Surface Functionalization by Stimuli-Sensitive Microgels for Effective Enzyme Uptake and Rational Design of Biosensor Setups
Abstract
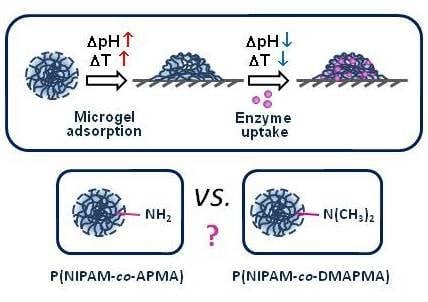
:1. Introduction
2. Materials and Methods
2.1. Laser Microelectrophoresis
2.2. Potentiometric/Conductometric Titration
2.3. Dynamic Light Scattering (DLS)
2.4. Electrokinetic Analysis (EKA)
2.5. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
2.6. Preparation of Surfaces
2.7. Microgel/Enzyme Film Assembling
2.8. Electrochemical Assay
3. Results and Discussion
3.1. Stimuli-Dependent Behavior of Microgels in Aqueous Solutions
3.2. Microgel and Microgel/Enzyme Film Assembling on Solid Surfaces
3.3. Sensor Responses of Microgel/Enzyme Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Retsch, M.; Walther, A.; Loos, K.; Müller, A.H.E. Synthesis of dense poly(acrylic acid) brushes and their interaction with amine-functional silsesquioxane nanoparticles. Langmuir 2008, 24, 9421–9429. [Google Scholar] [CrossRef] [PubMed]
- Pahal, S.; Gakhar, R.; Raichur, A.M.; Varma, M.M. Polyelectrolyte multilayers for bio-applications: Recent advancements. IET Nanobiotechnol. 2017, 11, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Jiang, X.Y.; Yamane, M.; Lee, M.; Goodman, S.; Yang, F. Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J. Mater. Chem. B 2015, 3, 8757–8770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maerten, C.; Jierry, L.; Schaaf, P.; Boulmedais, F. Review of electrochemically triggered macromolecular film buildup processes and their biomedical applications. ACS Appl. Mater. Interfaces 2017, 9, 28117–28138. [Google Scholar] [CrossRef] [PubMed]
- Sigolaeva, L.V.; Pergushov, D.V.; Synatschke, C.V.; Wolf, A.; Dewald, I.; Kurochkin, I.N.; Fery, A.; Müller, A.H.E. Co-assemblies of micelle-forming diblock copolymers and enzymes on graphite substrate for an improved design of biosensor systems. Soft Matter 2013, 9, 2858–2868. [Google Scholar] [CrossRef]
- Sigolaeva, L.V.; Günther, U.; Pergushov, D.V.; Gladyr, S.Yu.; Kurochkin, I.N.; Schacher, F.H. Sequential pH-dependent adsorption of ionic amphiphilic diblock copolymer micelles and choline oxidase onto conductive substrates: Toward the design of biosensors. Macromol. Biosci. 2014, 14, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Gromova, M.S.; Sigolaeva, L.V.; Fastovets, M.A.; Evtushenko, E.G.; Babin, I.A.; Pergushov, D.V.; Amitonov, S.V.; Eremenko, A.V.; Kurochkin, I.N. Improved adsorption of choline oxidase on a polyelectrolyte lbl film in the presence of iodide anions. Soft Matter 2011, 7, 7404–7409. [Google Scholar] [CrossRef]
- Sigolaeva, L.V.; Mergel, O.; Evtushenko, E.G.; Gladyr, S.Yu.; Gelissen, A.P.H.; Pergushov, D.V.; Kurochkin, I.N.; Plamper, F.A.; Richtering, W. Engineering systems with spatially separated enzymes via dual-stimuli-sensitive properties of microgels. Langmuir 2015, 31, 13029–13039. [Google Scholar] [CrossRef] [PubMed]
- Sigolaeva, L.V.; Dubacheva, G.V.; Porus, M.V.; Eremenko, A.V.; Rudakova, E.V.; Makhaeva, G.F.; Richardson, R.J.; Kurochkin, I.N. A layer-by-layer tyrosinase biosensor for assay of carboxylesterase and neuropathy target esterase activities in blood. Anal. Methods 2013, 5, 3872–3879. [Google Scholar] [CrossRef]
