Immobilizing Laccase on Modified Cellulose/CF Beads to Degrade Chlorinated Biphenyl in Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
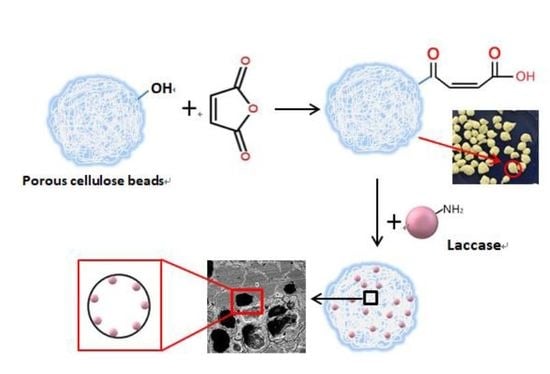
2.2. Preparation and Modification of the Cellulose/CF Beads
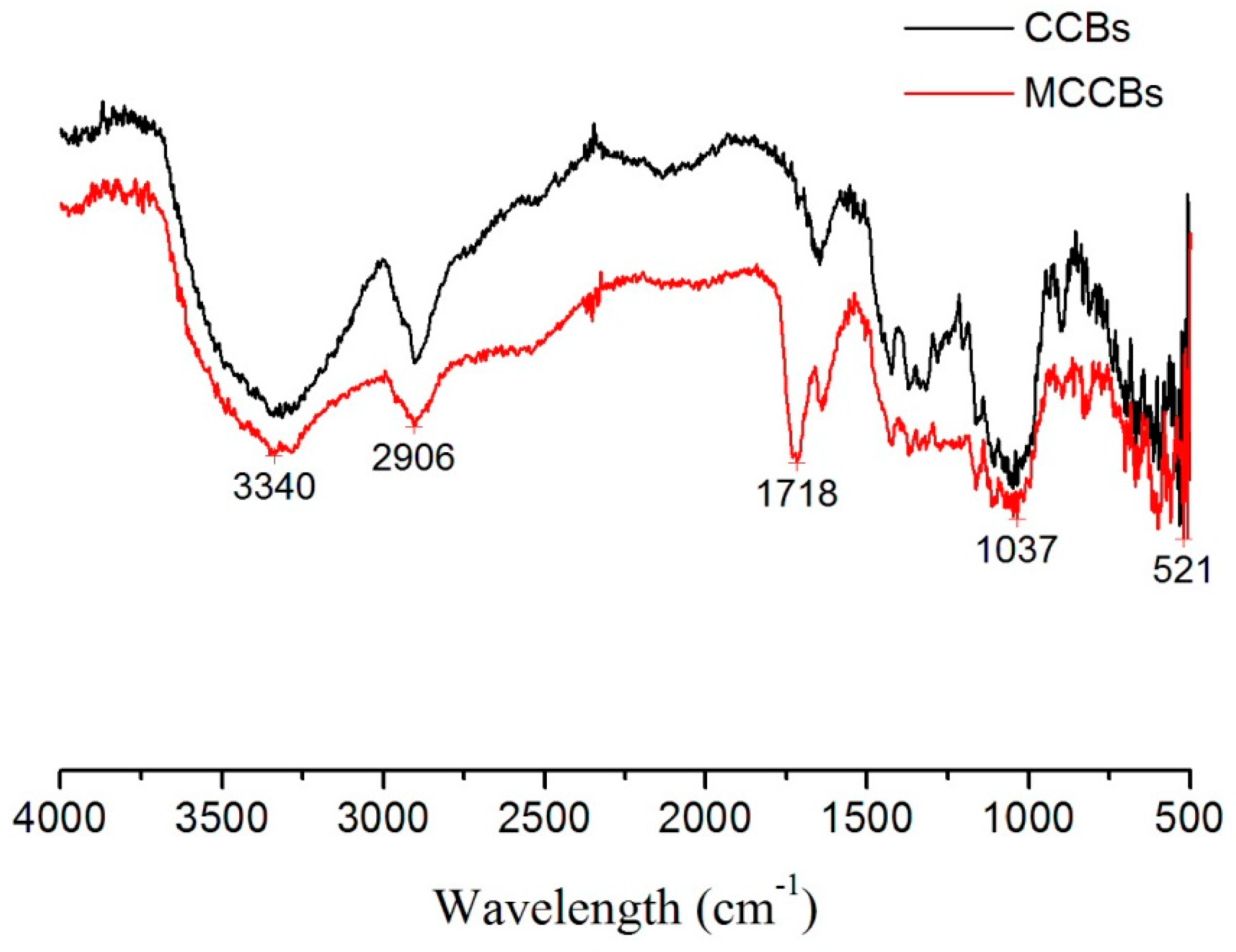
2.3. Characterization of MCCBs
2.4. Determination of Laccase Activity
2.5. Immobilization of Laccase
2.6. Thermal and Operational Stability of Immobilized Laccase
2.7. Degradation of the PCBs
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of MCCBs
3.2. Optimum Laccase Immobilization Conditions
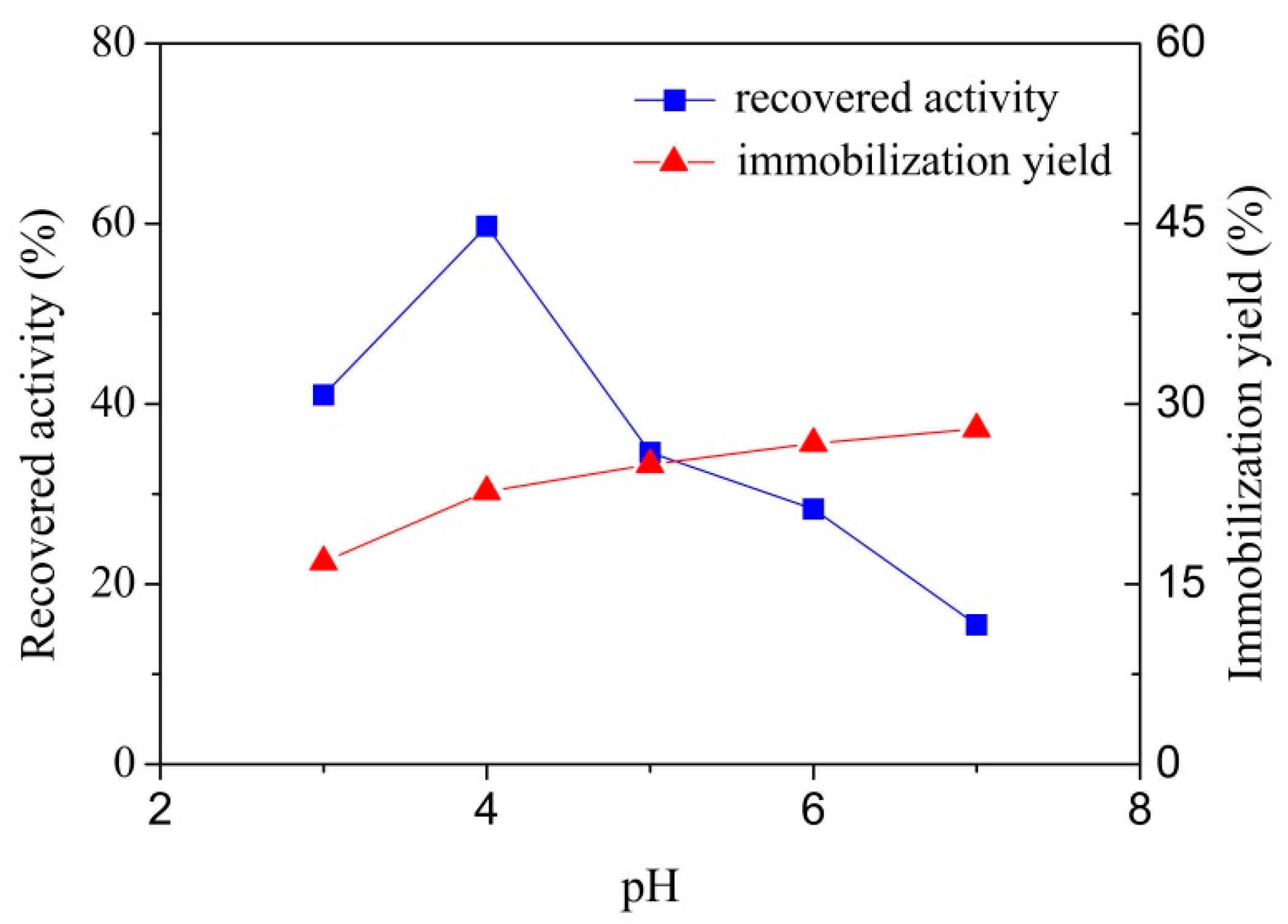
3.2.1. Effect of pH on Laccase Immobilization
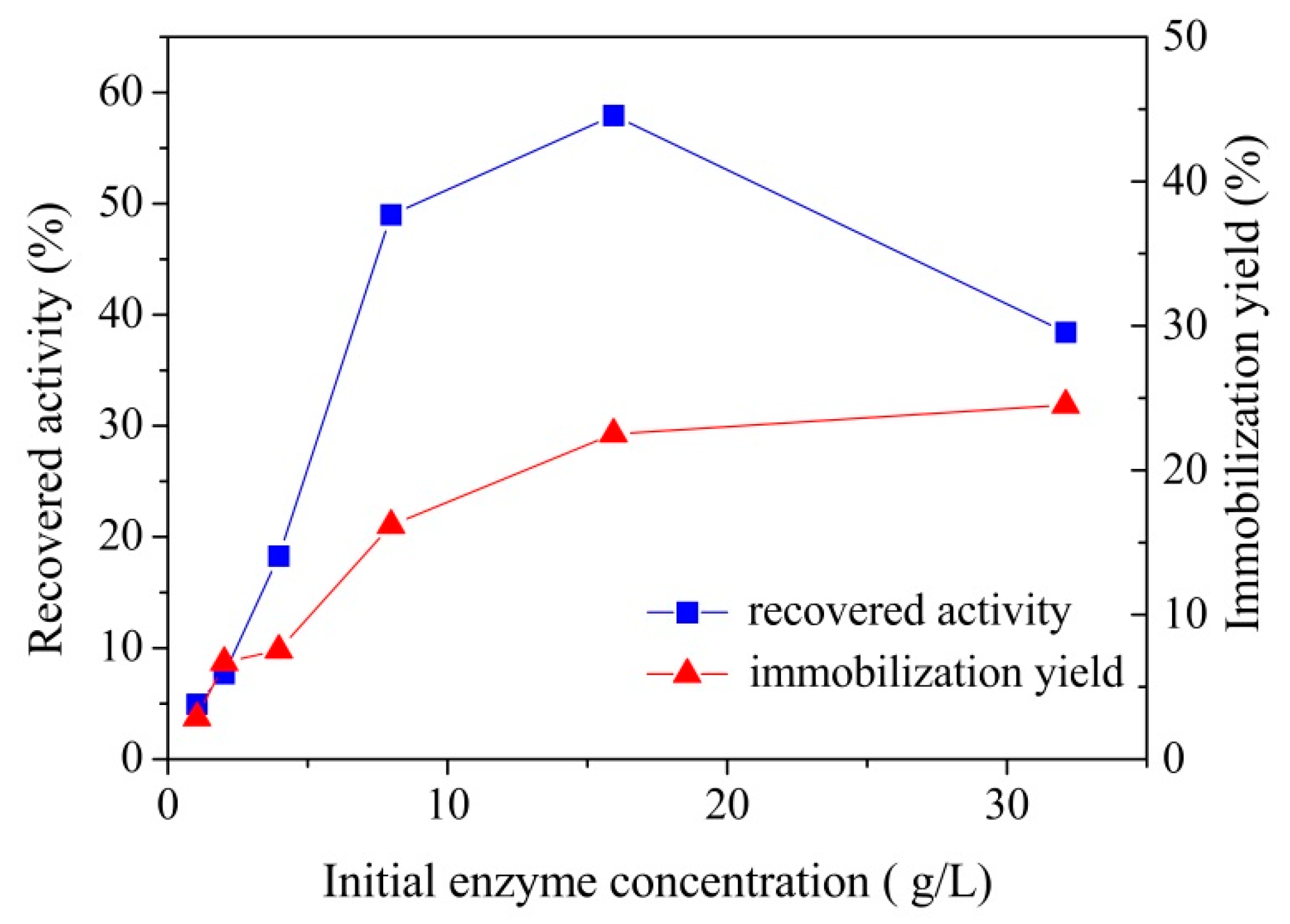
3.2.2. Effect of Initial Enzyme Concentration on Laccase Immobilization
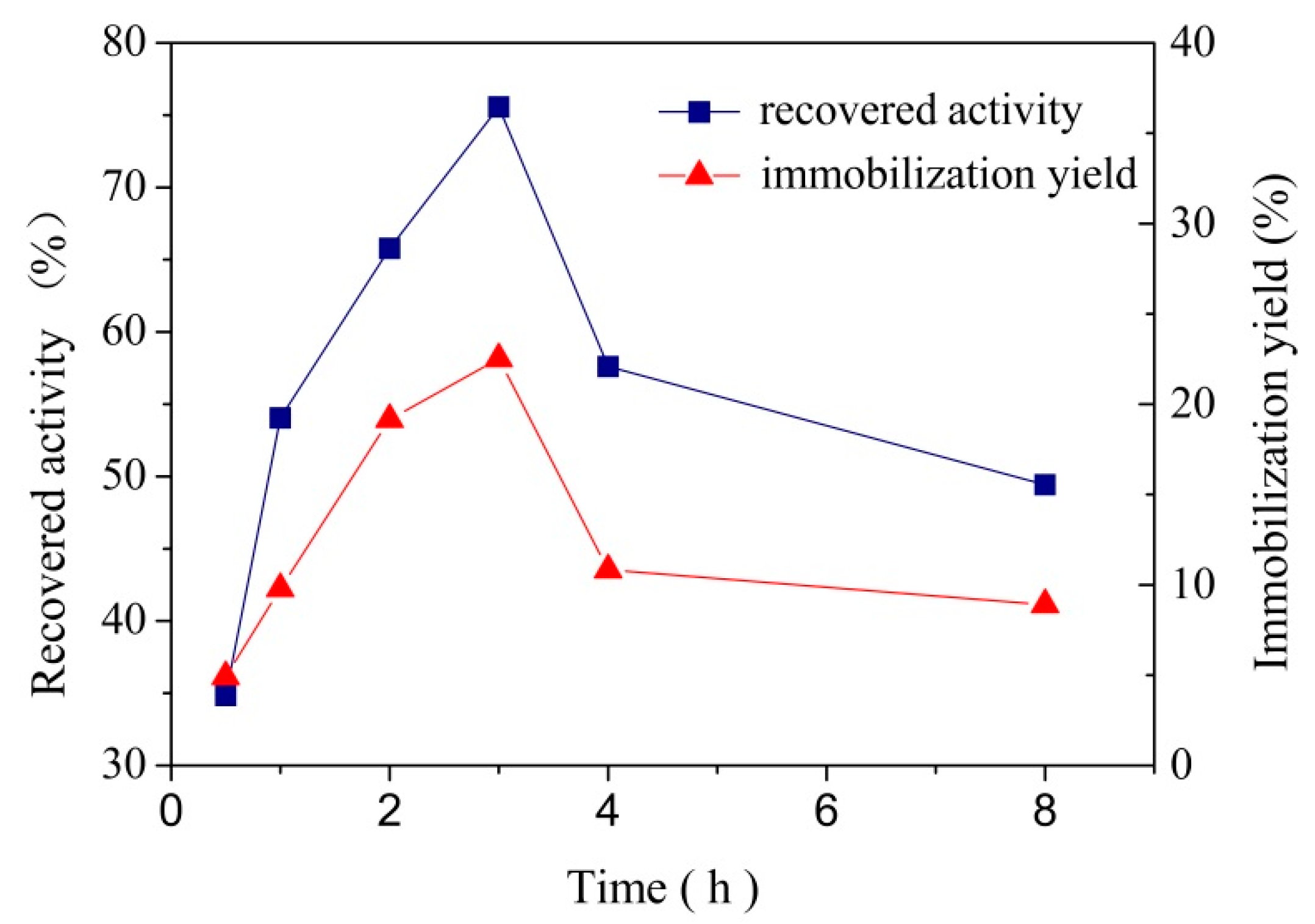
3.2.3. Effect of Contact Time on Laccase Immobilization
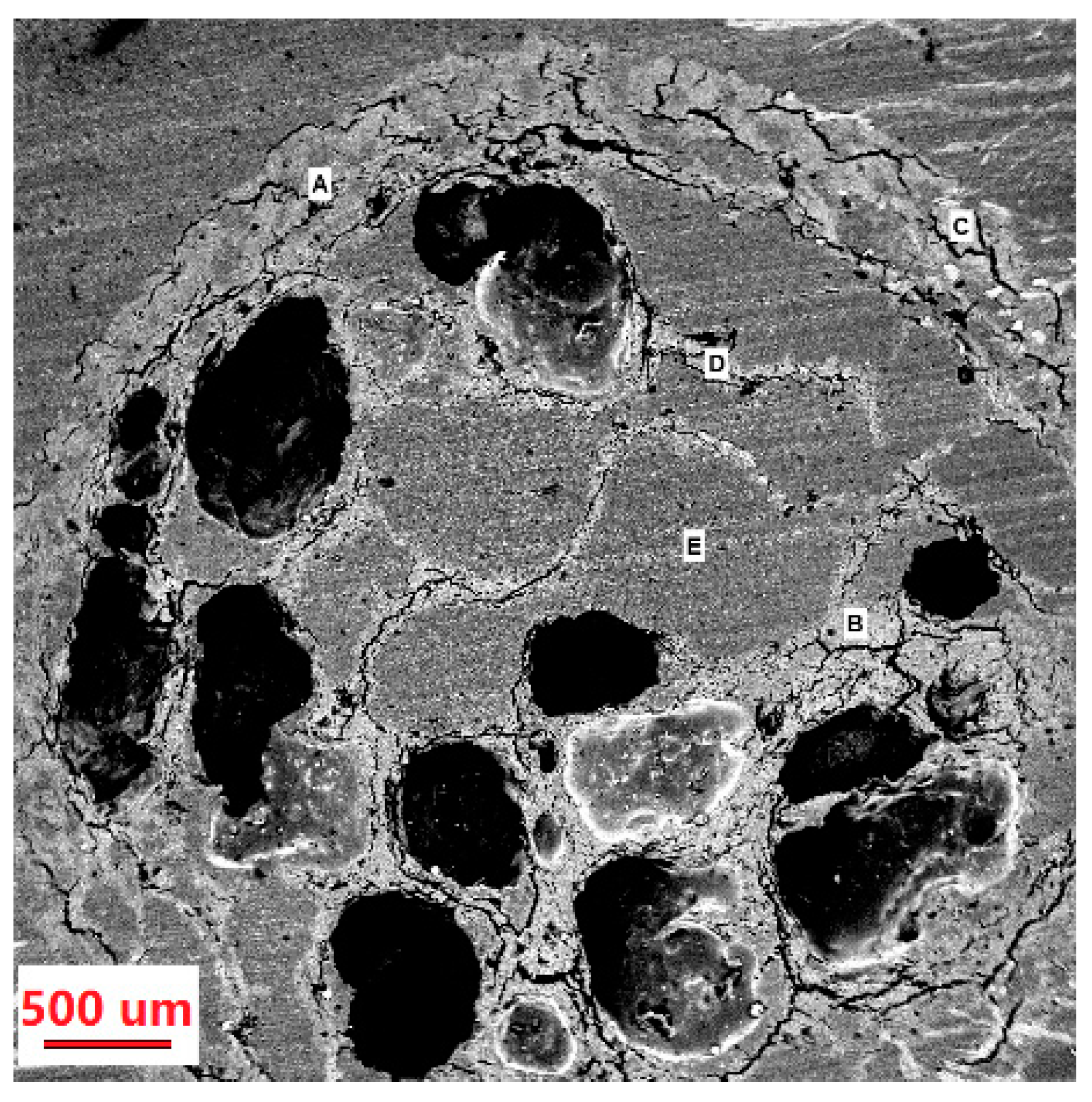
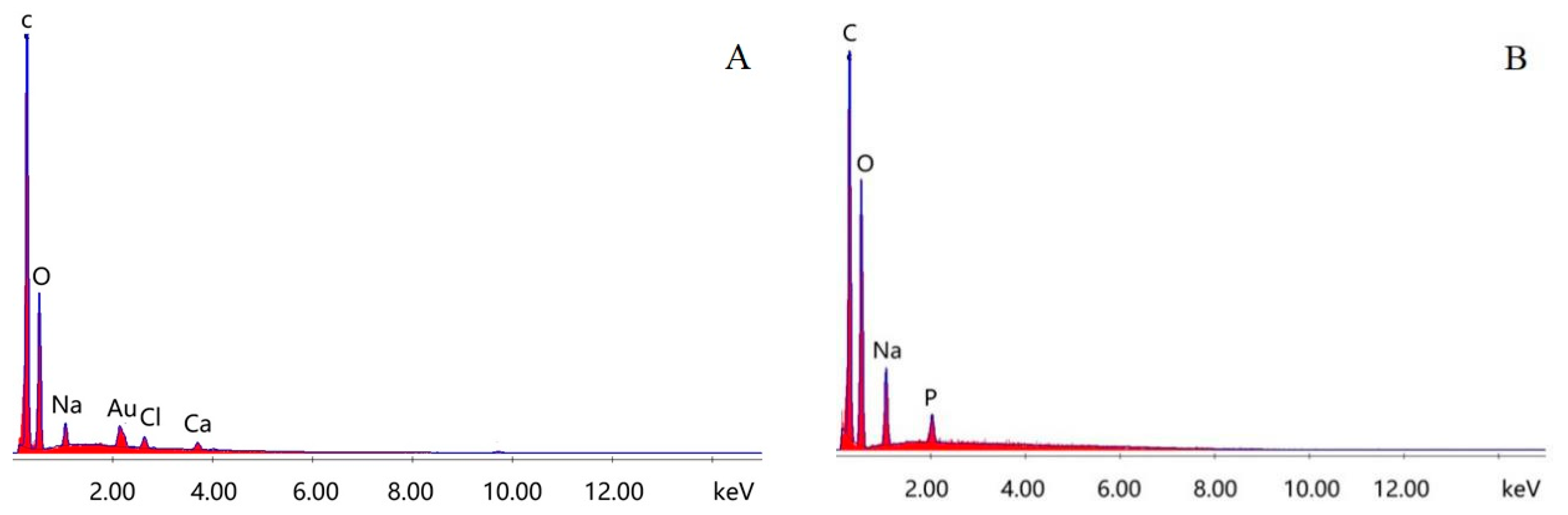
3.3. EDS Analysis of Immobilized Laccase MCCBs
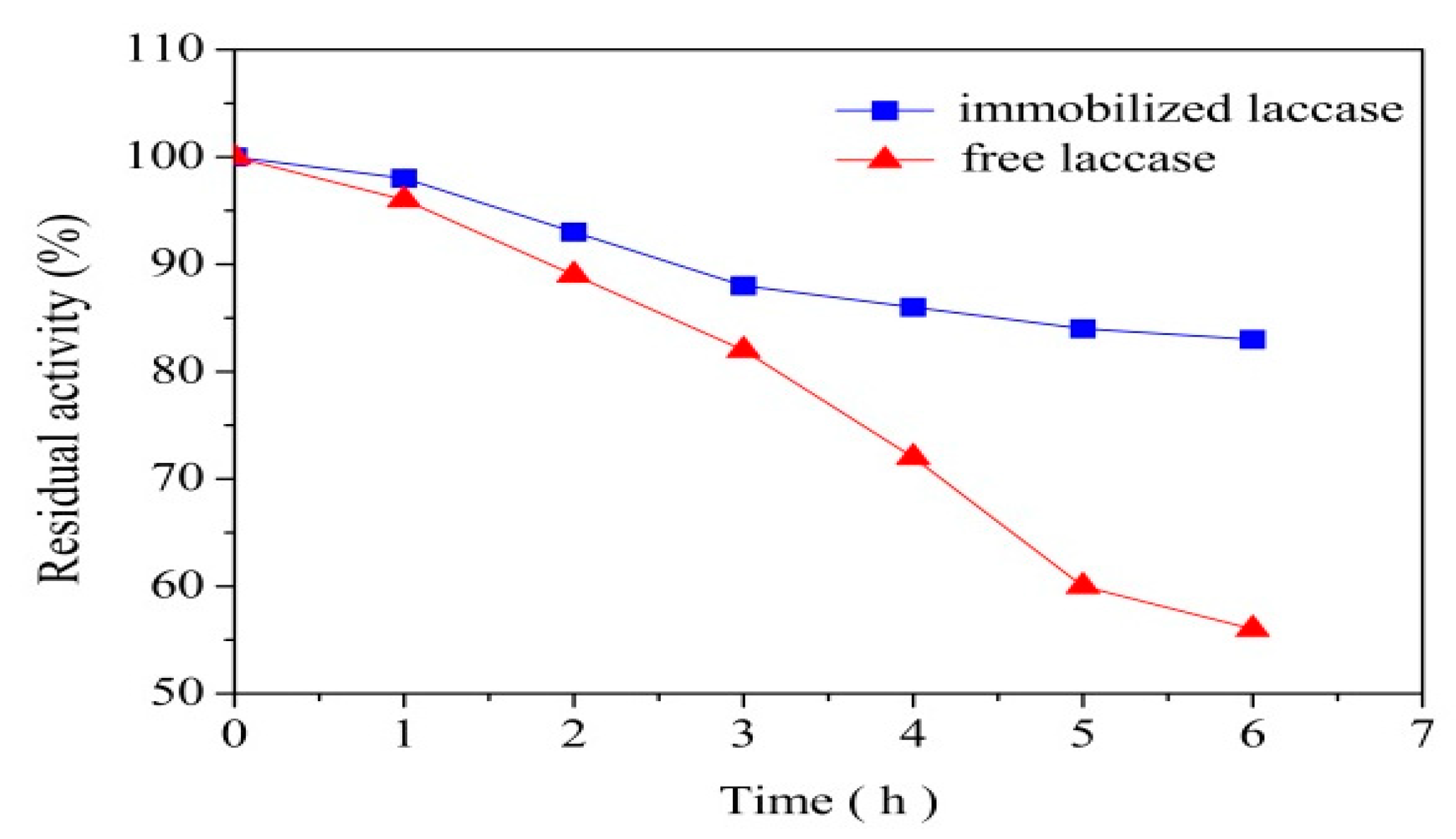
3.4. Thermal and Operational Stability of Immobilization Laccase
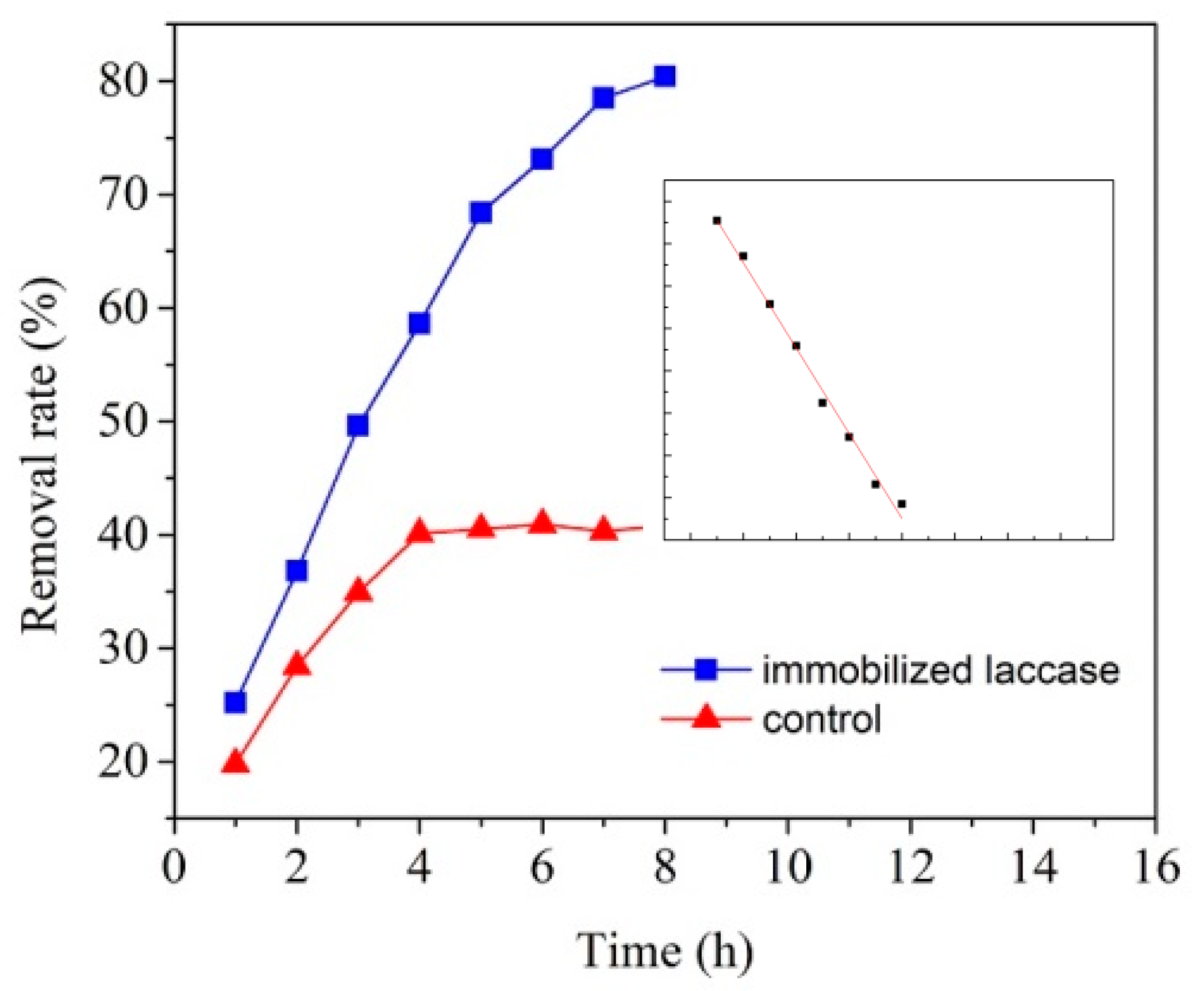
3.5. Degradation of PCBs by Immobilized Laccase
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robertson, L.W.; Hansen, L.G. PCBs: Recent Advances in Environmental Toxicology and Health Effects; University Press of Kentucky: Lexington, KY, USA, 2015. [Google Scholar]
