A Robust Fabrication Method for Amphiphilic Janus Particles via Immobilization on Polycarbonate Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Buffers
2.2. Preparation of Polycarbonate/Polymethylsilsesquioxane (PC/PMSQ) Microspheres
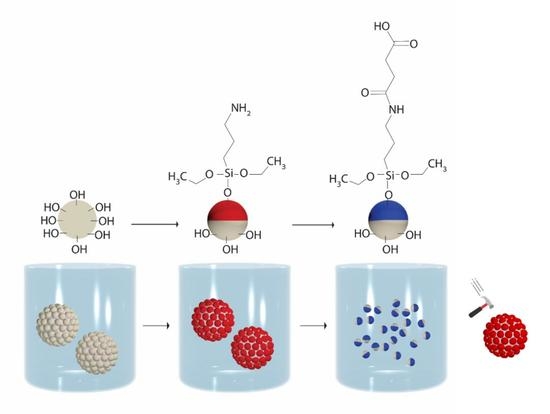
2.3. Fabrication of PMSQ Amphiphilic Janus Particles
2.3.1. Fabrication of PMSQ-NH2 Amphiphilic Janus Particles
2.3.2. Fabrication of PMSQ-COOH Amphiphilic Janus Particles
2.4. Fluorescent Labeling of PMSQ-COOH Amphiphilic Janus Particles
2.5. Synthesis of Amine Functionalized Silica Nanoparticles
2.6. Coupling of Amine Functionalized Silica Nanoparticles(NPs) to the PMSQ-COOH Amphiphilic Janus Particles
2.7. Characterization of PMSQ Amphiphilic Janus Particles
3. Results and Discussion
3.1. Preparation of PC/PMSQ Microspheres
3.2. Fabrication of Amphiphilic Janus Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noguchi, T.G.; Iwashita, Y.; Kimura, Y. Dependence of the Internal Structure on Water/Particle Volume Ratio in an Amphiphilic Janus Particle–Water–Oil Ternary System: From Micelle-like Clusters to Emulsions of Spherical Droplets. Langmuir 2017, 33, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Andala, D.M.; Shin, S.H.R.; Lee, H.-Y.; Bishop, K.J.M. Templated Synthesis of Amphiphilic Nanoparticles at the Liquid–Liquid Interface. ACS Nano 2012, 6, 1044–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Park, B.J.; Tu, F.; Lee, D. Amphiphilic Janus particles at fluid interfaces. Soft Matter 2013, 9, 6604–6617. [Google Scholar] [CrossRef]
- Walther, A.; Müller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Müller, A.H.E. Janus particles. Soft Matter 2008, 4, 663–668. [Google Scholar] [CrossRef]
- Walther, A.; André, X.; Drechsler, M.; Abetz, V.; Müller, A.H.E. Janus Discs. J. Am. Chem. Soc. 2007, 129, 6187–6198. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P.G. Soft matter. Rev. Mod. Phys. 1992, 64, 645–648. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Q.; Tripathy, M.; Luijten, E.; Schweizer, K.S.; Granick, S. Janus Particle Synthesis and Assembly. Adv. Mater. 2010, 22, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, R.; Zhang, M.; Böker, A.; Zettl, H.; Abetz, C.; Frederik, P.; Krausch, G.; Abetz, V.; Müller, A.H.E. Amphiphilic Janus Micelles with Polystyrene and Poly(methacrylic acid) Hemispheres. J. Am. Chem. Soc. 2003, 125, 3260–3267. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, R.; Böker, A.; Zettl, H.; Kaya, H.; Pyckhout-Hintzen, W.; Krausch, G.; Abetz, V.; Müller, A.H.E. Janus Micelles. Macromolecules 2001, 34, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Kraft, D.J.; Ni, R.; Smallenburg, F.; Hermes, M.; Yoon, K.; Weitz, D.A.; van Blaaderen, A.; Groenewold, J.; Dijkstra, M.; Kegel, W.K. Surface roughness directed self-assembly of patchy particles into colloidal micelles. Proc. Natl. Acad. Sci. USA 2012, 109, 10787–10792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, T.; Gokmen, M.T.; Rinnen, S.; Arlinghaus, H.F.; Du Prez, F.; Ravoo, B.J. Bifunctional Janus beads made by “sandwich” microcontact printing using click chemistry. J. Mater. Chem. 2012, 22, 6190–6199. [Google Scholar] [CrossRef]
