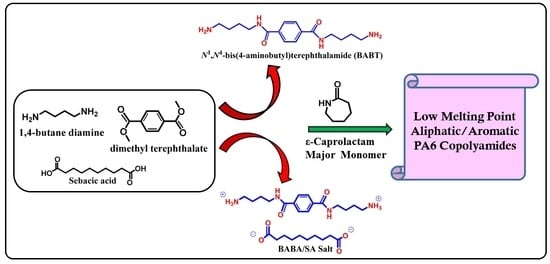
3.2. Synthesis and Structure Analysis of Aliphatic-Aromatic Copolyamides
For the first time, new aliphatic-aromatic copolyamides were synthesized via melt polymerization of different wt % of long-chain aromatic BABT/SA salts with CPL, as described in detail in the above experimental section (
Scheme 3,
Scheme 4 and
Scheme 5). Three expected mechanisms for the preparation of PA6-BABT/SA random copolymides are proposed and shown in
Scheme 3,
Scheme 4 and
Scheme 5. The prepolymerization was first allowed to proceed at 235 °C for three hours. In
Scheme 3, three step reactions are required for the PA6 copolymerization. In Step 1, 6-aminocaproic acid (ACS) acts as an activator to initiate the polymerization reaction. In this typical reaction (Step 1), the free proton of COOH present in the ACS first transfers to the carbonyl of CPL to form an active center. Then, the amine nitrogen or amine end group of the growing chain of ACS attack the carbonium ion of CPL through a nucleophilic addition reaction and rapidly initiate the following poly-addition. In Step 2, the NH
3+ (quaternary ammonium) group in the BABT/SA salt reacts with a COO
− group through a self-condensation reaction and initiate the prepolymerization. In Step 3, by increasing the temperature to 260 °C, the chain is terminated by the poly-condensation of amine and carboxylic end groups.
As shown in
Scheme 4, the BABT/SA salt acted as a catalyst and initiated the copolymerization reaction. In this typical reaction, in the presence of BABT/SA salt, the free proton of NH
3+ in the BABT/SA salt first transfers to the carbonyl of ε-caprolactam and rapidly initiates the poly-addition. The two amino groups of BABT more likely initiate the nucleophilic addition to extend the molecular chain on both sides (
Scheme 5). Following that, poly-condensation occurs between the linear molecules and SA unit to further increase the molecular weight of the polymer. SA might be randomly arranged in the copolyamides after amide exchange [
35,
36].
Scheme 3,
Scheme 4 and
Scheme 5 outline the most likely synthetic paths based on the polymerization chemistry.
The chemical structure of all polyamides were confirmed by
1H NMR, FT-IR, and
13C NMR spectroscopy.
Figure 4 shows the representative
1H NMR spectra of neat PA 6. The peaks in the region of 3.61 ppm and 2.82 ppm were ascribed to the chemical shifts of the methyl protons adjacent to the amino group (
CH2–NH–CO–) and the carbonyl group (NH–CO–
CH2) of PA6, respectively. The proton signals in the 2.08–1.30 ppm region are attributed to the other protons of the aliphatic methylene units of PA6. After the reaction of PA6 with BABT/SA, the new peaks appeared in the region of 7.78, 3.50, and 2.54 ppm, indicating the successful incorporation of BABT/SA salt into the PA6. The adding ratio of BABT/SA salt varied from 10 to 30 wt %, and the peak intensity of the region at 7.78 ppm and 2.54 ppm increased correspondingly. Based on the above discussions, the PA6-based copolymer was successfully synthesized, in which the BABT/SA segments were randomly arranged.
The FT-IR spectra of PA6 and PA6-BABT/SA copolymers are shown in
Figure 5. PA6-BABT/SA copolyamides exhibited almost identical infrared characteristics as PA6. The absorption band at 3302 cm
−1 is ascribed to the hydrogen bonded V
N–H stretching vibration. The coupled absorption band at 2857 (symmetric) and 2952 cm
−1 (asymmetric) are attributed to the stretching vibration of V
C–H. The signals at 1630, 1536, 1460, 1255, and 686 cm
−1 are attributed to amide-I (V
C=O), amide-II (V
C–N stretch and V
N–H band), amide-III (V
C–N and δ
C–H), amide-IV (V
C–C=O), and amide-V (V
N–H, out-of-plane bonding vibration), respectively [
37]. Nonetheless, for the copolyamides with an increasing weight ratio of BABT/SA, these amide absorption bands appeared weaker, which denotes a reduction in the crystallinity order. The FT-IR and
1H NMR results suggest that the desirable PA6-BABT/SA copolyamides were successfully synthesized.
In order to obtain more detailed information about the structure of PA6-BABT/SA copolyamides,
13C NMR was performed.
13C NMR spectra were also in good agreement with the expected structures, as shown in
Figure 6 and
Figure 7. The chemical shift and assignments are summarized in
Table 1.
