Controlling the Internal Structures of Polymeric Microspheres via the Introduction of a Water-Soluble Organic Solvent
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
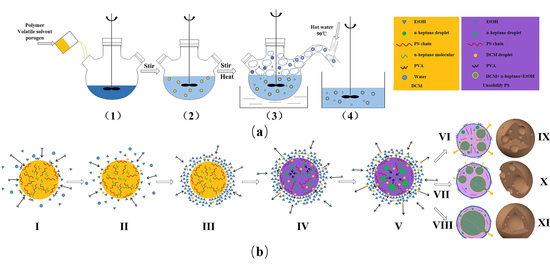
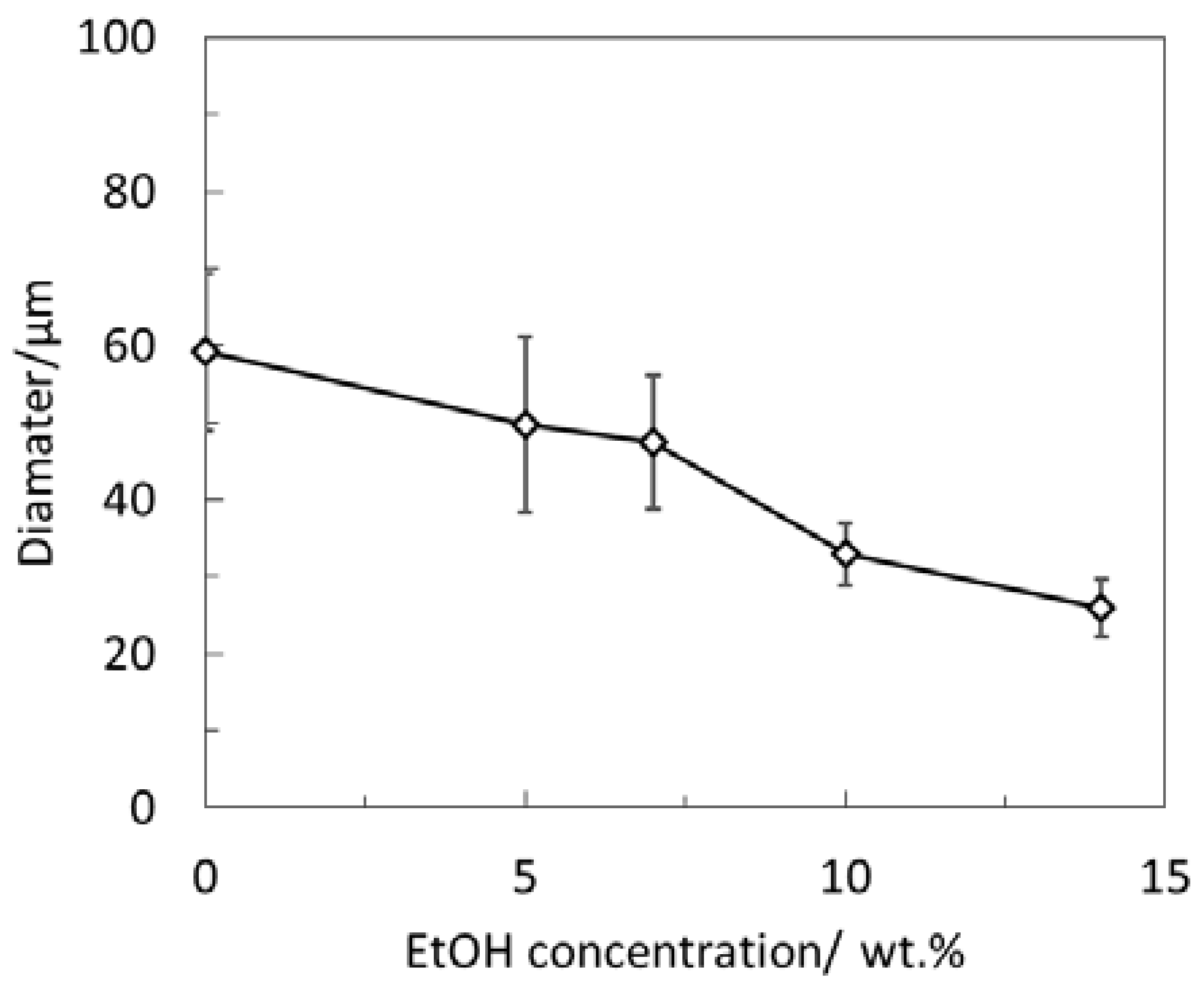
3.1. The Effect of Introducing a Water-Soluble Organic Solvent on the Internal Structures of Polymeric Microspheres
3.2. The Mechanism Resulting in Controlling the Internal Structures of Polymeric Microspheres
3.3. Controlling the Thickness of Hollow Polymeric Microsphere Shells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gokmen, M.T.; Prez, F.E.D. Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zou, F.; Liu, K.; Qiang, Z.; Taubert, C.J.; Ustriyana, P.; Vogt, B.D.; Zhu, Y. A binary metal organic framework derived hollow Ni3S2/Co9S8/N-doped carbon composite with superior sodium storage performance. J. Mater. Chem. A 2017, 5, 11781–11787. [Google Scholar] [CrossRef]
- Wang, S.; Tangvijitsakul, P.; Qiang, Z.; Bhaway, S.M.; Lin, K.; Cavicchi, K.A.; Soucek, M.D.; Vogt, B.D. Role of Amphiphilic Block Copolymer Composition on Pore Characteristics of Micelle-Templated Mesoporous Cobalt Oxide Films. Langmuir 2016, 32, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Qiang, Z.; Lecorchick, W.; Cavicchi, K.A.; Vogt, B.D. Nanoporous Nonwoven Fibril-Like Morphology by Cooperative Self-Assembly of Poly(ethylene oxide)-block-Poly(ethyl acrylate)-block-Polystyrene and Phenolic Resin. Langmuir 2014, 30, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Xu, F.; Sun, B.; Fu, R.W.; He, H.K.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Makuta, T.; Tamakawa, Y.; Endo, J. Hollow microspheres fabricated from instant adhesive. Mater. Lett. 2011, 65, 3415–3417. [Google Scholar] [CrossRef]
- Schmidt, W.; Roessling, G. Novel manufacturing process of hollow polymer microspheres. Chem. Eng. Sci. 2006, 61, 4973–4981. [Google Scholar] [CrossRef]
- Bilalis, P.; Boukos, N.; Kordas, G.C. Novel PEGylated pH-sensitive polymeric hollow microspheres. Mater. Lett. 2012, 67, 180–183. [Google Scholar] [CrossRef]
- Jang, T.-S.; Lee, E.-J.; Kim, H.-E.; Koh, Y.-H. Hollow porous poly(ε-caprolactone) microspheres by emulsion solvent extraction. Mater. Lett. 2012, 72, 157–159. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Li, X.; Zhang, S. Preparation of Hollow/Porous Polymeric Microspheres based on OH-HPEEK and PVA. Mater. Lett. 2012, 74, 85–88. [Google Scholar] [CrossRef]
- Ji, S.; Srivastava, D.; Parker, N.J.; Lee, I. Transitional behavior of polymeric hollow microsphere formation in turbulent shear flow by emulsion diffusion method. Polymer 2012, 53, 205–212. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Zheng, F. Polyaniline hollow microspheres synthesized via self-assembly method in a polymer acid aqueous solution. Mater. Lett. 2015, 148, 34–36. [Google Scholar] [CrossRef]