- Sigolaeva, L.V.; Gladyr, S.Y.; Gelissen, A.P.H.; Mergel, O.; Pergushov, D.V.; Kurochkin, I.N.; Plamper, F.A.; Richtering, W. Dual-stimuli-sensitive microgels as a tool for stimulated spongelike adsorption of biomaterials for biosensor applications. Biomacromolecules 2014, 15, 3735–3745. [Google Scholar] [CrossRef] [PubMed]
- Sigolaeva, L.; Makhaeva, G.; Rudakova, E.; Boltneva, N.; Porus, M.; Dubacheva, G.; Eremenko, A.; Kurochkin, I.; Richardson, R.J. Biosensor analysis of blood esterases for organophosphorus compounds exposure assessment: Approaches to simultaneous determination of several esterases. Chem.-Biol. Interact. 2010, 187, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Sigolaeva, L.V.; Gladyr, S.Y.; Mergel, O.; Gelissen, A.P.H.; Noyong, M.; Simon, U.; Pergushov, D.V.; Kurochkin, I.N.; Plamper, F.A.; Richtering, W. Easy-preparable butyrylcholinesterase/microgel construct for facilitated organophosphate biosensing. Anal. Chem. 2017, 89, 6092–6099. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Gao, Y.F.; Li, X.; Serpe, M.J. Responsive polymers for biosensing and protein delivery. J. Mater. Chem. B 2014, 2, 2444–2451. [Google Scholar] [CrossRef]
- Islam, M.R.; Serpe, M.J. Polyelectrolyte mediated intra and intermolecular crosslinking in microgel-based etalons for sensing protein concentration in solution. Chem. Commun. 2013, 49, 2646–2648. [Google Scholar] [CrossRef] [PubMed]
- Plamper, F.A.; Richtering, W. Functional microgels and microgel systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wellert, S.; Richter, M.; Hellweg, T.; von Klitzing, R.; Hertle, Y. Responsive microgels at surfaces and interfaces. Z. Phys. Chem.-Int. J. Res. Phys. Chem. Chem. Phys. 2015, 229, 1225–1250. [Google Scholar] [CrossRef]
- Serpe, M.J.; Kim, J.; Lyon, L.A. Colloidal hydrogel microlenses. Adv. Mater. 2004, 16, 184–187. [Google Scholar] [CrossRef]
- Fernandes, P.A.L.; Schmidt, S.; Zeiser, M.; Fery, A.; Hellweg, T. Swelling and mechanical properties of polymer gels with cross-linking gradient. Soft Matter 2010, 6, 3455–3458. [Google Scholar] [CrossRef]
- Schmidt, S.; Zeiser, M.; Hellweg, T.; Duschl, C.; Fery, A.; Möhwald, H. Adhesion and mechanical properties of PNIPAM microgel films and their potential use as switchable cell culture substrates. Adv. Funct. Mater. 2010, 20, 3235–3243. [Google Scholar] [CrossRef]
- Mergel, O.; Gelissen, A.P.H.; Wünnemann, P.; Böker, A.; Simon, U.; Plamper, F.A. Selective packaging of ferricyanide within thermoresponsive microgels. J. Phys. Chem. C 2014, 118, 26199–26211. [Google Scholar] [CrossRef]
- Mergel, O.; Wünnemann, P.; Simon, U.; Böker, A.; Plamper, F.A. Microgel size modulation by electrochemical switching. Chem. Mater. 2015, 27, 7306–7312. [Google Scholar] [CrossRef]
- Walta, S.; Pergushov, D.V.; Oppermann, A.; Steinschulte, A.A.; Geisel, K.; Sigolaeva, L.V.; Plamper, F.A.; Wöll, D.; Richtering, W. Microgels enable capacious uptake and controlled release of architecturally complex macromolecular species. Polymer 2017, 119, 50–58. [Google Scholar] [CrossRef]