Figure 8A displays the
13C NMR spectra of BABT/SA homopolymer, neat PA6, and PA6-BABA
30. Based on the carbon resonances of BABT-SA and PA6, the five methylene carbon peaks located at 41.6, 42.3, 42.7, 42.9, and 43.4 ppm are present in the spectra of the copolyamides (
Figure 8), which correspond to the five possible dyad arrangements of the PA6 and BABT/SA units (BABT, CPL-CPL, CPL-SA, BABT-CPL, and BABT-SA) along the polymer chain. The chemical structures and peak areas of CPL-CPL, BABT, BABT-SA, BABT-CPL, and CPL-SA dyad sequences in the PA6-BABT/SA copolyamides are shown in
Figure 8B. These results enabled the calculation of the degree of randomness (R) of PA6-BABT/SA copolyamides using Equations (2)–(5) [
23]:
Then, Equation (6) was used to calculate the molar percentage of BABT/SA salt present in the PA6-BABT/SA copolymers:
where
,
, and
represent the molar fractions of the total amounts of PA6-BABT/SA copolyamides, CPL, and BABT/SA salt, respectively.
,
PA6 and B represent the peak areas of the corresponding shifts. R represents the degree of randomness. The corresponding calculated results are listed in
Table 2.
An R value greater than one indicates that the units have a more alternating tendency. When R is less than one, the units tend to cluster in homogenous sequences and thus the copolymer exhibits a block character. When R is two, then a completely alternating copolymer is present. In the case of R being zero, the copolymer is a pure block copolymer, or the system is a mixture of two polyamides. As presented in the last column of
Table 2, the total degree of randomness (R) for PA6-BABT/SA
20 and PA6-BABT/SA
30 was calculated as 1.05 and 1.03, respectively, which reveals that the random copolyamides were prepared.
It was not possible to calculate the composition of PA6-BABT/SA10 copolyamide from 13C NMR due to the very low intensities of the BABT/SA salt peaks.
3.3. Intrinsic Viscosity and Molecular Weight
The viscosity method was used to measure the single point intrinsic viscosities of PA6-BABT/SA copolyamides in concentrated sulfuric acid (96%) using an Ubbelohde viscometer.
Mn,
Mw,
Mp, and PDI (polydispersity index) were measured using GPC.
Mp is the molecular weight corresponding to that of the maximum of the chromatographic peak. Additionally, Mark-Houwink constants
K and
α, in Equation (7), were obtained from log-log plot of single point intrinsic viscosity versus
Mw (GPC measured):
where
K and α are constants associated with the interaction between the polymer and the solvent. Mark-Houwink constants were obtained from the slope and intercept of the plot as
K = 2.169 × 10
−4 and α = 0.778 for sulfuric acid (96%). An α value greater than 0.75 is typical for good solvents.
Table 3 shows the results obtained from the PA6-BABT/SA copolyamides, whereas
Figure 9 shows the chromatographs for the neat PA6 and PA6-BABT/SA copolyamides.
Intrinsic viscosities and molecular weights of the copolyamides increased with the increasing incorporation of BABT/SA, reaching a maximum value when BABT/SA content was 10 wt %. The value then began to decrease when BABT/SA was above 20 to 30 wt %. Because the polymerization rate of the aromatic BABT/SA salt was faster than that of the neat PA6, the copolymerization rate thus increased with the incorporation of 10 wt % aromatic BABT/SA salt, which resulted in a higher molecular weight copolymer, reflected by the greater intrinsic viscosity. However, the copolymerization may occur in the presence of an excessive amount of BABT/SA salt, inhibiting the block-polymerization process and reducing the molecular mass of the copolyamides. On the other hand, polyamide salts from BABT and SA have a catalytic influence on the polymerization of CPL. Thus, the polymerization reaction with an increasing amount of BABT/SA was much faster, and the molecular weight and intrinsic viscosity were lower than those of the neat PA6. Moreover, by varying the weight content of BABT/SA in the reactants, the intrinsic viscosity of the final products ranged from 1.33 to 0.94 dL/g and the
Mn as well as
Mw varied from 30,900 to 23,600 Da and 77,800 to 48,700 Da, respectively. The range of PDI for the PA6-BABT/SA copolyamides was between 2.11 and 2.06. Generally, PA6 with
Mn higher than 13,000, endows good physical and mechanical properties, which specifically befits applications in plastic, membrane, and textile yarn, and so on [
38].
3.4. Thermal Properties
DSC and TGA techniques were applied to investigate the melting temperature and thermal stability of BABT, BABT/SA, and polyamides, and the results are summarized in
Table 4. The polymerization process strongly depends on the thermal properties of the synthesized monomers.