- Kaufmann, T.; Gokmen, M.T.; Wendeln, C.; Schneiders, M.; Rinnen, S.; Arlinghaus, H.F.; Bon, S.A.F.; Du Prez, F.E.; Ravoo, B.J. “Sandwich” Microcontact Printing as a Mild Route Towards Monodisperse Janus Particles with Tailored Bifunctionality. Adv. Mater. 2011, 23, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Breiner, T.; Mori, H.; Müller, A.H.E. Amphiphilic cylindrical brushes with poly(acrylic acid) core and poly(n-butyl acrylate) shell and narrow length distribution. Polymer 2003, 44, 1449–1458. [Google Scholar] [CrossRef]
- Cheng, G.; Böker, A.; Zhang, M.; Krausch, G.; Müller, A.H.E. Amphiphilic Cylindrical Core−Shell Brushes via a “Grafting From” Process Using ATRP. Macromolecules 2001, 34, 6883–6888. [Google Scholar] [CrossRef]
- Casagrande, C.; Fabre, P.; Raphaël, E.; Veyssié, M. “Janus Beads”: Realization and Behaviour at Water/Oil Interfaces. Europhys. Lett. EPL 1989, 9, 251–255. [Google Scholar] [CrossRef]
- Glaser, N.; Adams, D.J.; Böker, A.; Krausch, G. Janus Particles at Liquid−Liquid Interfaces. Langmuir 2006, 22, 5227–5229. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Lee, D.; Shum, H.C.; Weitz, D.A. Colloid Surfactants for Emulsion Stabilization. Adv. Mater. 2008, 20, 3239–3243. [Google Scholar] [CrossRef]
- Choi, J.; Zhao, Y.; Zhang, D.; Chien, S.; Lo, Y.-H. Patterned Fluorescent Particles as Nanoprobes for the Investigation of Molecular Interactions. Nano Lett. 2003, 3, 995–1000. [Google Scholar] [CrossRef]
- Anker, J.N.; Behrend, C.J.; Huang, H.; Kopelman, R. Magnetically-modulated optical nanoprobes (MagMOONs) and systems. J. Magn. Magn. Mater. 2005, 293, 655–662. [Google Scholar] [CrossRef]
- Howse, J.R.; Jones, R.A.L.; Ryan, A.J.; Gough, T.; Vafabakhsh, R.; Golestanian, R. Self-Motile Colloidal Particles: From Directed Propulsion to Random Walk. Phys. Rev. Lett. 2007, 99, 048102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlick, R.A.; Sengupta, S.; McFadden, T.; Zhang, H.; Sen, A. A Polymerization-Powered Motor. Angew. Chem. Int. Ed. 2011, 50, 9374–9377. [Google Scholar] [CrossRef] [PubMed]
- Perro, A.; Reculusa, S.; Ravaine, S.; Bourgeat-Lami, E.; Duguet, E. Design and synthesis of Janus micro- and nanoparticles. J. Mater. Chem. 2005, 15, 3745–3760. [Google Scholar] [CrossRef]
- Nisisako, T.; Torii, T.; Takahashi, T.; Takizawa, Y. Synthesis of Monodisperse Bicolored Janus Particles with Electrical Anisotropy Using a Microfluidic Co-Flow System. Adv. Mater. 2006, 18, 1152–1156. [Google Scholar] [CrossRef]
- Roh, K.-H.; Martin, D.C.; Lahann, J. Biphasic Janus particles with nanoscale anisotropy. Nat. Mater. 2005, 4, nmat1486. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Gates, B.D.; Wolfe, D.B.; Paul, K.E.; Whitesides, G.M. Fabrication and Wetting Properties of Metallic Half-Shells with Submicron Diameters. Nano Lett. 2002, 2, 891–894. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Ren, M.; Yang, W. Preparation of Polymeric Janus Particles by Directional UV-Induced Reactions. Langmuir 2009, 25, 11048–11053. