Modification of PSf/SPSf Blended Porous Support for Improving the Reverse Osmosis Performance of Aromatic Polyamide Thin Film Composite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication of Porous Support Membrane
2.3. Fabrication of Polyamide TFC Reverse Osmosis Membrane
2.4. Permeability Tests
2.5. Characterization of PSf/SPSf Porous Support and Polyamide RO Membrane
3. Results
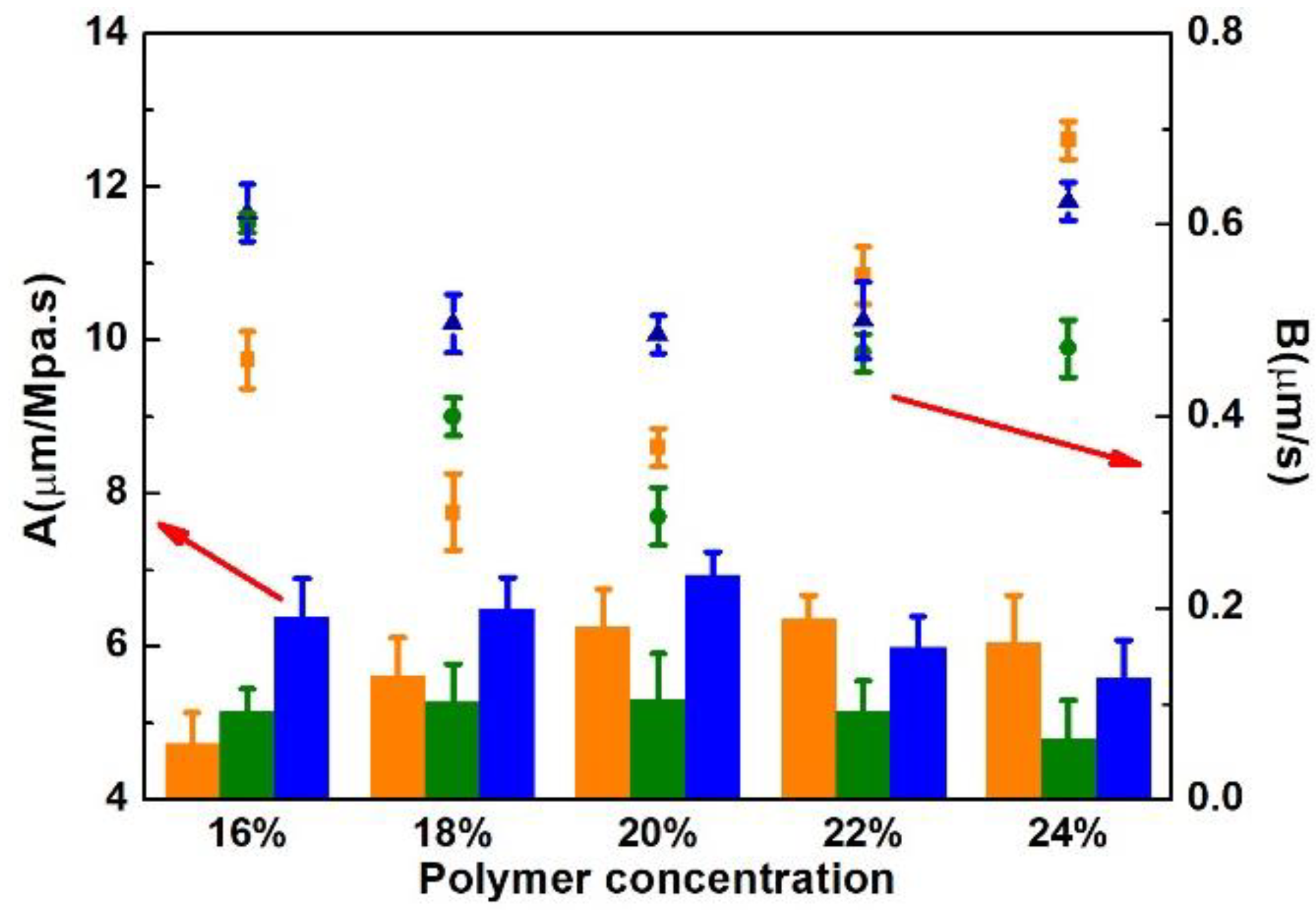
3.1. Effects of Solvent and Polymer Concentration on the Porous Support and RO Membranes
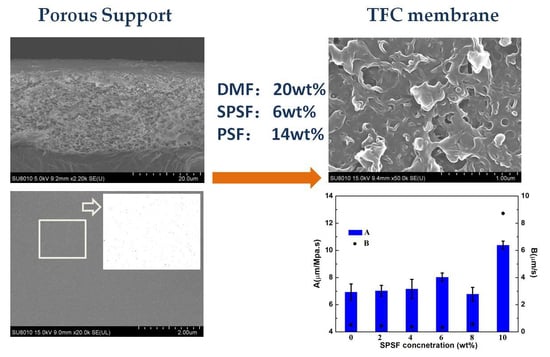
3.1.1. Morphology of the Membranes
3.1.2. Membrane Separation Performances
3.2. Effects of Doping Content of SPSf on the Porous Support and RO Membrane
3.2.1. Viscosity of the Casting Solution, Thickness and Pore Nature of the Porous Support
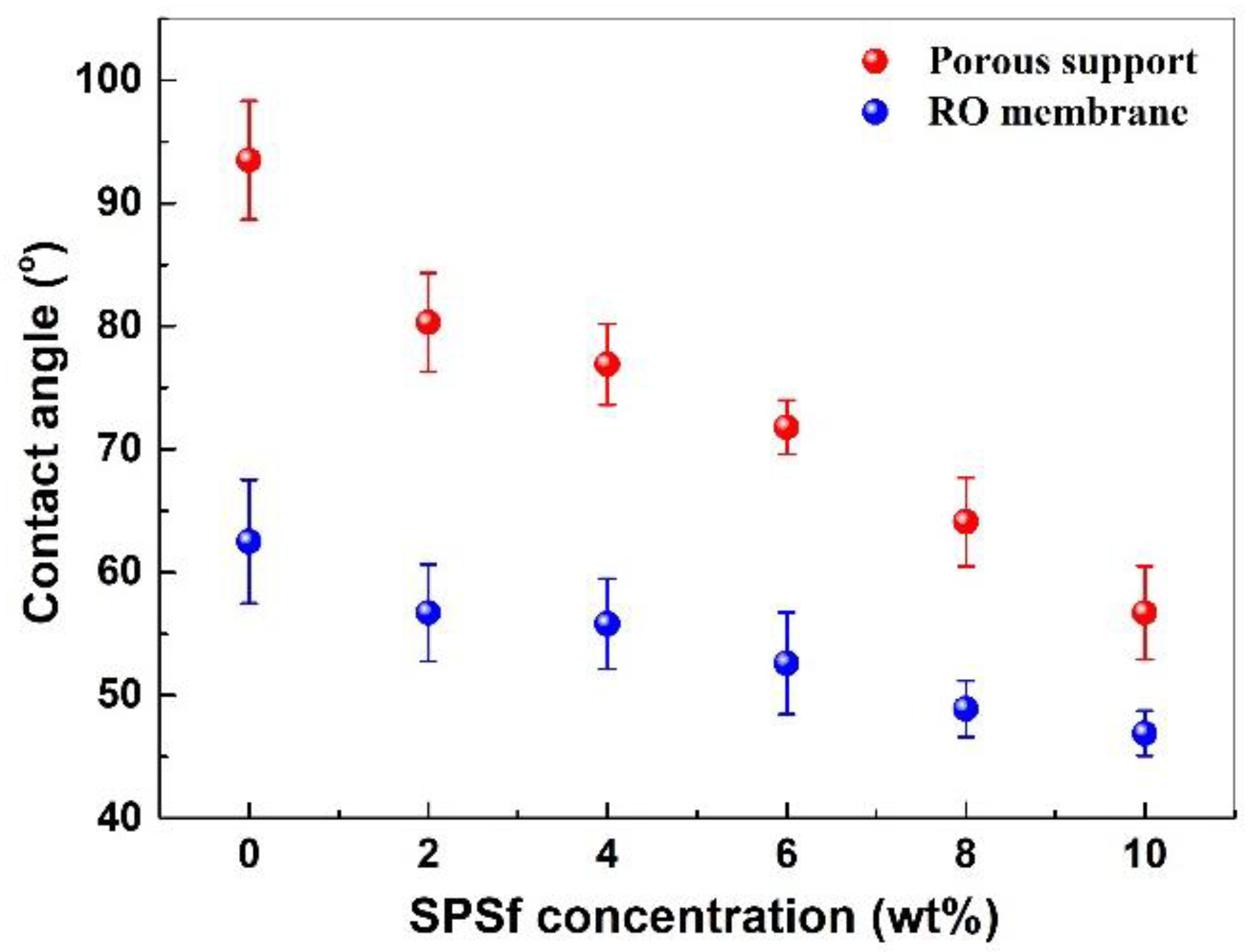
3.2.2. Surface Morphology and Wettability of the Membranes
3.2.3. Membrane Separation Performances
4. Discussion
4.1. Effects of Solvent and Polymer Concentration of the Casting Solution on the Porous Support and Polyamide TFC RO Membrane
4.1.1. Morphology of the PSf Porous Support and Polyamide RO Membrane
4.1.2. Separation Performance of the Porous Support and Polyamide TFC RO Membrane
4.2. Effects of the Doping Content of SPSf in the Casting Solution on the Structure and Separation Performance of the Porous Support and Polyamide TFC RO Membrane
4.2.1. Viscosity of the Casting Solution and the Thickness of the Porous Support
4.2.2. Pore Nature of the Porous Support
4.2.3. Surface Morphologies of the Porous Supports and TFC RO Membranes
4.2.4. Surface Wettability of the Porous Support and TFC RO Membrane
4.2.5. Separation Performances of the Porous Support and TFC RO Membrane
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Misdan, N.; Lau, W.J.; Ismail, A.F.; Matsuura, T. Formation of thin film composite nanofiltration membrane: Effect of polysulfone substrate characteristics. Desalination 2013, 329, 9–18. [Google Scholar] [CrossRef]
- Zarrabi, N.; Yekavalangi, M.E.; Vatanpour, V.; Shockravi, A.; Safarpour, M. Improvement in desalination performance of thin film nanocomposite nanofiltration membrane using amine-functionalized multiwalled carbon nanotube. Desalination 2016, 394, 83–90. [Google Scholar] [CrossRef]
