Advances in the Phototriggered Synthesis of Single-Chain Polymer Nanoparticles
Abstract
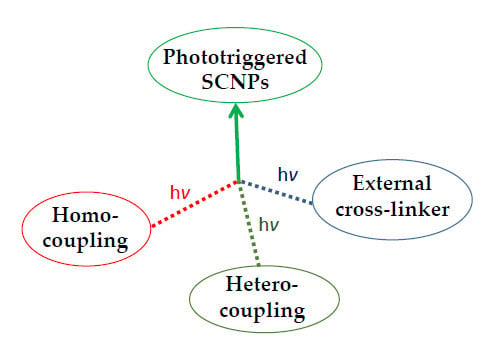
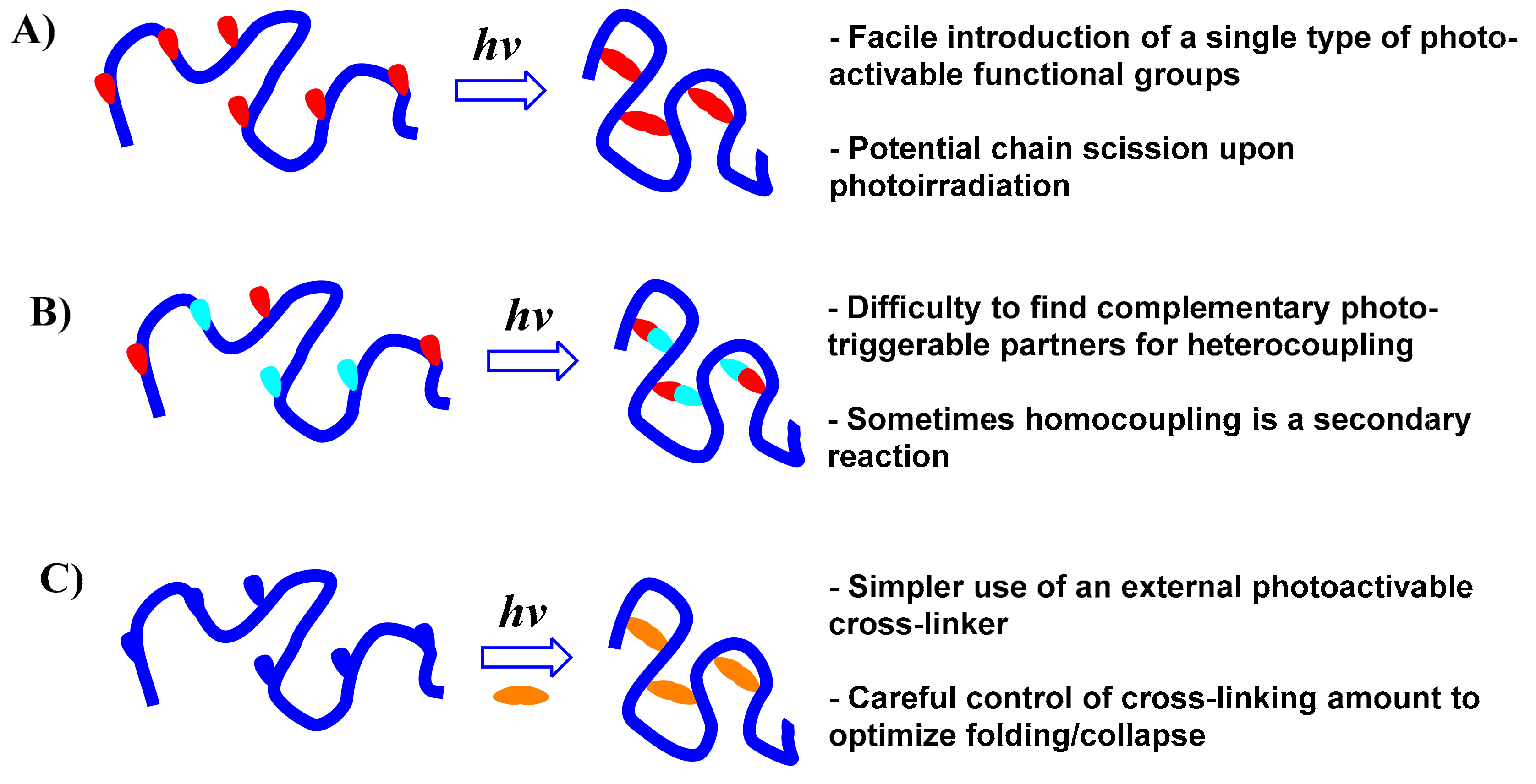
:1. Introduction
2. Synthesis of Single-Chain Polymer Nanoparticles via Photoactivated Intrachain Homocoupling
2.1. Photodimerization
2.1.1. Cinnamoyl Functional Groups
2.1.2. Coumarin Functional Groups