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.Y.; Phang, I.Y.; Acikgoz, C.; Yilmaz, M.D.; Hempenius, M.A.; Vancso, G.J.; Huskens, J. Janus Particles with Controllable Patchiness and Their Chemical Functionalization and Supramolecular Assembly. Angew. Chem. Int. Ed. 2009, 48, 7677–7682. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.T.; Muir, B.W.; Such, G.K.; Postma, A.; McLean, K.M.; Caruso, F. Fabrication of asymmetric “Janus” particles via plasma polymerization. Chem. Commun. 2010, 46, 5121–5123. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, S.Y.; Yang, S.-M. Janus Microspheres for a Highly Flexible and Impregnable Water-Repelling Interface. Angew. Chem. Int. Ed. 2010, 49, 2535–2538. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.D.; Kraeutler, M.J.; Yang, S.; Composto, R.J. Patchy and Multiregion Janus Particles with Tunable Optical Properties. Nano Lett. 2010, 10, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zu, X.; Li, Y.; Mu, W.; Deng, Y. Janus particles with tunable coverage of zinc oxide nanowires. J. Mater. Chem. 2011, 21, 2067–2069. [Google Scholar] [CrossRef]
- Hong, L.; Jiang, S.; Granick, S. Simple Method to Produce Janus Colloidal Particles in Large Quantity. Langmuir 2006, 22, 9495–9499. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-C.; Chen, W.-S.; Shie, T.-Y.; Lin, J.-N.; Kuo, C. Novel Fabrication of Janus Particles from the Surfaces of Electrospun Polymer Fibers. Langmuir 2008, 24, 5663–5666. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Liao, C.-W.; Chao, Y.-C.; Kuo, C. Fabrication and Characterization of Asymmetric Janus and Ternary Particles. ACS Appl. Mater. Interfaces 2010, 2, 3185–3191. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; de Vries, M.H.; Picchioni, F.; Loos, K. A novel method of preparing metallic Janus silica particles using supercritical carbon dioxide. Nanoscale 2013, 5, 10420–10427. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Granick, S. Controlling the Geometry (Janus Balance) of Amphiphilic Colloidal Particles. Langmuir 2008, 24, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Huang, J.; Sun, W.; Wei, Y.; Liu, Y.; Ding, L.; Bao, J.; Chen, Z.-R. Exploration of selective decoration of Janus silica particles within polymeric patterned pore arrays. RSC Adv. 2016, 6, 55860–55866. [Google Scholar] [CrossRef]
- Zhang, J.; Grzybowski, B.A.; Granick, S. Janus Particle Synthesis, Assembly, and Application. Langmuir 2017, 33, 6964–6977. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.H.; Lee, Y.J.; Yi, G.-R.; Jang, S.G.; Schmidt, B.V.K.J.; Liao, K.; Klinger, D.; Hawker, C.J.; Kim, B.J. Shape-Tunable Biphasic Janus Particles as pH-Responsive Switchable Surfactants. Macromolecules 2017, 50, 9276–9285. [Google Scholar] [CrossRef]
- Cao, Z.; Bian, Q.; Chen, Y.; Liang, F.; Wang, G. Light-Responsive Janus-Particle-Based Coatings for Cell Capture and Release. ACS Macro Lett. 2017, 6, 1124–1128. [Google Scholar] [CrossRef]
- Satoh, H.; Yabu, H. One-Pot Preparation of Organic-Inorganic Composite Microspheres Comprising Silica Nanoparticles and End-Functionalized Polymers. Macromol. Mater. Eng. 2016, 301, 279–286. [Google Scholar] [CrossRef]