- Liu, P. Stabilization of layer-by-layer engineered multilayered hollow microspheres. Adv. Colloid Interf. 2014, 207, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Li, Z.; Liu, F.; Wei, W.; Wang, X.; Liu, Z. Study of the organic solvent template double emulsions for porous microspheres production with adjustable structures. Chem. Eng. Sci. 2018, 184, 229–238. [Google Scholar] [CrossRef]
- He, Y.; Guo, W.; Shan, M.; Zhu, L.; Si, T.; Wang, H.; Sun, Y. Technical improvement for producing porous polymer microspheres in foam phase. Mater. Lett. 2017, 201, 54–57. [Google Scholar] [CrossRef]
- He, M.; Zhang, H.; Cheng, W.; Dong, X. Polymer Physics, 3rd ed.; Fudan University Press: Shanghai, China, 2007; pp. 52–54. ISBN 9787309054156. [Google Scholar]




| Sample | PS g | n-Heptane g | DCM g | PVA g | Aqueous Phase 100.0 g | Temperature Gradient °C/min |
|---|---|---|---|---|---|---|
| E0 | 10.0 | 6.0 | 60.0 | 1.0 | Water | 1.0 |
| E1 | 10.0 | 6.0 | 60.0 | 1.0 | 5.0 wt % EtOH solution | 1.0 |
| E2 | 10.0 | 6.0 | 60.0 | 1.0 | 7.0 wt % EtOH solution | 1.0 |
| E3 | 10.0 | 6.0 | 60.0 | 1.0 | 10.0 wt % EtOH solution | 1.0 |
| E4 | 10.0 | 6.0 | 60.0 | 1.0 | 14.0 wt % EtOH solution | 1.0 |
| E5 | 10.0 | 6.0 | 60.0 | 1.0 | 20.0 wt % EtOH solution | 1.0 |
| T1 | 3.0 | 6.0 | 60.0 | 1.0 | 10.0 wt % EtOH solution | 1.0 |
| T2 | 6.0 | 6.0 | 60.0 | 1.0 | 10.0 wt % EtOH solution | 1.0 |
| T3 | 10.0 | 6.0 | 60.0 | 1.0 | 10.0 wt % EtOH solution | 1.0 |
| T4 | 15.0 | 6.0 | 60.0 | 1.0 | 10.0 wt % EtOH solution | 1.0 |
| P1 | 3.0 | 6.0 | 60.0 | 1.0 | Water | 1.0 |
| P2 | 6.0 | 6.0 | 60.0 | 1.0 | Water | 1.0 |
| P3 | 10.0 | 6.0 | 60.0 | 1.0 | Water | 1.0 |
| P4 | 15.0 | 6.0 | 60.0 | 1.0 | Water | 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Li, X.; Zhu, T.; Shan, M.; Zhu, L.; Si, T.; Wang, H.; Sun, Y. Controlling the Internal Structures of Polymeric Microspheres via the Introduction of a Water-Soluble Organic Solvent. Polymers 2018, 10, 789. https://doi.org/10.3390/polym10070789
He Y, Li X, Zhu T, Shan M, Zhu L, Si T, Wang H, Sun Y. Controlling the Internal Structures of Polymeric Microspheres via the Introduction of a Water-Soluble Organic Solvent. Polymers. 2018; 10(7):789. https://doi.org/10.3390/polym10070789
Chicago/Turabian StyleHe, Yanping, Xin Li, Tianci Zhu, Mengxing Shan, Linhua Zhu, Tian Si, Hong Wang, and Yanlin Sun. 2018. "Controlling the Internal Structures of Polymeric Microspheres via the Introduction of a Water-Soluble Organic Solvent" Polymers 10, no. 7: 789. https://doi.org/10.3390/polym10070789