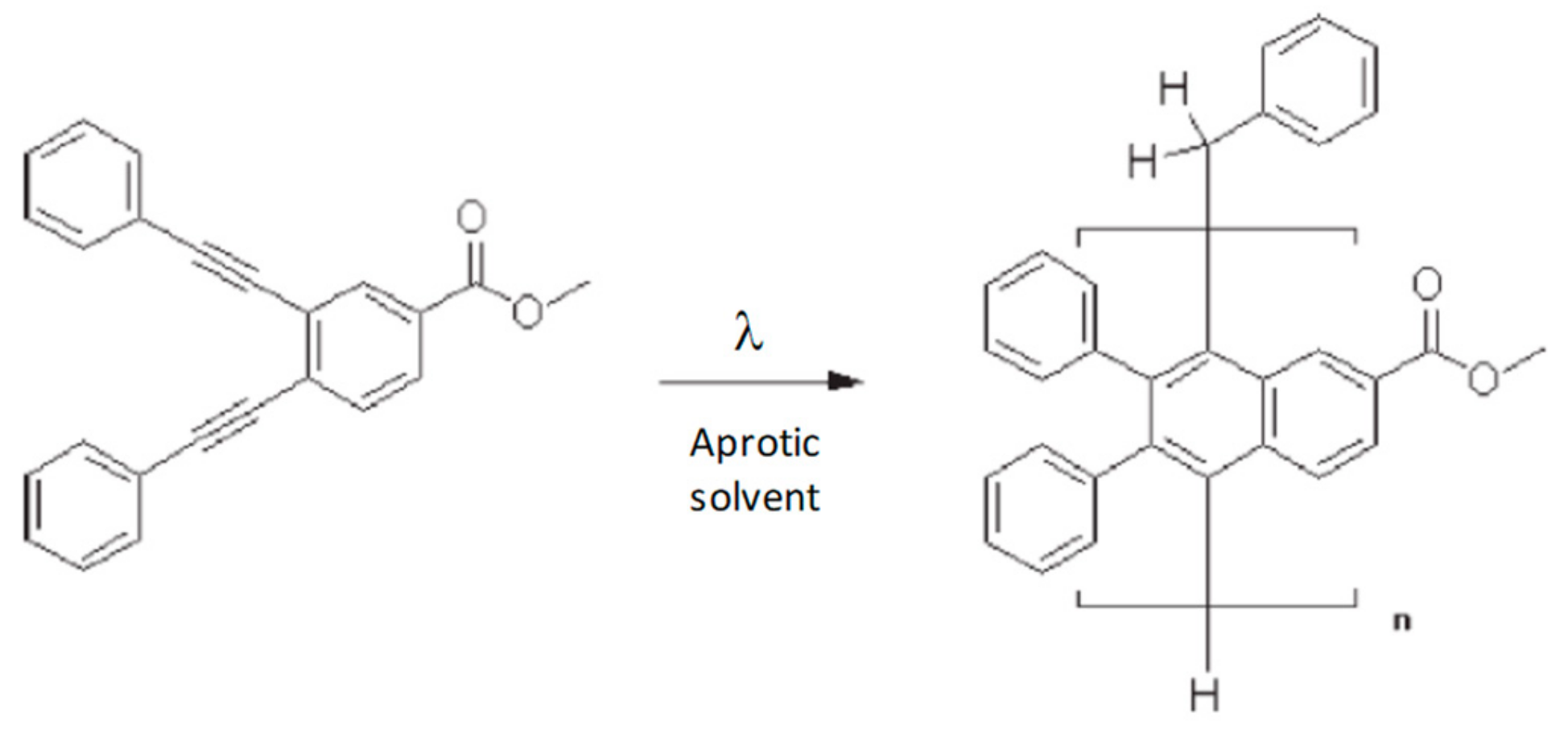
2.1.3. Anthracene Functional Groups
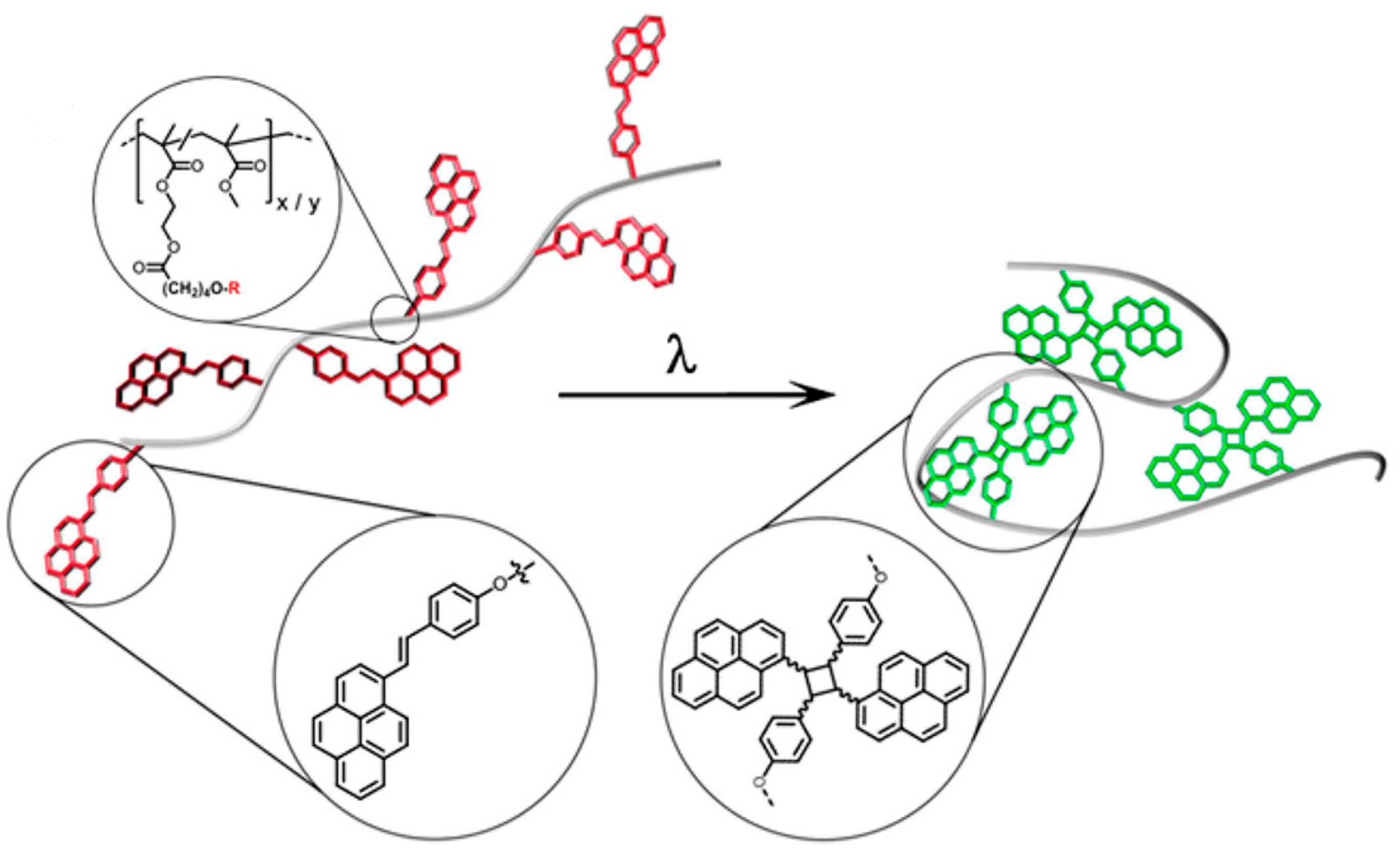
2.1.4. Styrylpyrene Functional Groups
2.1.5. Stilbene Functional Groups
2.1.6. Ketyl Radical Functional Groups
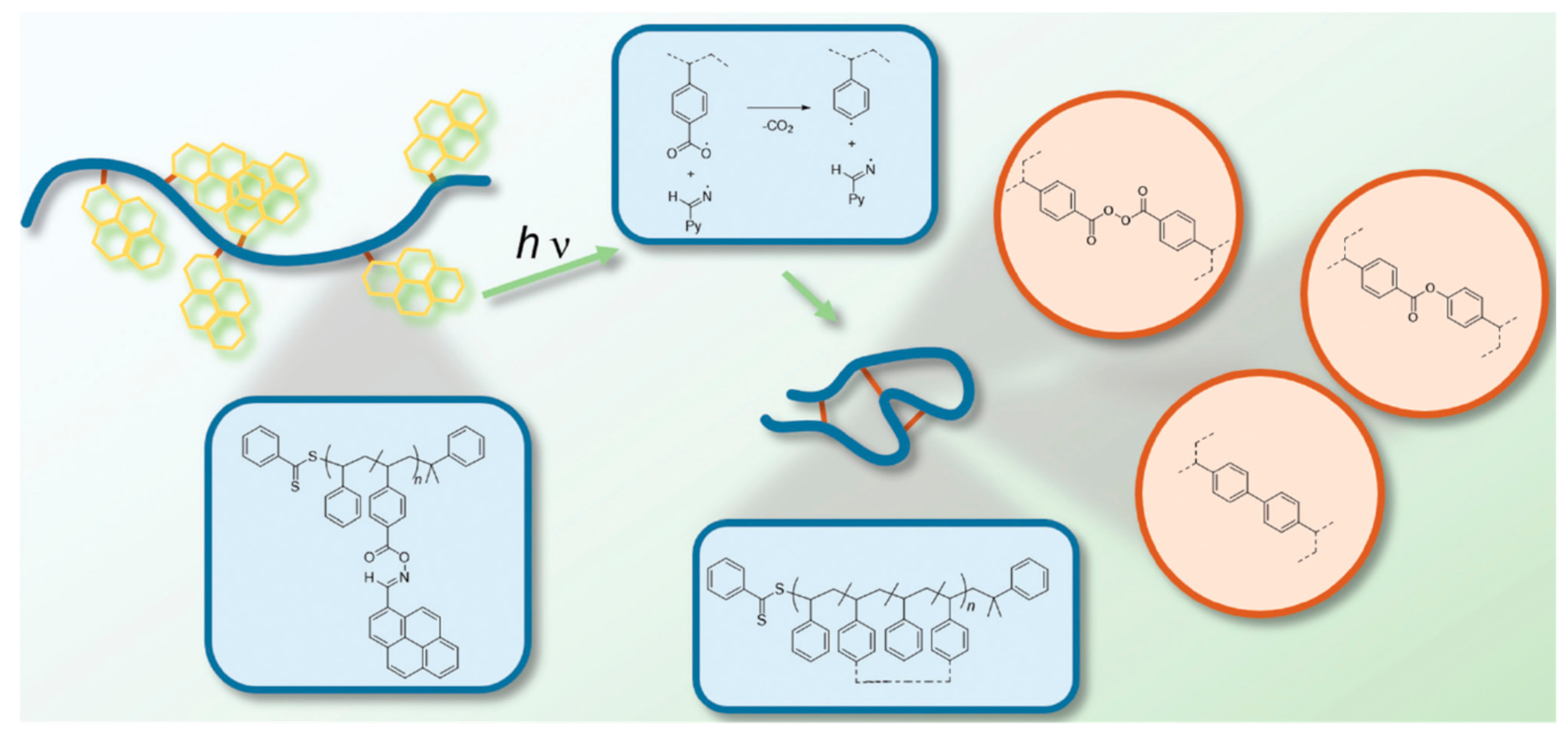
2.1.7. Carboxyl Radical Functional Groups
2.1.8. Nitrile Imine Functional Groups
2.2. Photocyclization