- Balmer, B.C.; Ylitalo, G.M.; Mcgeorge, L.E.; Baugh, K.L.; Boyd, D.; Mullin, K.D.; Rosel, P.E.; Sinclair, C.; Wells, R.S.; Zolman, E.S. Persistent organic pollutants (POPs) in blubber of common bottlenose dolphins (Tursiops truncatus) along the northern Gulf of Mexico coast. Sci. Total Environ. 2015, 527–528, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Urai, M.; Yoshizaki, H.; Anzai, H.; Ogihara, J.; Iwabuchi, N.; Harayama, S.; Sunairi, M.; Nakajima, M. Structural analysis of an acidic, fatty acid ester-bonded extracellular polysaccharide produced by a pristane-assimilating marine bacterium, Rhodococcus erythropolis PR4. Carbohydr. Res. 2007, 342, 933–942. [Google Scholar] [CrossRef]
- Ballinas-Casarrubias, L.; Villanueva-Solís, L.; Espinoza-Hicks, C.; Camacho-Dávila, A.; Castillo, H.A.P.; Pérez, S.B.; Villa, E.D.; Hernández, M.D.D.; González-Sánchez, G. Effect of Laccase-Mediated Biopolymer Grafting on Kraft Pulp Fibers for Enhancing Paper’s Mechanical Properties. Polymers 2017, 9, 570. [Google Scholar] [CrossRef]
- Sarma, R.; Islam, M.S.; Running, M.P.; Bhattacharyya, D. Multienzyme Immobilized Polymeric Membrane Reactor for the Transformation of a Lignin Model Compound. Polymers 2018, 10, 463. [Google Scholar] [CrossRef]
