Fabric Coated with Shape Memory Polyurethane and Its Properties
Abstract
:1. Introduction
2. Material and Method
2.1. SMPU Synthesis
2.2. Sample Preparation
2.3. Characterization
3. Result and Discussion
3.1. Water Resistance/Contact Angle
3.2. Water Vapor Transmission of Coated Fabric
3.3. Mechanism of Water Transmittance and Resistance Property of the Coated Fabric
3.4. Thermo-Mechanical Properties
3.5. Mechanical Properties
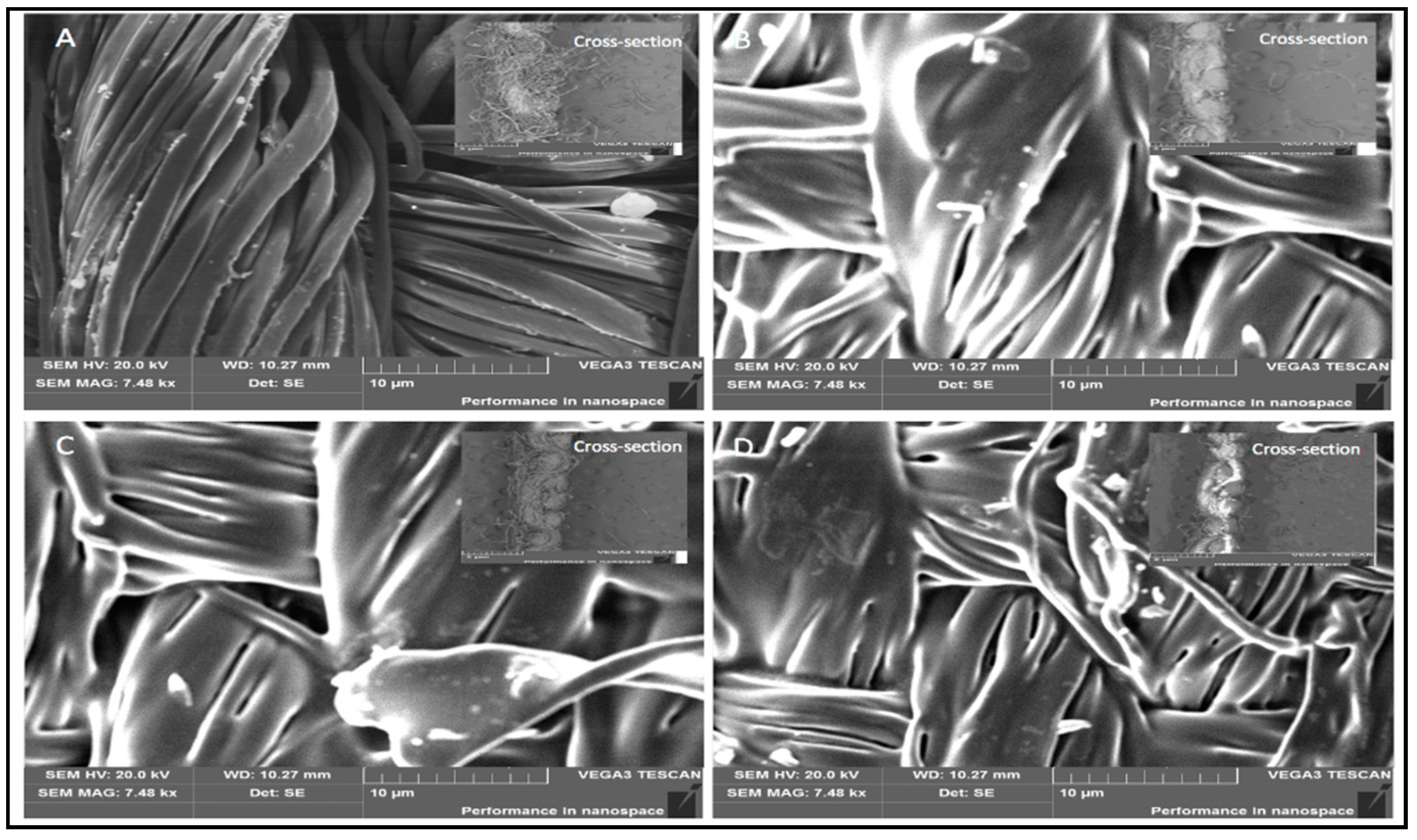
3.6. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mustafa, E.; Gözde, E.; Arzu, M. Analysis of thermal comfort properties of fabrics for protective applications. J. Text. Inst. 2017, 109, 1091–1098. [Google Scholar] [CrossRef]
- Li, Y. The Science of Clothing Comfort. Text. Prog. 2001, 1, 31. [Google Scholar] [CrossRef]
- Hu, J.L.; Mondal, S. Temperature Sensitive Shape Memory Polymer for Smart Textile Application; Woodhead in Association with the Textile Institute: Cambridge, UK, 2006; pp. 104–121. [Google Scholar]
- Wong, K.H. Study of Water Vapor Permeability of Fabric Coated with Shape Memory Polyurethane. Bachelor’s Thesis, The Hong Kong Polytechnic University, Hong Kong, China, 2016. [Google Scholar]
- Bartels, V.T.; Umbach, K.H. Water Vapors Transport through Protective Textiles at Low Temperatures. Text. Res. J. 2002, 72, 899–905. [Google Scholar] [CrossRef]
- Parsons, K.C. Human Thermal Environments; Taylor & Francis Group: London, UK, 1993. [Google Scholar]
- Xu, X.; Karis, A.J.; Buller, M.J.; Santee, W.R. Relationship between core temperature, skin temperature, and heat flux during exercise in heat. Eur. J. Appl. Phys. 2013, 113, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Hu, J.L.; Yang, Z.; Liu, Y.; Szeto, Y.S. Shape Memory Polyurethane for Smart Garment. Res. J. Text. Appar. 2002, 6, 75–83. [Google Scholar] [CrossRef]
- Zhang, P.; Watanabe, Y.; Kim, S.H.; Tokura, H.; Gong, R.H. Thermoregulatory Responses to Different Moisture-transfer Rates of Clothing Materials during Exercise. J. Text. Inst. 2001, 92, 372–378. [Google Scholar]
- Mondal, S.; Hu, J.L. Water vapor permeability of cotton fabrics coated with shape memory polyurethane. Carbohydr. Polym. 2007, 67, 282–287. [Google Scholar] [CrossRef]
