Synthesis and Characterization of Fully Conjugated Ladder Naphthalene Bisimide Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
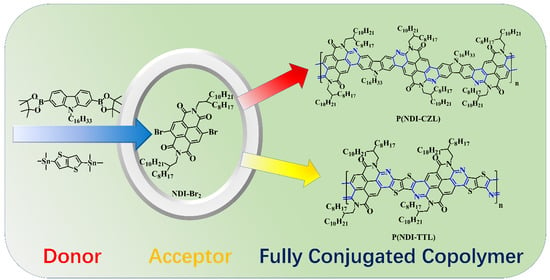
2.3. General Synthesis for Compound NDI-CZL and Polymer P(NDI-CZL)
2.4. General Synthesis for Compound NDI-TTL and Polymer P(NDI-TTL)
3. Results
3.1. Synthetic Route Discussion
3.2. SEC Trace for Copolymers
3.3. Thermal Properties of the Copolymers
3.4. FTIR Spectra of Copolymers
3.5. Photophysical Properties of Compounds and Copolymers
3.6. Electrochemical Properties of Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Kalin, A.J.; Wang, C.; Early, J.T.; Al-Hashimi, M.; Fang, L. Donor-acceptor conjugated ladder polymer via aromatization-driven thermodynamic annulation. Polym. Chem. 2018, 9, 1603–1609. [Google Scholar] [CrossRef]
- Daigle, M.; Morin, J.-F. Helical Conjugated Ladder Polymers: Tuning the Conformation and Properties through Edge Design. Macromolecules 2017, 50, 9257–9264. [Google Scholar] [CrossRef]
- Congzhi, Z.; Lei, F. Locking the Coplanar Conformation of π-Conjugated Molecules and Macromolecules Using Dynamic Noncovalent Bonds. Macromol. Rapid Commun. 2018, 39, 1700241. [Google Scholar] [CrossRef]
- Lai, H.W.H.; Teo, Y.C.; Xia, Y. Functionalized Rigid Ladder Polymers from Catalytic Arene-Norbornene Annulation Polymerization. ACS Macro Lett. 2017, 6, 1357–1361. [Google Scholar] [CrossRef]
- Lee, J.; Li, H.; Kalin, A.J.; Yuan, T.; Wang, C.; Olson, T.; Li, H.; Fang, L. Extended Ladder-Type Benzo k tetraphene-Derived Oligomers. Angew. Chem. Int. Ed. 2017, 56, 13727–13731. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ding, Y.; Agarwal, S.; Greiner, A.; Zhang, H.; Hou, H. Nanofibre preparation of non-processable polymers by solid-state polymerization of molecularly self-assembled monomers. Nanoscale 2017, 9, 18169–18174. [Google Scholar] [CrossRef] [PubMed]
- CTeo, Y.C.; Lai, H.W.H.; Xia, Y. Synthesis of Ladder Polymers: Developments, Challenges, and Opportunities. Chem. Eur. J. 2017, 23, 14101–14112. [Google Scholar] [CrossRef]
- Schlüter, A.-D. Ladder Polymers: The new generation. Adv. Mater. 1991, 3, 282–291. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, R.; Zhu, J.; Huang, W.; Chang, S.Y.; Meng, L.; Sun, P.; Cheng, H.W.; Qin, M.; Zhu, C.; et al. Ternary System with Controlled Structure: A New Strategy toward Efficient Organic Photovoltaics. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Grimsdale, A.C.; Mullen, K. Oligomers and polymers based on bridged phenylenes as electronic materials. Macromol. Rapid Commun. 2007, 28, 1676–1702. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Fei, Z.; Bo, Z. Spiro-bridged ladder-type poly(p-phenylene)s: Towards structurally perfect light-emitting materials. J. Am. Chem. Soc. 2008, 130, 7192–7193. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Rui, X.; Long, G.; Chen, W.; Yan, Q.; Zhang, Q. Pushing Up Lithium Storage through Nanostructured Polyazaacene Analogues as Anode. Angew. Chem. Int. Ed. Engl. 2015, 54, 7354–7358. [Google Scholar] [CrossRef] [PubMed]
- Olvera, L.I.; Rodríguez-Molina, M.; Ruiz-Treviño, F.A.; Zolotukhin, M.G.; Fomine, S.; Cárdenas, J.; Gaviño, R.; Alexandrova, L.; Toscano, R.A.; Martínez-Mercado, E. A Highly Soluble, Fully Aromatic Fluorinated 3D Nanostructured Ladder Polymer. Macromolecules 2017, 50, 8480–8486. [Google Scholar] [CrossRef]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Ku, S.Y.; Brady, M.A.; Treat, N.D.; Cochran, J.E.; Robb, M.J.; Kramer, E.J.; Chabinyc, M.L.; Hawker, C.J. A modular strategy for fully conjugated donor-acceptor block copolymers. J. Am. Chem. Soc. 2012, 134, 16040–16046. [Google Scholar] [CrossRef] [PubMed]