- Luna-Acosta, A.; Bustamante, P.; Budzinski, H.; Huet, V.; Thomas-Guyon, H. Persistent organic pollutants in a marine bivalve on the Marennes–Oléron Bay and the Gironde Estuary (French Atlantic Coast)—Part 2: Potential biological effects. Sci. Total Environ. 2015, 514, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Laccase immobilization on titania nanoparticles and titania-functionalized membranes. J. Membr. Sci. 2014, 452, 229–240. [Google Scholar] [CrossRef]
- Bautista, L.F.; Morales, G.; Sanz, R. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by laccase from Trametes versicolor covalently immobilized on amino-functionalized SBA-15. Chemosphere 2015, 136, 273–280. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, S.-G.; Sun, H.J.; Ha, S.; Kim, J. Precipitated and chemically-crosslinked laccase over polyaniline nanofiber for high performance phenol sensing. Chemosphere 2016, 143, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Huber, D.; Pellis, A.; Daxbacher, A.; Nyanhongo, G.S.; Guebitz, G.M. Polymerization of Various Lignins via Immobilized Myceliophthora thermophila Laccase (MtL). Polymers 2016, 8, 280. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Paul, U.C.; Fragouli, D.; Bayer, I.S.; Athanassiou, A. Functionalized cellulose networks for efficient oil removal from oil–water emulsions. Polymers 2016, 8, 52. [Google Scholar] [CrossRef]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly(N-isopropylacrylamide)-cellulose nanocrystals hybrid hydrogels for wound dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef]