Figure 10A shows that the
Tm of BABT is 217.1 °C. From the TGA thermogram (
Figure 10B), BABT shows a decomposition temperature (
Td) at 5 wt % loss (
T5%) at 194.25 °C (elimination of water) and 56 wt % loss at 431 °C. DTG (differential thermogravimetric) curves of BABT (
Figure 10B) showed two main peaks with maximum degradation temperatures (
Tmax) of 203.3 and 447.7 °C.
Figure 10C,D demonstrate that the
Tm of BABT/SA is 231.4 °C and the
T5% (5 wt % loss) is 221.8 °C (elimination of water). BABT/SA presents a
Tmax at 223.1 and 457.6 °C (
Figure 10D).
The thermal and melt crystallization properties of the polymers directly influence their usage and processing performance, which were also analyzed by DSC. The DSC traces are shown in
Figure 11 and the data extracted from these traces are summarized in
Table 4. The
Tm of the copolymer decreased from 210.7 to 152.5 °C (
Figure 11A–D) when the addition of BABT/SA salt increased from 10 to 30 wt %. Similarly, the observed melting enthalpies (Δ
Hm, and hence the crystallinity X
c%) were lower (46.9 to 38.6 J/g) compared to that of neat PA6 (51.1 J/g). Furthermore, the melting endotherms of copolyamides containing 20 to 30 wt % BABT/SA were broad in contrast to the reference PA6. The broadening of the melting endotherms is related to a larger distribution of crystallinity and crystal perfection. These results indicate that neat PA6 is not co-crystalline with the BABT/SA unit. The reduction in the
Tm of the copolyamides may be ascribed to the existence of an aromatic moiety in the polymer main chain, which interrupts the intermolecular hydrogen bonding and destroys the molecular chain regularity, therefore resulting in a crystallization reduction. This indicates that BABT/SA series up to 30 wt % act as impurities, which inhibit the construction of PA6 crystals. The lower
Tm may contribute to the mobility of copolyamides and improve its processability.
We noticed that the DSC curves of the copolymers with 10 to 30 wt % BABA/SA salt displayed a narrow crystallization peak from 171.6 to 113.4 °C, as shown in
Figure 11A–D. Beyond that, the DSC traces in
Table 4 show that the crystallinity of all PA6-BABT/SA copolyamides is lower than that of neat PA6, and decreases with escalating BABT/SA weight content. As such, good transparency of these copolymers is highly expected. The large steric hindrance of aromatic radicals and the copolymerization disrupting the well-defined structures and hydrogen bonding may be responsible for this observation. In addition, the
Tm of the commercially available PA11 and PA12 are about 190 and 180 °C [
39], respectively, which is almost the same as the PA6-BABT/SA
10 and PA6-BABT/SA
20 copolyamides.
The thermal stability of the synthesized copolyamides was assessed using TGA analysis. The temperature of 5% weight loss (
T5%) and the maximum decomposition temperature (
Tmax) of these copolyamides are listed in
Table 4. No significant transformation was observed in the curves of
Figure 12A by varying BABT/SA weight ratios, which indicated that the incorporation of aromatic moieties into the PA6 backbone was noticeably favorable to the thermal stability of the resulting copolymers. The apparent weight loss occurred in the range of 150 to 180 °C due to the water molecules absorbing on the surface of the polymer [
40]. The copolyamides were thermally degraded, mainly through a simple step process with a 5% weight loss above 350 °C and a 95% weight loss above 430 °C. DTG curves (
Figure 12B) show that the
Tmax of the copolyamides was observed in the range of 453.4 to 446.3 °C. The thermal stability of the copolyamides is similar to the commercially available PA6 because of the presence of an aromatic radical in the main chain of the copolymer, which indicates the novel series of copolyamides are suitable for most common applications.
3.6. Thermo-Mechanical Properties of Copolyamides
DMA is an important and effectual method to study the thermo-mechanical properties of the polymers. The glass transition temperature (
Tg), the most salient index to polyamides, is determined from the tan δ maximum.
Tg is intimately related with the composition and comonomer sequence of a copolymer, which is a microscopic expression of internal molecules varying between moving and freezing states [
43]. As shown in
Figure 14A, the
Tg of PA6 is 68.5 °C, which is higher than long chain aliphatic polyamides. For example, the
Tg of PA 1010, PA 1012, and PA 510 are only in the range of 40 to 50 °C [
44]. The molecular structure of PA6 contains more amide bonds with a semicrystalline nature, which limit the appearance of potential molecular chain compatibility to reduce the flexibility of the polymer chain. Therefore, more power is consumed to move the molecular chain from a freezing state, which increases the
Tg value of PA6. When 10 to 30 wt % BABT/SA was introduced into PA6, the reduction in crystallinity decreased the
Tg of PA6-BABT/SA copolyamides. The aromatic region of the BABT/SA units is reflected in the
Tg values, where three copolyamides exhibit different
Tg temperatures, ranging from 49.1 to 31.2 °C (
Figure 14B–D). The
Tg of the copolyamides are around human body temperature, which makes a polymer useful for shape memory applications in the medical field [
45]. The transition of
Tg to the room temperature range by varying the weight ratio of comonomer (BABT/SA) helps to adjust the working temperature range of the shape memory polymers. Furthermore, the tan δ values are almost the same as obtained by the loss modulus (E”) shown in
Figure 15A. The values are listed in
Table 5. In addition, the lower storage modulus (E’) of the BABT/SA based copolyamides compared to the linear aliphatic reference polyamide (PA6) indicates that the incorporation of BABT/SA decreases the crystallinity and enhances the flexibility of the copolymers. These results clearly show that the chemistry of BABT/SA salt plays a major role in tuning the thermal properties of the copolyamides.