- Harisha, R.S.; Hosamani, K.M.; Keri, R.S.; Nataraj, S.K.; Aminabhavi, T.M. Arsenic removal from drinking water using thin film composite nanofiltration membrane. Desalination 2010, 252, 75–80. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling control—A review. Desalination 2009, 245, 321–348. [Google Scholar] [CrossRef]
- Mehdipour, S.; Vatanpour, V.; Kariminia, H.R. Influence of ion interaction on lead removal by a polyamide nanofiltration membrane. Desalination 2015, 362, 84–92. [Google Scholar] [CrossRef]
- Ismail, A.F.; Lau, W. Influence of feed conditions on the rejection of salt and dye in aqueous solution by different characteristics of hollow fiber nanofiltration membranes. Desalin. Water Treat. 2009, 6, 281–288. [Google Scholar] [CrossRef]
- Pacheco, F.; Sougrat, R.; Reinhard, M.; Leckie, J.O.; Pinnau, I. 3D visualization of the internal nanostructure of polyamide thin films in RO membranes. J. Membr. Sci. 2016, 501, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.E.; Lee, J.S.; Park, S.H.; Kim, I.T.; Chan, E.P.; Kwon, Y.N.; Lee, J.H. Tailoring interlayer structure of molecular layer-by-layer assembled polyamide membranes for high separation performance. Appl. Surf. Sci. 2015, 356, 659–667. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite Reverse-Osmosis and Nanofiltration Membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Rangarajan, R.; Desai, N.V.; Mody, R.C.; Mohan, D.; Rao, A.V. Development of Fabric Reinforced Polysulfone Membranes. Desalination 1991, 85, 81–92. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Yan, H.; Miao, X.; Xu, J.; Pan, G.; Zhang, Y.; Shi, Y.; Guo, M.; Liu, Y. The porous structure of the fully-aromatic polyamide film in reverse osmosis membranes. J. Membr. Sci. 2015, 475, 504–510. [Google Scholar] [CrossRef]
- Klaysom, C.; Hermans, S.; Gahlaut, A.; Van Craenenbroeck, S.; Vankelecom, I.F. Polyamide/Polyacrylonitrile (PA/PAN) thin film composite osmosis membranes: Film optimization, characterization and performance evaluation. J. Membr. Sci. 2013, 445, 25–33. [Google Scholar] [CrossRef]