- Pan, Y.; Farmahini-Farahani, M.; O’Hearn, P.; Xiao, H.; Ocampo, H. An overview of bio-based polymers for packaging materials. J. Bioresour. Bioprod. 2016, 1, 106–113. [Google Scholar]
- Wu, X.; Zhao, F.; Varcoe, J.R.; Thumser, A.E.; Avignone-Rossa, C.; Slade, R.C. Direct electron transfer of glucose oxidase immobilized in an ionic liquid reconstituted cellulose–carbon nanotube matrix. Bioelectrochemistry 2009, 77, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Jin, J.N.; Kan, E.; Kim, K.J.; Sang, H.L. Bacterial cellulose-chitosan composite hydrogel beads for enzyme immobilization. Biotechnol. Bioprocess Eng. 2017, 22, 89–94. [Google Scholar] [CrossRef]
- Frazão, C.J.R.; Silva, N.H.C.; Freire, C.S.R.; Silvestre, A.J.D.; Xavier, A.M.R.B.; Tavares, A.P.M. Bacterial cellulose as carrier for immobilization of laccase: Optimization and characterization. Eng. Life Sci. 2015, 14, 500–508. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Kamala-Kannan, S.; Min, C.; Kim, J.S.; Hadibarata, T.; Salim, M.R.; Oh, B.T. Laccase immobilization on cellulose nanofiber: The catalytic efficiency and recyclic application for simulated dye effluent treatment. J. Mol. Catal. B Enzym. 2014, 100, 111–120. [Google Scholar] [CrossRef]