3.7. Mechanical Properties
The typical stress-strain curves for the PA6-BABT/SA and reference PA6 polymers are shown in
Figure 15B. The results of the tensile testing are listed in
Table 5. PA6 has good tensile strength but the ductility and elasticity are poor. The reason for adding the long-chain aromatic polyamide salt is to improve the tensile strength and ductility of PA6. The mechanical properties of the PA6-BABT/SA copolyamides principally depend on the composition and molecular weight of the copolymers. Therefore, the effect of different weight contents of BABT/SA salt segments on their mechanical properties was analyzed using tensile measurements. As shown in
Figure 15B, PA6 has a tensile strength of 69.3 MPa. The addition of 10 wt % BABT/SA salt into the PA6 matrix increases the tensile strength of the copolymer (PA6-BABT/SA
10) up to 105.5 MPa due to the high reinforcing ability of aromatic BABT/SA salt. The improvement in the ductility of copolyamides could be ascribed to the insertion of partially aromatic long chain comonomer into PA6 [
46]. For PA6-BABT/SA copolyamides, the stress-strain curves showed a similar shape, and the tensile strength gradually dropped (105.2 to 46.6 MPa) with increasing weight content of BABT/SA salt segments. However, a superior strain at break was observed with the increased BABT/SA salt segments. The strains at break of PA6-BABT/SA copolyamides were 30.1%, 136.7% and 218.3% when the weight content of BABT/SA units was 10, 20 and 30 wt %, respectively. The strain at break of PA6-BABT/SA
30 improved by 200% and the tensile strength dropped by about 36%, when compared to neat PA6, due to the random structure of PA6-BABT/SA
20 and PA6-BABA/SA
30 copolyamides. Inserting increasing weight content of BABT/SA segments randomly arranged the PA6 backbone. Therefore, the tensile strength gradually decreased. It is reasonable that the tensile strength would drop with the addition of above 10 wt % BABT/SA salt. Besides, the reduction in crystallinity and the change observed from the α phase to γ phase, in agreement with the DSC and XRD results, could explain the significant reduction in the tensile strength [
41]. Nevertheless, loss in tensile strength is exchanged for a considerable increase in the strain at break. These results clearly show that PA6-BABT/SA copolyamides have outstanding mechanical properties compared to neat PA6 and commercially available PA66 [
47].
3.9. Transparency of Copolyamides
The UV spectrum of PA6-BABT/SA copolyamides are shown in
Figure 16A. The traces revealed that, along with the increased incorporation ratio of BABT/SA, both the intensity and area of the UV absorption peak decreased substantially. The transmittance value measured using a UV-transmission spectra is illustrated in Figure16B. The cut of the wave length values (λ
0) of these copolymides were in the range of 280 to 310 nm. The transmittance of all the polyamide films was measured at 325 nm. The results shown in
Figure 16C indicate that the transmittance of the copolymer films essentially continued to increase, and the maximum was 83.5% as the weight content of BABT/SA increased to 30 wt %. For all different ratios, the transparency of the as-synthesized copolymer was better than that of neat PA6. The traces are consistent with the XRD analysis and crystallinity data obtained from DSC of copolyamides. This also demonstrates that the transparency of the copolyamides could be improved considerably by adding 10 to 30 wt % BABT/SA segments. Specifically, as-synthesized copolyamides and the neat PA6, shaped to films with the same thickness of 30 μm, were stuck on a colored picture and photographed. The transparency images of representative PA6-BABT/SA copolymers and the reference PA6 are shown in
Figure 17. Compared with the original picture shown in
Figure 17A, all the copolymer films were colorless, whereas the reference film (PA6) was very slightly yellow, as illustrated in
Figure 17B. This indicates that the PA6 homopolymer is easily oxidized at high temperatures. Additionally, as shown in
Figure 17C, the polymer film had little wrinkles. When the content of BABT/SA salt increased from 10 to 30 wt %, the wrinkles disappeared and the copolymer films became more transparent [
34].