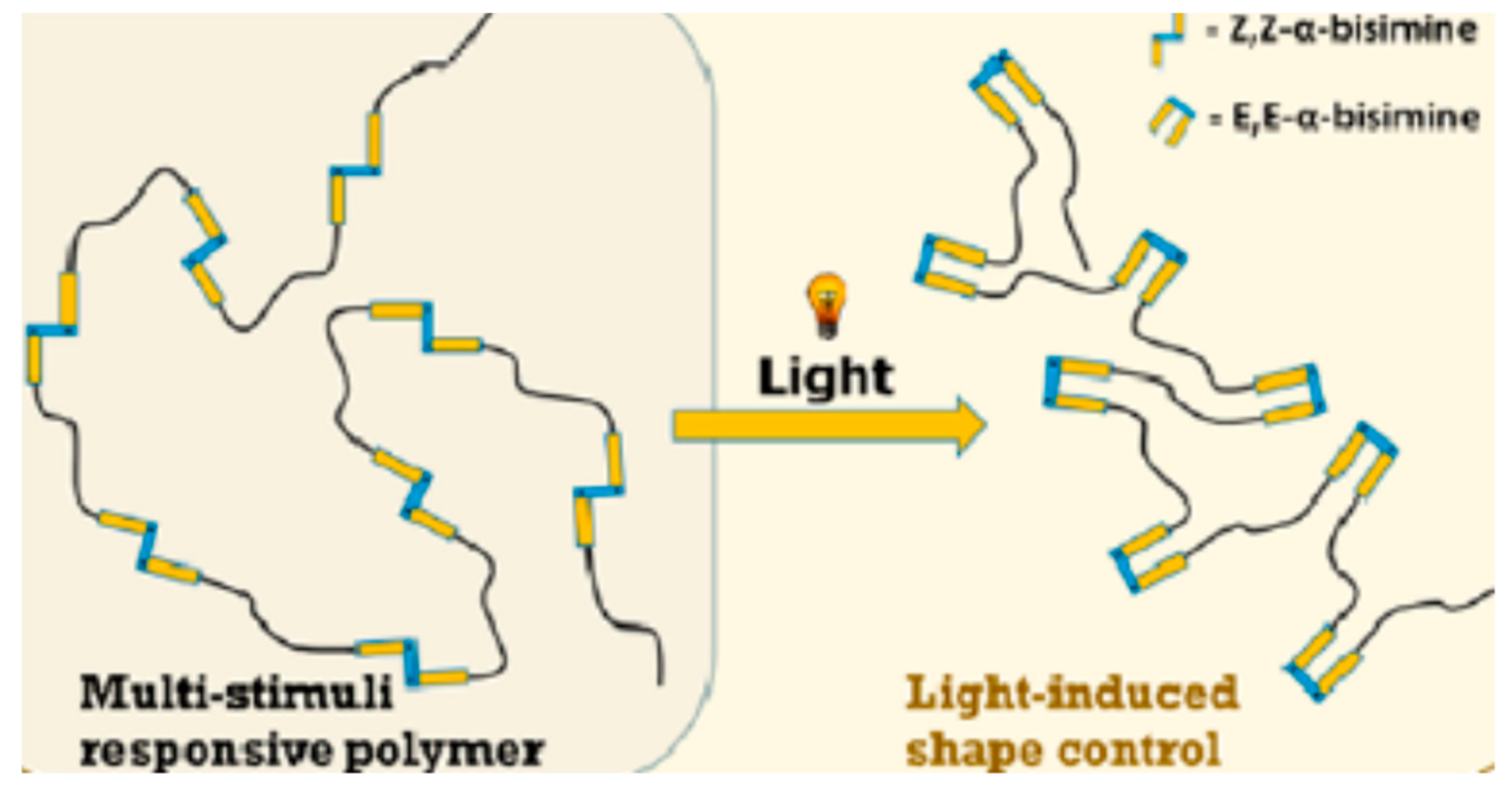
2.3. Photoisomerization
2.4. Photogeneration of Reactive Species
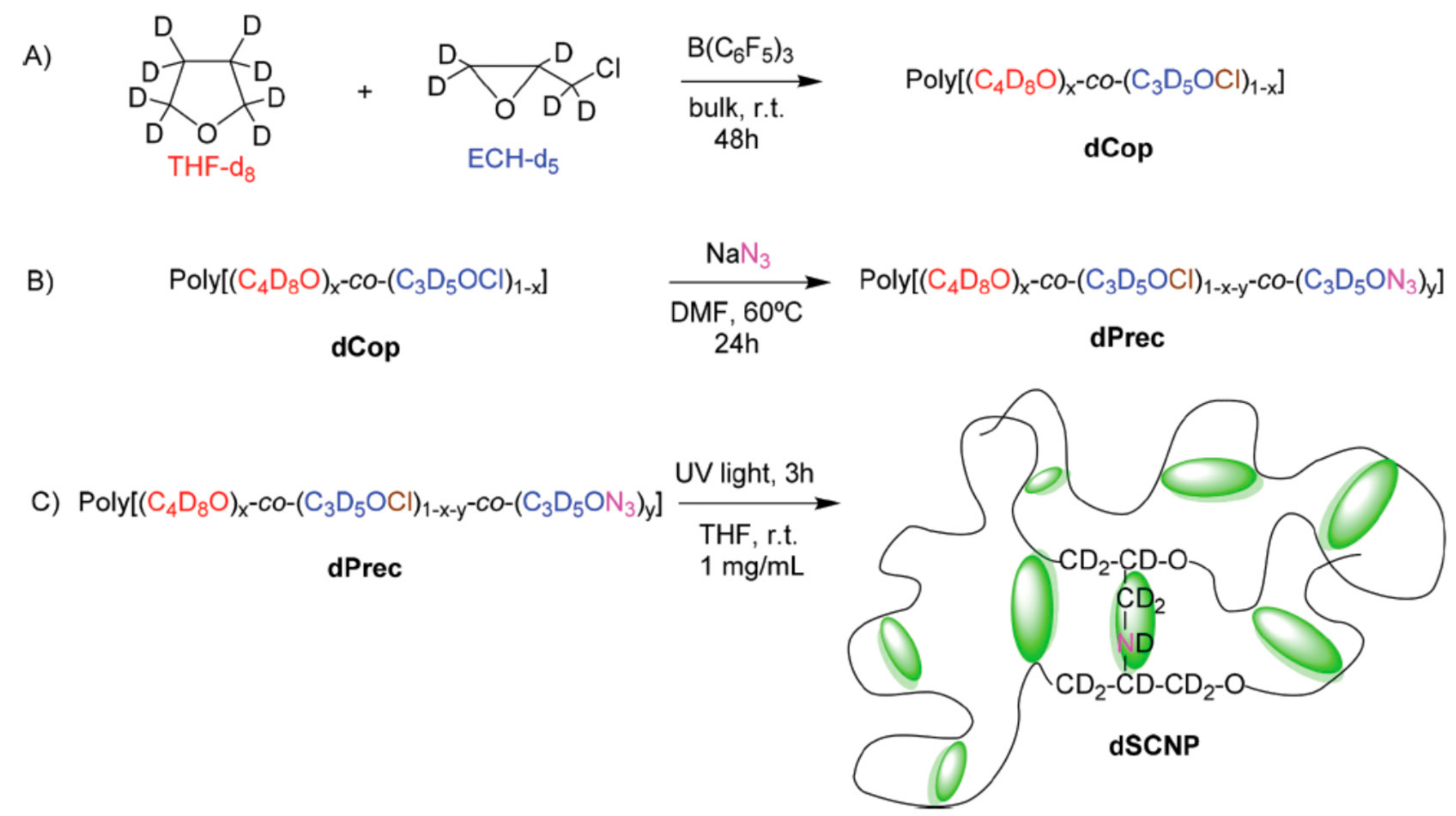
2.4.1. Nitrenes
2.4.2. Carbenes
2.5. Self-Assembly Induced by Photodeprotection
2.5.1. Hydrogen-Bonded Dimers
2.5.2. Hydrogen-Bonded Helical Stacks
3. Synthesis of Single-Chain Polymer Nanoparticles via Phototriggered Intrachain Heterocoupling