- Bui, N.N.; McCutcheon, J.R. Hydrophilic Nanofibers as New Supports for Thin Film Composite Membranes for Engineered Osmosis. Environ. Sci. Technol. 2013, 47, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hill, A.; Kentish, S. Formation of a thick aromatic polyamide membrane by interfacial polymerization. Sep. Purif. Technol. 2013, 104, 276–283. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J. Membr. Sci. 2015, 489, 43–54. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, S.S. Plasma treatment of polypropylene and polysulfone supports for thin film composite reverse osmosis membrane. J. Membr. Sci. 2006, 286, 193–201. [Google Scholar] [CrossRef]
- Pendergast, M.T.M.; Nygaard, J.M.; Ghosh, A.K.; Hoek, E.M. Using nanocomposite materials technology to understand and control reverse osmosis membrane compaction. Desalination 2010, 261, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Tiraferri, A.; Yip, N.Y.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. Relating performance of thin-film composite forward osmosis membranes to support layer formation and structure. J. Membr. Sci. 2011, 367, 340–352. [Google Scholar] [CrossRef] [Green Version]
- Yakavalangi, M.E.; Rimaz, S.; Vatanpour, V. Effect of surface properties of polysulfone support on the performance of thin film composite polyamide reverse osmosis membranes. J. Appl. Polym. Sci. 2016, 134. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Jeong, B.H.; Huang, X.; Hoek, E.M. Impacts of reaction and curing conditions on polyamide composite reverse osmosis membrane properties. J. Membr. Sci. 2008, 311, 34–45. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E.M. Impacts of support membrane structure and chemistry on polyamide-polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Singh, P.S.; Joshi, S.V.; Trivedi, J.J.; Devmurari, C.V.; Rao, A.P.; Ghosh, P.K. Probing the structural variations of thin film composite RO membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions. J. Membr. Sci. 2006, 278, 19–25. [Google Scholar] [CrossRef]
- Li, X.; Wang, K.Y.; Helmer, B.; Chung, T.S. Thin-Film Composite Membranes and Formation Mechanism of Thin-Film Layers on Hydrophilic Cellulose Acetate Propionate Substrates for Forward Osmosis Processes. Ind. Eng. Chem. Res. 2012, 51, 10039–10050. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A. Effect of lag time in interfacial polymerization on polyamide composite membrane with different hydrophilic sub layers. Desalination 2012, 284, 32–41. [Google Scholar] [CrossRef]