- Yabu, H.; Ohshima, H.; Saito, Y. Double-Phase-Functionalized Magnetic Janus Polymer Microparticles Containing TiO2 and Fe2O3 Nanoparticles Encapsulated in Mussel-Inspired Amphiphilic Polymers. ACS Appl. Mater. Interfaces 2014, 6, 18122–18128. [Google Scholar] [CrossRef] [PubMed]
- Yabu, H.; Kanahara, M.; Shimomura, M.; Arita, T.; Harano, K.; Nakamura, E.; Higuchi, T.; Jinnai, H. Polymer Janus Particles Containing Block-Copolymer Stabilized Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 3262–3266. [Google Scholar] [CrossRef] [PubMed]
- Yabu, H.; Koike, K.; Motoyoshi, K.; Higuchi, T.; Shimomura, M. A Novel Route for Fabricating Metal-Polymer Composite Nanoparticles with Phase-Separated Structures. Macromol. Rapid Commun. 2010, 31, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Arita, T.; Kanahara, M.; Motoyoshi, K.; Koike, K.; Higuchi, T.; Yabu, H. Localization of polymer-grafted maghemite nanoparticles in a hemisphere of Janus polymer particles prepared by a self-organized precipitation (SORP) method. J. Mater. Chem. C 2013, 1, 207–212. [Google Scholar] [CrossRef]
- Motoyoshi, K.; Tajima, A.; Higuchi, T.; Yabu, H.; Shimomura, M. Static and dynamic control of phase separation structures in nanoparticles of polymer blends. Soft Matter 2010, 6, 1253–1257. [Google Scholar] [CrossRef]
- Higuchi, T.; Tajima, A.; Yabu, H.; Shimomura, M. Spontaneous formation of polymer nanoparticles with inner micro-phase separation structures. Soft Matter 2008, 4, 1302–1305. [Google Scholar] [CrossRef]
- Lince, F.; Marchisio, D.L.; Barresi, A.A. Strategies to control the particle size distribution of poly-ε-caprolactone nanoparticles for pharmaceutical applications. J. Colloid Interface Sci. 2008, 322, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhang, L.; Wang, Z.; Yu, X.; Long, Y.; Zhang, X.; Zhao, N.; Xu, J. Multifunctional polymethylsilsesquioxane (PMSQ) surfaces prepared by electrospinning at the sol–gel transition: Superhydrophobicity, excellent solvent resistance, thermal stability and enhanced sound absorption property. J. Colloid Interface Sci. 2011, 359, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Feifel, S.C.; Lisdat, F. Silica nanoparticles for the layer-by-layer assembly of fully electro-active cytochrome c multilayers. J. Nanobiotechnol. 2011, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Grabarek, Z.; Gergely, J. Zero-length crosslinking procedure with the use of active esters. Anal. Biochem. 1990, 185, 131–135. [Google Scholar] [CrossRef]
- Khayata, N.; Abdelwahed, W.; Chehna, M.F.; Charcosset, C.; Fessi, H. Preparation of vitamin E loaded nanocapsules by the nanoprecipitation method: From laboratory scale to large scale using a membrane contactor. Int. J. Pharm. 2012, 423, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-loaded PLGA nanoparticles: Systematic study of particle size and drug content. Int. J. Pharm. 2007, 336, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Bernet, N.; de la Roche, S.; Hahn, H.T. Processing of Iron Oxide-epoxy Vinyl Ester Nanocomposites. J. Compos. Mater. 2003, 37, 465–476. [Google Scholar] [CrossRef]