3.1. Photoinduced Diels–Alder (DA) Reaction
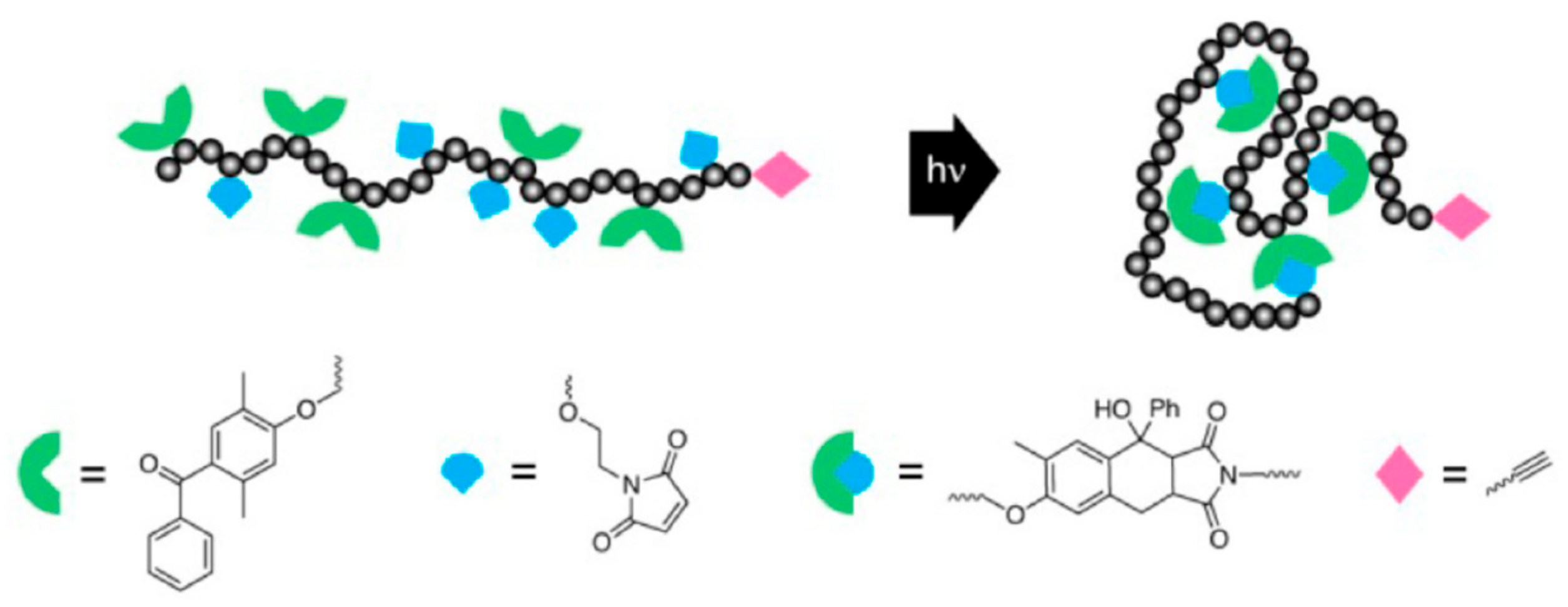
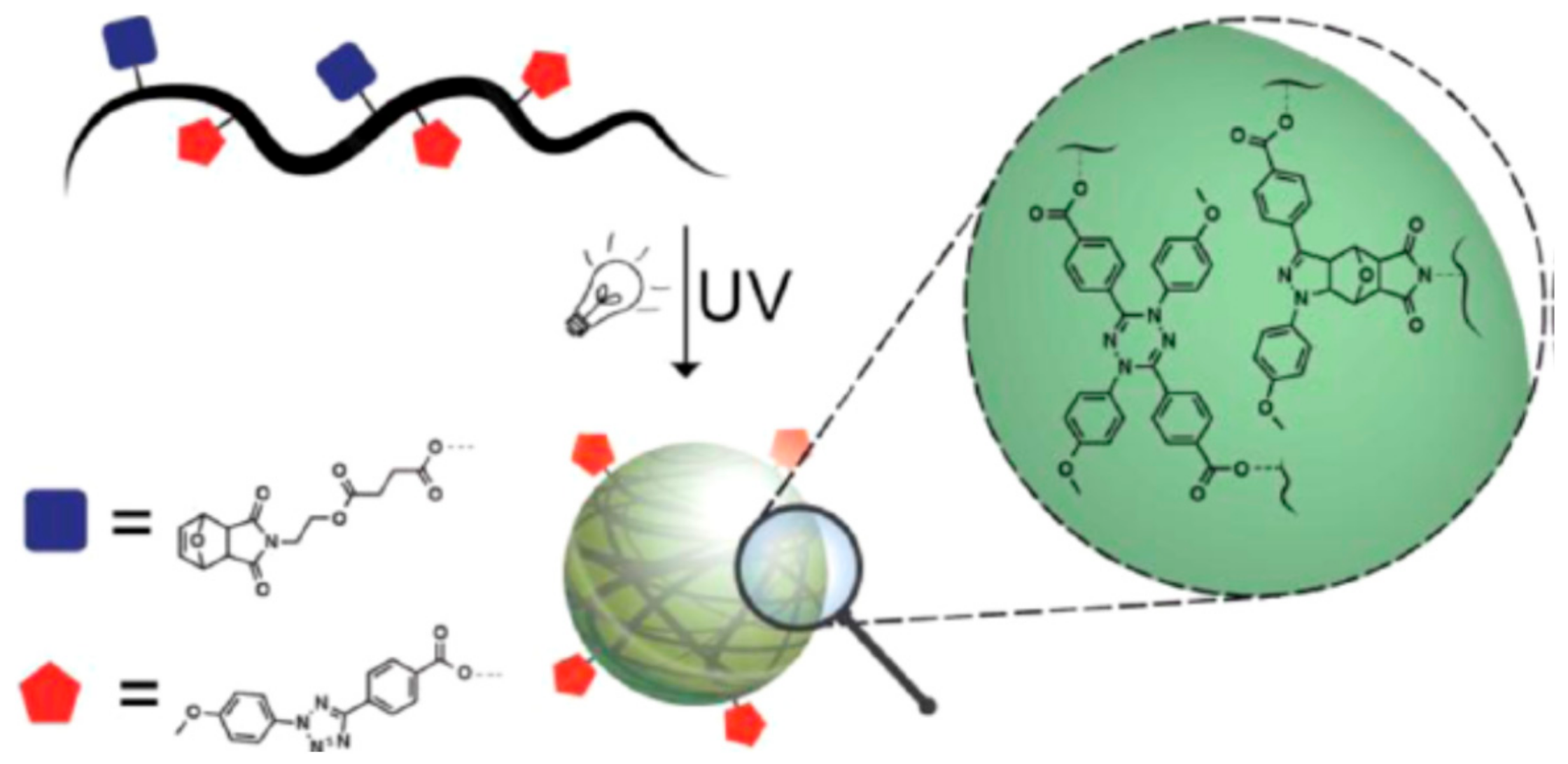
3.2. Photoinduced Nitrile Imine-Mediated Tetrazole-ene Cycloaddition (NITEC)
3.3. Photoinduced Nitrile Imine–Carboxylic Acid Ligation (NICAL)
4. Synthesis of Single-Chain Polymer Nanoparticles via Photogenerated Collapse Induced by External Cross-Linkers