- Moccelini, S.K.; Franzoi, A.C.; Vieira, I.C.; Dupont, J.; Scheeren, C.W. A novel support for laccase immobilization: Cellulose acetate modified with ionic liquid and application in biosensor for methyldopa detection. Biosens. Bioelectron. 2011, 26, 3549–3554. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, H.; Chen, M.; Song, Z.; Zhao, Y. Absorbents based on maleic anhydride-modified cellulose fibers/diatomite for dye removal. J. Mater. Sci. 2014, 49, 6696–6704. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, H.; Pan, Y.; Wang, L. Novel Composite Adsorbent Consisting of Dissolved Cellulose Fiber/Microfibrillated Cellulose for Dye Removal from Aqueous Solution. ACS Sustain. Chem. Eng. 2018, 6, 6994–7002. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, H.; Xiong, J.; Zhang, C.; Li, Y. Preparation and application of novel chitosan-cellulose composite materials to adsorb Pb (Ⅱ) and Cr (Ⅵ) ions from water. J. Bioresour. Bioprod. 2017, 2, 175–183. [Google Scholar]
- Eggert, C.; Temp, U.; Eriksson, K.-E. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: Purification and characterization of the laccase. Appl. Environ. Microbiol. 1996, 62, 1151–1158. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Skoronski, E.; Souza, D.H.; Ely, C.; Broilo, F.; Fernandes, M.; Fúrigo, A.; Ghislandi, M.G. Immobilization of laccase from Aspergillus oryzae on graphene nanosheets. Int. J. Biol. Macromol. 