | 1.53 | 3.24 | 6.53 | 10.37 |
| B | 0.44 | 1.65 | 3.65 | 4.69 |
| C | 0.42 | 0.83 | 1.74 | 1.93 |
| D | 0.29 | 0.53 | 1.03 | 1.62 |
| Nanocomposite | Degradation Temperature (°C) | ||||
|---|---|---|---|---|---|
| Polymer | 1P:0.5Ag+ (1) | 1P:1Ag+ (2) | 1P:2Ag+ (3) | 1P:3Ag+ (4) | |
| A | 400 | 350 | 340 | 335 | 330 |
| B | 400 | 360 | 355 | 340 | 335 |
| C | 400 | 380 | 365 | 355 | 345 |
| D | 400 | 390 | 375 | 365 | 350 |
| Nanocomposite | Glass Transition Temperature (°C) | ||||
|---|---|---|---|---|---|
| Polymer | 1P:0.5Ag+ (1) | 1P:1Ag+ (2) | 1P:2Ag+ (3) | 1P:3Ag+ (4) | |
| A | 119 | 124 | 126 | 126 | 126 |
| B | 128 | 129 | 130 | 127 | 127 |
| C | 133 | 135 | 135 | 134 | 131 |
| D | 133 | 135 | 135 | 123 | 129 |
| Nanocomposite | Lower Critical Solubility Temperature (°C) | ||||
|---|---|---|---|---|---|
| Polymer | 1P:0.5Ag+ (1) | 1P:1Ag+ (2) | 1P:2Ag+ (3) | 1P:3Ag+ (4) | |
| A | 36.1 | 37.7 | 38.1 | 39.2 | 48.5 |
| B | 35.5 | 38.5 | 39.4 | 42.3 | 42.6 |
| C | 35.4 | 38 | 38.2 | 40.5 | 41.1 |
| D | 35.6 | 36.5 | 36.8 | 37.5 | 38.1 |
| Nanocomposite | 1P:0.5Ag+ (1) | 1P:1Ag+ (2) | 1P:2Ag+ (3) | 1P:3Ag+ (4) | ||||
|---|---|---|---|---|---|---|---|---|
| A | 1.5 | >1 | 1.5 | >1 | 1 | >0.5 | 1 | >0.5 |
| B | 1.5 | >1 | 1.5 | >1 | 1 | >0.5 | 1 | >0.5 |
| C | >2 | >2 | >2 | >2 | 2 | 1.5 | 2 | 1.5 |
| D | >2 | >2 | >2 | >2 | >2 | 2 | >2 | 2 |
| 1P:0.5Ag+ (1) | 1P:1Ag+ (2) | 1P:2Ag+ (3) | 1P:3Ag+ (4) | |||||
|---|---|---|---|---|---|---|---|---|
| A | >2 | 1 | >2 | 1 | 1 | 0.5 | 1 | 0.5 |
| B | >2 | >1.5 | >2 | >1.5 | >1.5 | >1 | >1.5 | >1 |
| C | >2 | 2 | >2 | 2 | 2 | 1.5 | 2 | 1.5 |
| D | >2 | >2 | >2 | >2 | >2 | >2 | >2 | >2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, M.; Horvàth, L.; Brunetto, P.S.; Fromm, K.M. Trithiocarbonate-Functionalized PNiPAAm-Based Nanocomposites for Antimicrobial Properties. Polymers 2018, 10, 665. https://doi.org/10.3390/polym10060665
Tan M, Horvàth L, Brunetto PS, Fromm KM. Trithiocarbonate-Functionalized PNiPAAm-Based Nanocomposites for Antimicrobial Properties. Polymers. 2018; 10(6):665. https://doi.org/10.3390/polym10060665
Chicago/Turabian StyleTan, Milène, Lenke Horvàth, Priscilla S. Brunetto, and Katharina M. Fromm. 2018. "Trithiocarbonate-Functionalized PNiPAAm-Based Nanocomposites for Antimicrobial Properties" Polymers 10, no. 6: 665. https://doi.org/10.3390/polym10060665