- Wei, J.; Jian, X.G.; Wu, C.R.; Zhang, S.H.; Yan, C. Influence of polymer structure on thermal stability of composite membranes. J. Membr. Sci. 2005, 256, 116–121. [Google Scholar] [CrossRef]
- Kim, I.-C.; Jegal, J.; Lee, K.-H. Effect of aqueous and organic solutions on the performance of polyamide thin-film-composite nanofiltration membranes. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2151–2163. [Google Scholar] [CrossRef]
- Verissimo, S.; Peinemann, K.-V.; Bordado, J. Thin-film composite hollow fiber membranes: An optimized manufacturing method. J. Membr. Sci. 2005, 264, 48–55. [Google Scholar] [CrossRef]
- Korikov, A.R.; Kosaraju, R.; Sirkar, K.K. Interfacially polymerized hydrophilic microporous thin film composite membranes on porous polypropylene hollowfibers and flat film. J. Membr. Sci. 2006, 279, 588–600. [Google Scholar] [CrossRef]
- Liu, B.C.; Chen, C.; Zhao, P.J. Thin-film composite forward osmosis membranes with substrate layer composed of polysulfone blended with PEG or polysulfone grafted PEG methyl ether methacrylate. Front. Chem. Sci. Eng. 2016, 10, 562–574. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Li, X. Thin film composite forward osmosis membranes based on polydopamine modified polysulfone substrates with enhancements in both water flux and salt rejection. Chem. Eng. Sci. 2012, 80, 219–231. [Google Scholar] [CrossRef]
- Yu, C.H.; Kusumawardhana, I.; Lai, J.Y. PTFE/polyamide thin-film composite membranes using PTFE films modified with ethylene diamine polymer and interfacial polymerization: Preparation and pervaporation application. J. Colloid Interface Sci. 2009, 336, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A. Preparation and characterization of thin film composite reverses osmosis membranes with wet and dry support layer. Desalin. Water Treat. 2015, 56, 2284–2295. [Google Scholar] [CrossRef]
- Kosaraju, P.B.; Sirkar, K.K. Interfacially polymerized thin film composite membranes on microporous polypropylene supports for solvent-resistant nanofiltration. J. Membr. Sci. 2008, 321, 155–161. [Google Scholar] [CrossRef]