2017, 99, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, D.; Neeraj, G.; Swaroopini, R.; Shobana, R.; Kumar, V.V.; Cabana, H. Synergetic integration of laccase and versatile peroxidase with magnetic silica microspheres towards remediation of biorefinery wastewater. Environ. Sci. Pollut. Res. 2017, 24, 17993–18009. [Google Scholar] [CrossRef]
- Xu, F. Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J. Biol. Chem. 1997, 272, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.; Majcherczyk, A. Laccase activity tests and laccase inhibitors. J. Biotechnol. 2000, 78, 193–199. [Google Scholar] [CrossRef]
- Bonomo, R.; Cennamo, G.; Purrello, R.; Santoro, A.; Zappala, R. Comparison of three fungal laccases from Rigidoporus lignosus and Pleurotus ostreatus: Correlation between conformation changes and catalytic activity. J. Inorg. Biochem. 2001, 83, 67–75. [Google Scholar] [CrossRef]
- Rodakiewicz-Nowak, J.; Kasture, S.; Dudek, B.; Haber, J. Effect of various water-miscible solvents on enzymatic activity of fungal laccases. J. Mol. Catal. B Enzym. 2000, 11, 1–11. [Google Scholar] [CrossRef]
- Cristóvão, R.O.; Tavares, A.P.; Brígida, A.I.; Loureiro, J.M.; Boaventura, R.A.; Macedo, E.A.; Coelho, M.A.Z. Immobilization of commercial laccase onto green coconut fiber by adsorption and its application for reactive textile dyes degradation. J. Mol. Catal. B Enzym. 2011, 72, 6–12. [Google Scholar] [CrossRef]