- Drobizhev, M.; Stepanenko, Y.; Rebane, A.; Wilson, C.J.; Screen, T.E.O.; Anderson, H.L. Strong Cooperative Enhancement of Two-Photon Absorption in Double-Strand Conjugated Porphyrin Ladder Arrays. J. Am. Chem. Soc. 2006, 128, 12432–12433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollingsworth, W.R.; Lee, J.; Fang, L.; Ayzner, A.L. Exciton Relaxation in Highly Rigid Conjugated Polymers: Correlating Radiative Dynamics with Structural Heterogeneity and Wavefunction Delocalization. ACS Energy Lett. 2017, 2, 2096–2102. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Dong, J.; Wu, Y.; Li, W. Highly Efficient Synthesis of a Ladder-Type BN-Heteroacene and Polyheteroacene. Asian J. Org. Chem. 2018, 7, 465–470. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Q. Recent progress in rechargeable lithium batteries with organic materials as promising electrodes. J. Mater. Chem. A 2016, 4, 7091–7106. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, S.; Chen, D.; Guo, F.; Yu, G.; Zhao, L.; Zhang, Y. Synthesis of an indacenodithiophene-based fully conjugated ladder polymer and its optical and electronic properties. Polym. Chem. 2018, 9, 2227–2231. [Google Scholar] [CrossRef]
- Nehls, B.S.; Füldner, S.; Preis, E.; Farrell, T.; Scherf, U. Microwave-Assisted Synthesis of 1,5- and 2,6-Linked Naphthylene-Based Ladder Polymers. Macromolecules 2005, 38, 687–694. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Li, C.; Bo, Z. Synthesis of Fully Soluble Azomethine-Bridged Ladder-Type Poly(p-phenylenes) by Bischler−Napieralski Reaction. Macromolecules 2010, 43, 10216. [Google Scholar] [CrossRef]
- Huang, H.-H.; Prabhakar, C.; Tang, K.-C.; Chou, P.-T.; Huang, G.-J.; Yang, J.-S. Ortho-Branched Ladder-Type Oligophenylenes with Two-Dimensionally π-Conjugated Electronic Properties. J. Am. Chem. Soc. 2011, 133, 8028–8039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bi, H.; Zhang, Y.; Yao, D.; Gao, H.; Fan, Y.; Zhang, H.; Wang, Y.; Wang, Y.; Chen, Z.; et al. Luminescent Boron-Contained Ladder-Type π-Conjugated Compounds. Inorg. Chem. 2009, 48, 7230–7236. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Feng, X.; Yang, X.; Enkelmann, V.; Baumgarten, M.; Müllen, K. Conjugated Ladder-Type Heteroacenes Bearing Pyrrole and Thiophene Ring Units: Facile Synthesis and Characterization. J. Org. Chem. 2008, 73, 9207–9213. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Graf, M.; Grasse, F.; Jacob, J.; List, E.J.W.; Müllen, K. Blue-Emitting Carbon- and Nitrogen-Bridged Poly(ladder-type tetraphenylene)s. Chem. Mater. 2006, 18, 2879–2885. [Google Scholar] [CrossRef]
- Hou, I.C.-Y.; Hu, Y.; Narita, A.; Muellen, K. Diels-Alder polymerization: A versatile synthetic method toward functional polyphenylenes, ladder polymers and graphene nanoribbons. Polym. J. 2018, 50, 3–20. [Google Scholar] [CrossRef]
- Wu, J.; Rui, X.; Wang, C.; Pei, W.-B.; Lau, R.; Yan, Q.; Zhang, Q. Nanostructured Conjugated Ladder Polymers for Stable and Fast Lithium Storage Anodes with High-Capacity. Adv. Energy Mater. 2015, 5, 1402189. [Google Scholar] [CrossRef]