- Aoki, N.; Nishikawa, M.; Hattori, K. Synthesis of chitosan derivatives bearing cyclodextrin and adsorption of p-nonylphenol and bisphenol A. Carbohydr. Polym. 2003, 52, 219–223. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Rivillon Amy, S.; Chabal, Y.J. Attachment of 3-(Aminopropyl)triethoxysilane on Silicon Oxide Surfaces: Dependence on Solution Temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Hong, F.C.-N. Improved adhesion of PMSQ hard coatings on polymer substrates. J. Coat. Technol. Res. 2011, 8, 779–783. [Google Scholar] [CrossRef]
- Lu, Y.; Miller, J.D. Carboxyl Stretching Vibrations of Spontaneously Adsorbed and LB-Transferred Calcium Carboxylates as Determined by FTIR Internal Reflection Spectroscopy. J. Colloid Interface Sci. 2002, 256, 41–52. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Takahara, Y.K.; Ikeda, S.; Ishino, S.; Tachi, K.; Ikeue, K.; Sakata, T.; Hasegawa, T.; Mori, H.; Matsumura, M.; Ohtani, B. Asymmetrically Modified Silica Particles: A Simple Particulate Surfactant for Stabilization of Oil Droplets in Water. J. Am. Chem. Soc. 2005, 127, 6271–6275. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.G.; Zhao, Y.-P. Autonomously motile catalytic nanomotors by bubble propulsion. Appl. Phys. Lett. 2009, 94, 163104. [Google Scholar] [CrossRef]
- Wheat, P.M.; Marine, N.A.; Moran, J.L.; Posner, J.D. Rapid Fabrication of Bimetallic Spherical Motors. Langmuir 2010, 26, 13052–13055. [Google Scholar] [CrossRef] [PubMed]
- Ebbens, S.J.; Howse, J.R. In pursuit of propulsion at the nanoscale. Soft Matter 2010, 6, 726–738. [Google Scholar] [CrossRef]
- Johal, P.; Chaudhary, S. Electronic Paper Technology. Int. J. Adv. Res. Sci. Eng. 2013, 2, 106–110. [Google Scholar]
- Yu, C.; Zhang, J.; Granick, S. Selective Janus Particle Assembly at Tipping Points of Thermally-Switched Wetting. Angew. Chem. Int. Ed. 2014, 53, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kang, Y.C. One-pot facile synthesis of Janus-structured SnO2–CuO composite nanorods and their application as anode materials in Li-ion batteries. Nanoscale 2013, 5, 4662–4668. [Google Scholar] [CrossRef] [PubMed]








| Polymer Concentration (%) | PC/PMSQ Ratio with Yield (%) | ||
|---|---|---|---|
| - | 1:1 | 2:1 | 3:1 |
| 1 | 44.12 | - | - |
| 3 | 43.05 | - | - |
| 5 | 65.49 | 65.81 | 51.43 |
| 7 | 78.21 | 67.46 | 58.76 |
| 10 | 88.13 | 82.24 | 72.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, K.A.; Yaakov, N.; Itzhaik Alkotzer, Y.; Zelikman, E.; Mechrez, G. A Robust Fabrication Method for Amphiphilic Janus Particles via Immobilization on Polycarbonate Microspheres. Polymers 2018, 10, 900. https://doi.org/10.3390/polym10080900
Mani KA, Yaakov N, Itzhaik Alkotzer Y, Zelikman E, Mechrez G. A Robust Fabrication Method for Amphiphilic Janus Particles via Immobilization on Polycarbonate Microspheres. Polymers. 2018; 10(8):900. https://doi.org/10.3390/polym10080900
Chicago/Turabian StyleMani, Karthik Ananth, Noga Yaakov, Yafit Itzhaik Alkotzer, Evgeni Zelikman, and Guy Mechrez. 2018. "A Robust Fabrication Method for Amphiphilic Janus Particles via Immobilization on Polycarbonate Microspheres" Polymers 10, no. 8: 900. https://doi.org/10.3390/polym10080900