- Bautista, L.F.; Morales, G.; Sanz, R. Immobilization strategies for laccase from Trametes versicolor on mesostructured silica materials and the application to the degradation of naphthalene. Bioresour. Technol. 2010, 101, 8541–8548. [Google Scholar] [CrossRef]
- De Oliveira, P.C.; Alves, G.M.; de Castro, H.F. Immobilisation studies and catalytic properties of microbial lipase onto styrene–divinylbenzene copolymer. Biochem. Eng. J. 2000, 5, 63–71. [Google Scholar] [CrossRef]
- Tavares, A.P.; Silva, C.G.; Dražić, G.; Silva, A.M.; Loureiro, J.M.; Faria, J.L. Laccase immobilization over multi-walled carbon nanotubes: Kinetic, thermodynamic and stability studies. J. Colloid Interface Sci. 2015, 454, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.P.; Rodríguez, O.; Fernández-Fernández, M.; Domínguez, A.; Moldes, D.; Sanromán, M.A.; Macedo, E.A. Immobilization of laccase on modified silica: Stabilization, thermal inactivation and kinetic behaviour in 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid. Bioresour. Technol. 2013, 131, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Al-Adhami, A.J.; Bryjak, J.; Greb-Markiewicz, B.; Peczyńska-Czoch, W. Immobilization of wood-rotting fungi laccases on modified cellulose and acrylic carriers. Process Biochem. 2002, 37, 1387–1394. [Google Scholar] [CrossRef]