- Liu, S.; Jin, Z.; Teo, Y.C.; Xia, Y. Efficient synthesis of rigid ladder polymers via palladium catalyzed annulation. J. Am. Chem. Soc. 2014, 136, 17434–17437. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Forster, M.; Preis, E.; Wang, H.; Ma, Y.G.; Scherf, U. Simplified Synthesis of Spiro-Bridged Ladder-Type Poly(p-phenylene). J. Polym. Sci. Pol. Chem. 2009, 47, 5137–5143. [Google Scholar] [CrossRef]
- Asaoka, S.; Takeda, N.; Lyoda, T.; Cook, A.R.; Miller, J.R. Electron and hole transport to trap groups at the ends of conjugated polyfluorenes. J. Am. Chem. Soc. 2008, 130, 11912–11920. [Google Scholar] [CrossRef] [PubMed]
- Koldemir, U.; Puniredd, S.R.; Wagner, M.; Tongay, S.; McCarley, T.D.; Kamenov, G.D.; Muellen, K.; Pisula, W.; Reynolds, J.R. End Capping Does Matter: Enhanced Order and Charge Transport in Conjugated Donor-Acceptor Polymers. Macromolecules 2015, 48, 6369–6377. [Google Scholar] [CrossRef]
- Liu, L.; Yang, B.; Zhang, H.; Tang, S.; Xie, Z.; Wang, H.; Wang, Z.; Lu, P.; Ma, Y. Role of tetrakis(triphenylphosphine)palladium(0) in the degradation and optical properties of fluorene-based compounds. J. Phys. Chem. C 2008, 112, 10273–10278. [Google Scholar] [CrossRef]
- Adachi, T.; Vogelsang, J.; Lupton, J.M. Unraveling the Electronic Heterogeneity of Charge Traps in Conjugated Polymers by Single-Molecule Spectroscopy. J. Phys. Chem. Lett. 2014, 5, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yao, H.; Liu, Z.; Yang, C.; Zhong, C.; Qin, J.; Yu, G.; Liu, Y. Bent Ladder-Type Hexaphenylene with Carbazole Core and Spiro Linkage as Stable and Efficient Blue Emitter. Org. Lett. 2009, 11, 4132–4135. [Google Scholar] [CrossRef] [PubMed]
- Kass, K.J.; Forster, M.; Scherf, U. Incorporating an Alternating Donor-Acceptor Structure into a Ladder Polymer Backbone. Angew. Chem. 2016, 128, 7947–7951. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Tour, J.M. Alternating donor/acceptor repeat units in polythiophenes. Intramolecular charge transfer for reducing band gaps in fully substituted conjugated polymers. J. Am. Chem. Soc. 1998, 120, 5355–5362. [Google Scholar] [CrossRef]
- Bheemireddy, S.R.; Hautzinger, M.P.; Li, T.; Lee, B.; Plunkett, K.N. Conjugated Ladder Polymers by a Cyclopentannulation Polymerization. J. Am. Chem. Soc. 2017, 139, 5801–5807. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Vázquez, R.J.; Zhao, D.; Li, L.; Lo, W.-Y.; Zhang, N.; Wu, Q.; Keller, B.; Eshun, A.; Abeyasinghe, N.; et al. Two Photon Absorption Study of Low-Bandgap, Fully Conjugated Perylene Diimide-Thienoacene-Perylene Diimide Ladder-Type Molecules. Chem. Mater. 2017, 29, 6726–6732. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Zhang, Q.; Huang, W.; Zhu, J.; Wang, R.; Chang, S.-Y.; Sun, P.; Meng, L.; Zhao, H.; et al. Unique Energy Alignments of a Ternary Material System toward High-Performance Organic Photovoltaics. Adv. Mater. 2018, 30, 1801501. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Cheng, P.; Zhang, M.; Lau, T.K.; Wu, Y.; Jia, B.; Wang, J.; Yan, C.; Qin, M.; Lu, X.; Zhan, X. Realizing Small Energy Loss of 0.55 eV, High Open-Circuit Voltage >1 V and High Efficiency >10% in Fullerene-Free Polymer Solar Cells via Energy Driver. Adv. Mater. 2017, 29, 1605216. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Kim, F.S.; Seger, M.J.; Jenekhe, S.A.; Watson, M.D. Naphthalene Diimide-Based Polymer Semiconductors: Synthesis, Structure–Property Correlations, and n-Channel and Ambipolar Field-Effect Transistors. Chem. Mater. 2012, 24, 1434–1442. [Google Scholar] [CrossRef]