- Bysell, H.; Mansson, R.; Hansson, P.; Malmsten, M. Microgels and microcapsules in peptide and protein drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M.; Bysell, H.; Hansson, P. Biomacromolecules in microgels-opportunities and challenges for drug delivery. Curr. Opin. Colloid Interface Sci. 2010, 15, 435–444. [Google Scholar] [CrossRef]
- Herman, K.; Lang, M.E.; Pich, A. Tunable clustering of magnetic nanoparticles in microgels: Enhanced magnetic relaxivity by modulation of network architecture. Nanoscale 2018, 10, 3884–3892. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, S.F.; Filizzola, J.O.C.; Oliveira, P.F.M.; Silva, T.M.; Lara, B.R.; Lopes, M.V.; Rossi-Bergmann, B.; Elaissari, A.; Santos, A.M. Fabrication of biocompatible and stimuli-responsive hybrid microgels with magnetic properties via aqueous precipitation polymerization. Mater. Lett. 2016, 175, 296–299. [Google Scholar] [CrossRef]
- Müller, M.B.; Kuttner, C.; König, T.A.F.; Tsukruk, V.V.; Förster, S.; Karg, M.; Fery, A. Plasmonic library based on substrate-supported gradiential plasmonic arrays. ACS Nano 2014, 8, 9410–9421. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Tebbe, M.; Andreeva, D.V.; Karg, M.; Alvarez-Puebla, R.A.; Pazos-Perez, N.; Fery, A. Large-area organization of PNIPAM-coated nanostars as SERS platforms for polycyclic aromatic hydrocarbons sensing in gas phase. Langmuir 2012, 28, 9168–9173. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, A.; Ricciardi, A.; Giaquinto, M.; Micco, A.; Bobeico, E.; La Ferrara, V.; Ruvo, M.; Cutolo, A.; Cusano, A. Microgel assisted lab-on-fiber optrode. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gelissen, A.P.H.; Schmid, A.J.; Plamper, F.A.; Pergushov, D.V.; Richtering, W. Quaternized microgels as soft templates for polyelectrolyte layer-by-layer assemblies. Polymer 2014, 55, 1991–1999. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide)-experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Nagel, J.; Kroschwald, F.; Bellmann, C.; Schwarz, S.; Janke, A.; Heinrich, G. Immobilisation of different surface-modified silica nanoparticles on polymer surfaces via melt processing. Colloids Surf. A-Physicochem. Eng. Asp. 2017, 532, 208–212. [Google Scholar] [CrossRef]
- Dragan, E.S.; Schwarz, S.; Eichhorn, K.J. Specific effects of the counterion type and concentration on the construction and morphology of polycation/azo dye multilayers. Colloids Surf. A-Physicochem. Eng. Asp. 2010, 372, 210–216. [Google Scholar] [CrossRef]
- Schwarz, S.; Nagel, J.; Jaeger, W. Comparison of polyelectrolyte multilayers built up with polydiallyldimethylammonium chloride and poly(ethyleneimine) from salt-free solutions by in-situ surface plasmon resonance measurements. Macromol. Symp. 2004, 211, 201–216. [Google Scholar] [CrossRef]
- Voinova, M.V.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: Continuum mechanics approach. Phys. Scr. 1999, 59, 391–396. [Google Scholar] [CrossRef]