- Steffensen, C.L.; Andersen, M.L.; Degn, P.E.; Nielsen, J.H. Cross-linking proteins by laccase-catalyzed oxidation: Importance relative to other modifications. J. Agric. Food Chem. 2008, 56, 12002–12010. [Google Scholar] [CrossRef] [PubMed]
- Nollet, H.; Roels, M.; Lutgen, P.; Van der Meeren, P.; Verstraete, W. Removal of PCBs from wastewater using fly ash. Chemosphere 2003, 53, 655–665. [Google Scholar] [CrossRef]
- Choi, H.; Al-Abed, S.R.; Agarwal, S.; Dionysiou, D.D. Synthesis of reactive nano-Fe/Pd bimetallic system-impregnated activated carbon for the simultaneous adsorption and dechlorination of PCBs. Chem. Mater. 2008, 20, 3649–3655. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Xia, Q.; Li, Y.; Hou, X.; Niu, M.; Ping, Q.; Xiao, H. Immobilizing Laccase on Modified Cellulose/CF Beads to Degrade Chlorinated Biphenyl in Wastewater. Polymers 2018, 10, 798. https://doi.org/10.3390/polym10070798
Li N, Xia Q, Li Y, Hou X, Niu M, Ping Q, Xiao H. Immobilizing Laccase on Modified Cellulose/CF Beads to Degrade Chlorinated Biphenyl in Wastewater. Polymers. 2018; 10(7):798. https://doi.org/10.3390/polym10070798
Chicago/Turabian StyleLi, Na, Quiyang Xia, Yuan Li, Xiaobang Hou, Meihong Niu, Qingwei Ping, and Huining Xiao. 2018. "Immobilizing Laccase on Modified Cellulose/CF Beads to Degrade Chlorinated Biphenyl in Wastewater" Polymers 10, no. 7: 798. https://doi.org/10.3390/polym10070798