- Park, H.M.; Jee, K.Y.; Lee, Y.T. Preparation and characterization of a thin-film composite reverse osmosis membrane using a polysulfone membrane including metal-organic frameworks. J. Membr. Sci. 2017, 541, 510–518. [Google Scholar] [CrossRef]
- Hou, D.; Fan, H.; Jiang, Q.; Wang, J.; Zhang, X. Preparation and characterization of PVDF flat-sheet membranes for direct contact membrane distillation. Sep. Purif. Technol. 2014, 135, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Smolders, C.; Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef]
- Van de Witte, P.; Dijkstra, P.J.; Van den Berg, J.W.A.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; An, Q.; Ji, Y.; Qian, J.; Gao, C. Effect of zero shear viscosity of the casting solution on the morphology and permeability of polysulfone membrane prepared via the phase-inversion process. Desalination 2010, 260, 43–50. [Google Scholar] [CrossRef]
- Jin, Y.; Su, Z. Effects of polymerization conditions on hydrophilic groups in aromatic polyamide thin films. J. Membr. Sci. 2009, 330, 175–179. [Google Scholar] [CrossRef]











| No. | PSf (wt %) | SPSf (wt %) | Solvent (wt %) |
|---|---|---|---|
| 1 | 20 | 0 | 80 |
| 2 | 18 | 2 | 80 |
| 3 | 16 | 4 | 80 |
| 4 | 14 | 6 | 80 |
| 5 | 12 | 8 | 80 |
| 6 | 10 | 10 | 80 |
| Solution | Composition |
|---|---|
| Aqueous phase | 2 wt % MPD, 2 wt % TEA, 0.15 wt % SDS and 4 wt % CSA in water |
| Organic phase | 0.15 wt % TMC in hexane |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.-F.; Gu, X.-L.; Xie, X.; Li, R.-H.; Yu, C.-Y.; Song, X.-X.; Gao, C.-J. Modification of PSf/SPSf Blended Porous Support for Improving the Reverse Osmosis Performance of Aromatic Polyamide Thin Film Composite Membranes. Polymers 2018, 10, 686. https://doi.org/10.3390/polym10060686
Liu L-F, Gu X-L, Xie X, Li R-H, Yu C-Y, Song X-X, Gao C-J. Modification of PSf/SPSf Blended Porous Support for Improving the Reverse Osmosis Performance of Aromatic Polyamide Thin Film Composite Membranes. Polymers. 2018; 10(6):686. https://doi.org/10.3390/polym10060686
Chicago/Turabian StyleLiu, Li-Fen, Xing-Ling Gu, Xin Xie, Rui-Han Li, Chun-Yang Yu, Xiao-Xiao Song, and Cong-Jie Gao. 2018. "Modification of PSf/SPSf Blended Porous Support for Improving the Reverse Osmosis Performance of Aromatic Polyamide Thin Film Composite Membranes" Polymers 10, no. 6: 686. https://doi.org/10.3390/polym10060686