- Stieger, M.; Richtering, W.; Pedersen, J.S.; Lindner, P. Small-angle neutron scattering study of structural changes in temperature sensitive microgel colloids. J. Chem. Phys. 2004, 120, 6197–6206. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.; Bruno, N.; Vincent, B. Distribution of CdSe quantum dots within swollen polystyrene microgel particles using confocal microscopy. Langmuir 2005, 21, 2750–2753. [Google Scholar] [CrossRef] [PubMed]
- Eremeev, N.L.; Sigolaeva, L.V.; Kazanskaya, N.F. A formal kinetic description of the temperature behavior of alpha-chymotrypsin entrapped into N-isopropylacrylamide gel. Vestn. Mosk. Univ. Seriya 2 Khimiya 1992, 33, 511–515. [Google Scholar]
- Eremeev, N.L.; Sigolaeva, L.V.; Simakov, P.A.; Kazanskaya, N.F. Temperature dependences of equilibrium and kinetic constants of enzymatic-reactions during a phase-transition in a thermosensitive matrix. Biochem.-Moscow 1995, 60, 991–999. [Google Scholar]
- Sigolaeva, L.V.; Eremeev, N.L.; Kazanskaya, N.F. An anomalous temperature-dependence of immobilized alpha-chymotrypsin preparations. Bioorganicheskaya Khimiya 1994, 20, 268–273. [Google Scholar] [PubMed]






| P(NIPAM-co-APMA) | P(NIPAM-co-DMAPMA) | |||
|---|---|---|---|---|
| Temperature of the Microgel Adsorption | Mass of the Microgel Film, ng/cm2 | Mass of the Enzyme Film, ng/cm2 | Mass of the Microgel Film, ng/cm2 | Mass of the enzyme Film, ng/cm2 |
| 25 °C | 3960 ± 380 | 1840 ± 620 | 4770 ± 510 | 3510 ± 1300 |
| 50 °C | 2310 ± 830 1 5080 ± 650 2 | 5290 ± 1440 | 6410 ± 1360 1 10610 ± 1640 2 | 15780 ± 1100 |
| Temperature of the Microgel Adsorption | Time of the Enzyme Uptake | Sensor Response to 10−5 M of Choline, nA | |
|---|---|---|---|
| P(NIPAM-co-APMA)/ChO | P(NIPAM-co-DMAPMA)/ChO | ||
| 25 °C | 10 min | 36 ± 7 | 29 ± 3 |
| 50 °C | 10 min | 46 ± 11 | 100 ± 13 |
| 45 min | 85 ± 34 | – | |
| Biosensor Construct | Treatment 1 | Operational Stability, ∆, % |
|---|---|---|
| SPE/MnO2/P(NIPAM-co-APMA)/ChO | + GA | −0.16 ± 0.13 |
| − GA | −3.53 ± 1.12 | |
| SPE/MnO2/P(NIPAM-co-DMAPMA)/ChO | + GA | −4.20 ± 0.79 |
| − GA | −4.21 ± 0.78 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigolaeva, L.V.; Pergushov, D.V.; Oelmann, M.; Schwarz, S.; Brugnoni, M.; Kurochkin, I.N.; Plamper, F.A.; Fery, A.; Richtering, W. Surface Functionalization by Stimuli-Sensitive Microgels for Effective Enzyme Uptake and Rational Design of Biosensor Setups. Polymers 2018, 10, 791. https://doi.org/10.3390/polym10070791
Sigolaeva LV, Pergushov DV, Oelmann M, Schwarz S, Brugnoni M, Kurochkin IN, Plamper FA, Fery A, Richtering W. Surface Functionalization by Stimuli-Sensitive Microgels for Effective Enzyme Uptake and Rational Design of Biosensor Setups. Polymers. 2018; 10(7):791. https://doi.org/10.3390/polym10070791
Chicago/Turabian StyleSigolaeva, Larisa V., Dmitry V. Pergushov, Marina Oelmann, Simona Schwarz, Monia Brugnoni, Ilya N. Kurochkin, Felix A. Plamper, Andreas Fery, and Walter Richtering. 2018. "Surface Functionalization by Stimuli-Sensitive Microgels for Effective Enzyme Uptake and Rational Design of Biosensor Setups" Polymers 10, no. 7: 791. https://doi.org/10.3390/polym10070791