- Cho, J.W.; Jung, Y.C.; Chun, B.C.; Chung, Y.C. Water Vapor Permeability and Mechanical Properties of Fabrics Coated with Shape-Memory Polyurethane. J. Appl. Polym. Sci. 2004, 92, 2812–2816. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape Memory Polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Sinem, G.; Ebru, C.; Mehmet, T. The improved breathability of polyurethane coated cotton fabric via micro-cracking. J. Text. Inst. 2017, 108, 1815–1821. [Google Scholar]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. [Google Scholar]
- Chen, S.J.; Hu, J.L.; Zhuo, H.T. Properties and Mechanism of Two-way Shape Memory Polyurethane Composites. Compos. Sci. Technol. 2010, 70, 1437–1443. [Google Scholar] [CrossRef]
- Hu, J.L.; Ding, X.M.; Tao, X.M. Shape Memory Polymers and Their Applications to Smart. Text. Prod. J. China Text. Univ. 2002, 19, 89–93. [Google Scholar]
- Jeong, H.M.; Ahn, B.K.; Cho, S.M.; Kim, B.K. Water Vapor Permeability of Shape Memory Polyurethane with Amorphous Reversible Phase. J. Polym. Sci. 2000, 38, 3009–3017. [Google Scholar] [CrossRef]
- Yang, J.; Wen, H.; Zhuo, H.; Chen, S.; Ban, J. A New Type of Photo-Thermo Staged-Responsive Shape-Memory Polyurethanes Network. Polymers 2017, 9, 287. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kim, H.Y.; Choi, J.W.; Chun, B.C. Graft Polymerization of Polyacrylonitrile or Poly(methyl methacrylate) onto Polyurethane for the Improvement of Mechanical Properties and Water Vapor Permeability. Bull. Korean Chem. Soc. 2015, 36, 1418–1425. [Google Scholar] [CrossRef]
- Hu, J.L.; Zhuo, H. Shape Memory Polymers in Coating and Laminates for Textiles. In Smart Textile Coatings and Laminates; Woodhead in Association with the Textile Institute: Cambridge, UK, 2010; pp. 222–233. [Google Scholar]
- Xiao, Y.; Jiang, L.; Liu, Z.; Yuan, Y.; Yan, P.; Zhou, C.; Lei, J. Effect of phase separation on the crystallization of soft segments of green waterborne polyurethanes. Polym. Test. 2017, 60, 160–165. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Yeung, L.Y.; Lu, J.; Meng, Q.; Chen, S.; Yeung, K.W. Effect of steaming on shape memory polyurethane fibers with various hard segment contents. Smart Mater. Struct. 2007, 16, 969. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, J.L.; Chen, S. Study of Water Vapor Permeability of Shape Memory Polyurethane Nano fibrous Nonwovens. Text. Res. J. 2011, 81, 883–891. [Google Scholar]
- Hayashi, S. Room-temperature-functional Shape-memory Polymers. Plast. Eng. 1995, 51, 29–31. [Google Scholar]
- Hornbogen, E. Comparison of Shape Memory Metals and Polymers. Adv. Eng. Mater. 2006, 8, 101–106. [Google Scholar] [CrossRef]