- Durban, M.M.; Kazarinoff, P.D.; Luscombe, C.K. Synthesis and Characterization of Thiophene-Containing Naphthalene Diimide n-Type Copolymers for OFET Applications. Macromolecules 2010, 43, 6348–6352. [Google Scholar] [CrossRef]
- Kolhe, N.B.; Ashar, A.Z.; Narayan, K.S.; Asha, S.K. Naphthalene Diimide Copolymers with Oligo(p-phenylenevinylene) and Benzobisoxazole for Balanced Ambipolar Charge Transport. Macromolecules 2014, 47, 2296–2305. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Welford, A.; Komber, H.; Matsidik, R.; Thurn-Albrecht, T.; McNeill, C.R.; Sommer, M. Regioregular Polymer Analogous Thionation of Naphthalene Diimide–Bithiophene Copolymers. Macromolecules 2018, 51, 984–991. [Google Scholar] [CrossRef]
- Ye, L.; Jiao, X.; Zhang, H.; Li, S.; Yao, H.; Ade, H.; Hou, J. 2D-Conjugated Benzodithiophene-Based Polymer Acceptor: Design, Synthesis, Nanomorphology, and Photovoltaic Performance. Macromolecules 2015, 48, 7156–7163. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Earmme, T.; Courtright, B.A.E.; Eberle, F.N.; Jenekhe, S.A. n-Type Semiconducting Naphthalene Diimide-Perylene Diimide Copolymers: Controlling Crystallinity, Blend Morphology, and Compatibility Toward High-Performance All-Polymer Solar Cells. J. Am. Chem. Soc. 2015, 137, 4424–4434. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ueda, M.; Higashihara, T. Synthesis of All-Conjugated Donor–Acceptor–Donor ABA-Type Triblock Copolymers via Kumada Catalyst-Transfer Polycondensation. ACS Macro Lett. 2013, 2, 506–510. [Google Scholar] [CrossRef]
- Kim, Y.; Hong, J.; Oh, J.H.; Yang, C. Naphthalene Diimide Incorporated Thiophene-Free Copolymers with Acene and Heteroacene Units: Comparison of Geometric Features and Electron-Donating Strength of Co-units. Chem. Mater. 2013, 25, 3251–3259. [Google Scholar] [CrossRef]
- Durban, M.M.; Kazarinoff, P.D.; Segawa, Y.; Luscombe, C.K. Synthesis and Characterization of Solution-Processable Ladderized n-Type Naphthalene Bisimide Copolymers for OFET Applications. Macromolecules 2011, 44, 4721–4728. [Google Scholar] [CrossRef]









| Yield % | Mn × 103 g/mol a | Mw × 103 g/mol a | PDI a | T/°C b | HOMO/eV c | LUMO/eV d | ΔEgap/eV e | |
|---|---|---|---|---|---|---|---|---|
| P(NDI-CZ) | 96 | 16.4 | 26.5 | 1.62 | 430 | −5.24 (±0.10) | −3.38 | 1.86 |
| P(NDI-CZL) | 93 | 18.9 | 33.7 | 1.78 | 455 | −5.47 (±0.10) | −3.62 | 1.85 |
| P(NDI-TT) | 96 | 20.2 | 30.1 | 1.50 | 415 | −5.25 (±0.10) | −3.62 | 1.63 |
| P(NDI-TTL) | 93 | 26.0 | 45.7 | 1.76 | 422 | −5.68 (±0.10) | −3.85 | 1.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wu, Y.; Wang, C.; Ma, J.; Wu, F.; Zhang, Y.; Ba, X. Synthesis and Characterization of Fully Conjugated Ladder Naphthalene Bisimide Copolymers. Polymers 2018, 10, 790. https://doi.org/10.3390/polym10070790
Liu F, Wu Y, Wang C, Ma J, Wu F, Zhang Y, Ba X. Synthesis and Characterization of Fully Conjugated Ladder Naphthalene Bisimide Copolymers. Polymers. 2018; 10(7):790. https://doi.org/10.3390/polym10070790
Chicago/Turabian StyleLiu, Feng, Yonggang Wu, Chao Wang, Junshu Ma, Fan Wu, Ye Zhang, and Xinwu Ba. 2018. "Synthesis and Characterization of Fully Conjugated Ladder Naphthalene Bisimide Copolymers" Polymers 10, no. 7: 790. https://doi.org/10.3390/polym10070790