- Cui, B.; Wu, Q.Y.; Gu, L.; Shen, L.; Yu, H.B. High performance bio-based polyurethane elastomers: Effect of different soft and hard segments. Chin. J. Polym. Sci. 2016, 34, 901–909. [Google Scholar] [CrossRef]
- Hu, J.L. Shape Memory Polymers and Textiles; Woodhead in Association with the Textile Institute: Cambridge, UK, 2007. [Google Scholar]
- Jassal, M.; Khungar, A.; Bajaj, P.; Sinha, T.J.M. Waterproof Breathable Polymeric Coatings Based on Polyurethanes. J. Ind. Text. 2004, 33, 269. [Google Scholar] [CrossRef]
- Ruckman, J.E. Water Vapor Transfer in Waterproof Breathable Fabrics. J. Cloth. Sci. Technol. 1997, 9, 141–153. [Google Scholar] [CrossRef]
- Gu, L.; Cui, B.; Wu, Q.-Y.; Yu, H. Bio-based polyurethanes with shape memory behaviour at body temperature: Effect of different chain extenders. RSC Adv. 2016, 6, 17888–17895. [Google Scholar] [CrossRef]












| Sample | PEG (Polyol), moL | PTMG (Polyol), moL | PCL (Polyol), moL | MDI (Di-Isocyanate), moL | BDO (Chain Extender), moL | Soft:Hard Segment Ratio |
|---|---|---|---|---|---|---|
| Sample 1 | 0.082 | 0.282 | 0.200 | 65:35 | ||
| Sample 2 | 0.082 | 0.282 | 0.200 | 65:35 | ||
| Sample 3 | 0.082 | 0.282 | 0.200 | 65:35 |
| Sample Code | Description | Thickness (mm) |
|---|---|---|
| UF | Uncoated fabric (Control) | 0.20 ± 0.01 |
| CFPEG | Fabric coated with PEG | 0.30 ± 0.01 |
| CFPTMG | Fabric coated with PTMG | 0.32 ± 0.01 |
| CFPCL | Fabric coated with PCL | 0.33 ± 0.01 |
| Polyurethane | Shape Fixity (%) | Shape Recovery (%) | Plasticity (%) |
|---|---|---|---|
| PEG | 79.4 ± 1.5 | 85.3 ± 1.5 | 14.7 ± 1.5 |
| PTMG | 80.1 ± 1.5 | 86.7 ± 1.5 | 13.3 ± 1.5 |
| PCL | 79.5 ± 1.5 | 86.2 ± 1.5 | 13.8 ± 1.5 |
| Sample | Breaking Load (MPA) | Breaking Elongation (%) |
|---|---|---|
| UF (Control) | 27 ± 1 | 21 ± 1 |
| CFPEG | 36 ± 1 | 22 ± 1 |
| CFPTMG | 36 ± 1 | 21 ± 1 |
| CFPCL | 37 ± 1 | 20 ± 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahid, M.A.; Hu, J.; Wong, K.; Wu, Y.; Zhu, Y.; Sheng Luo, H.H.; Zhongmin, D. Fabric Coated with Shape Memory Polyurethane and Its Properties. Polymers 2018, 10, 681. https://doi.org/10.3390/polym10060681
Jahid MA, Hu J, Wong K, Wu Y, Zhu Y, Sheng Luo HH, Zhongmin D. Fabric Coated with Shape Memory Polyurethane and Its Properties. Polymers. 2018; 10(6):681. https://doi.org/10.3390/polym10060681
Chicago/Turabian StyleJahid, Md Anwar, Jinlian Hu, KwanHa Wong, You Wu, Yong Zhu, Hogan Hong Sheng Luo, and Deng Zhongmin. 2018. "Fabric Coated with Shape Memory Polyurethane and Its Properties" Polymers 10, no. 6: 681. https://doi.org/10.3390/polym10060681