5. Synthesis of Single-Chain Polymer Nanoparticles via Photoactivated Multifolding
5.1. Simultaneous Photoactivated Multifolding
5.2. Sequential Photoactivated Multifolding
5.2.1. Without External Cross-Linkers
5.2.2. With External Cross-Linkers
6. Phototriggered Disassembly of Single-Chain Polymer Nanoparticles
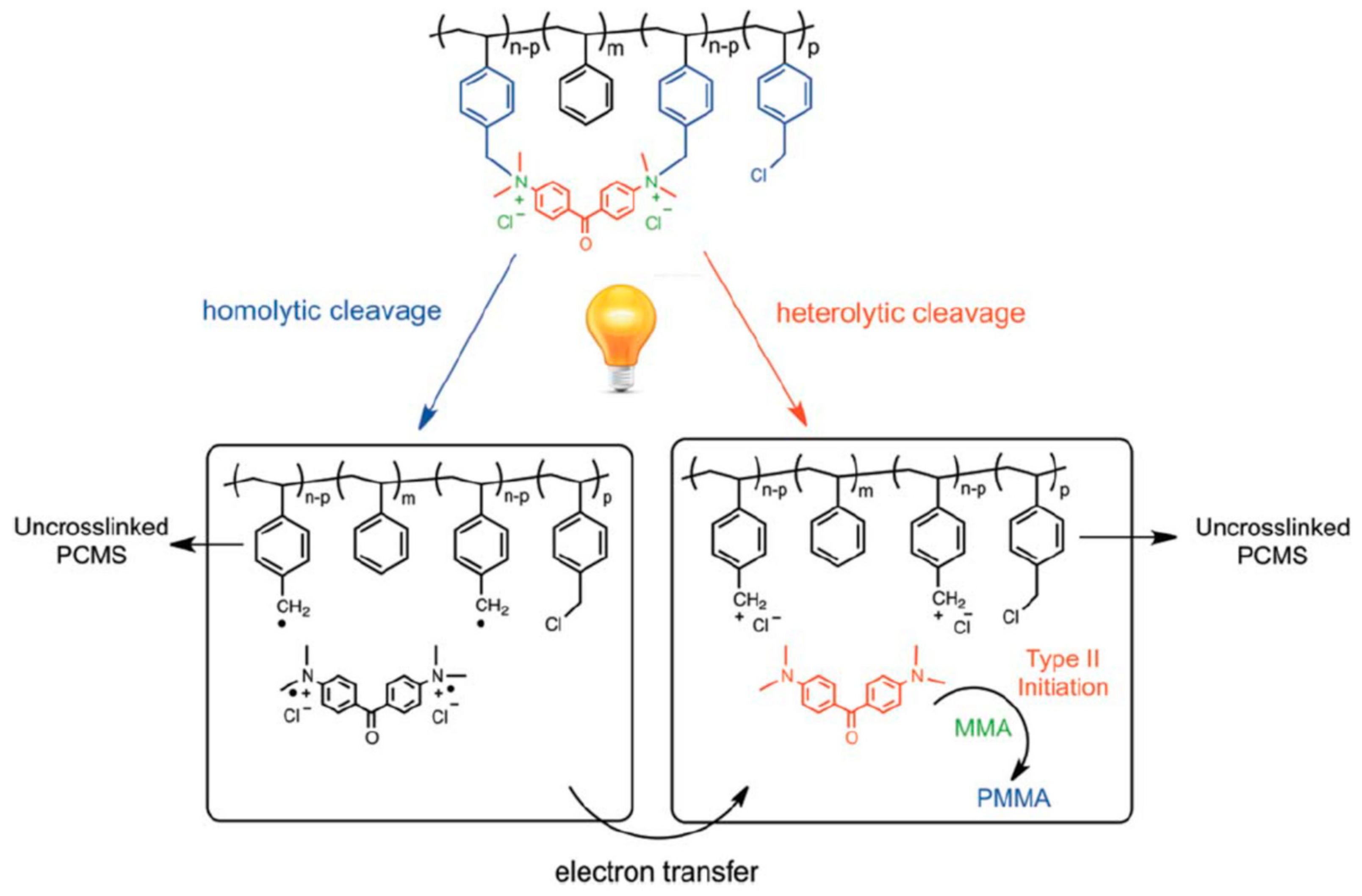
6.1. Main-Chain Disassembly
6.2. External Cross-Linker Disassembly
7. Applications of SCNPs Prepared from Photocross-linking
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albini, A.; Fagnoni, M. Green chemistry and photochemistry were born at the same time. Green Chem. 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Ciamician, G. Sur les actions chimiques de la lumiere. Bull. Soc. Chim. Fr. 1908, 4, 1–27. [Google Scholar]
- Ciamician, G. The Photochemistry of the Future. Science 1912, 36, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Klán, P.; Wirz, J. Photochemistry of Organic Compounds: From Concepts to Practice; John Wiley & Sons Ltd.: West Sussex, UK, 2009; pp. 44–67. [Google Scholar]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Waldeck, D.H. Photoisomerization dynamics of stilbenes. Chem. Rev. 1991, 91, 415–436. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.; Yin, G. Photochemical Nickel-Catalyzed Reductive Migratory Cross-Coupling of Alkyl Bromides with Aryl Bromides. Org. Lett. 2018, 20, 1880–1883. [Google Scholar] [CrossRef] [PubMed]
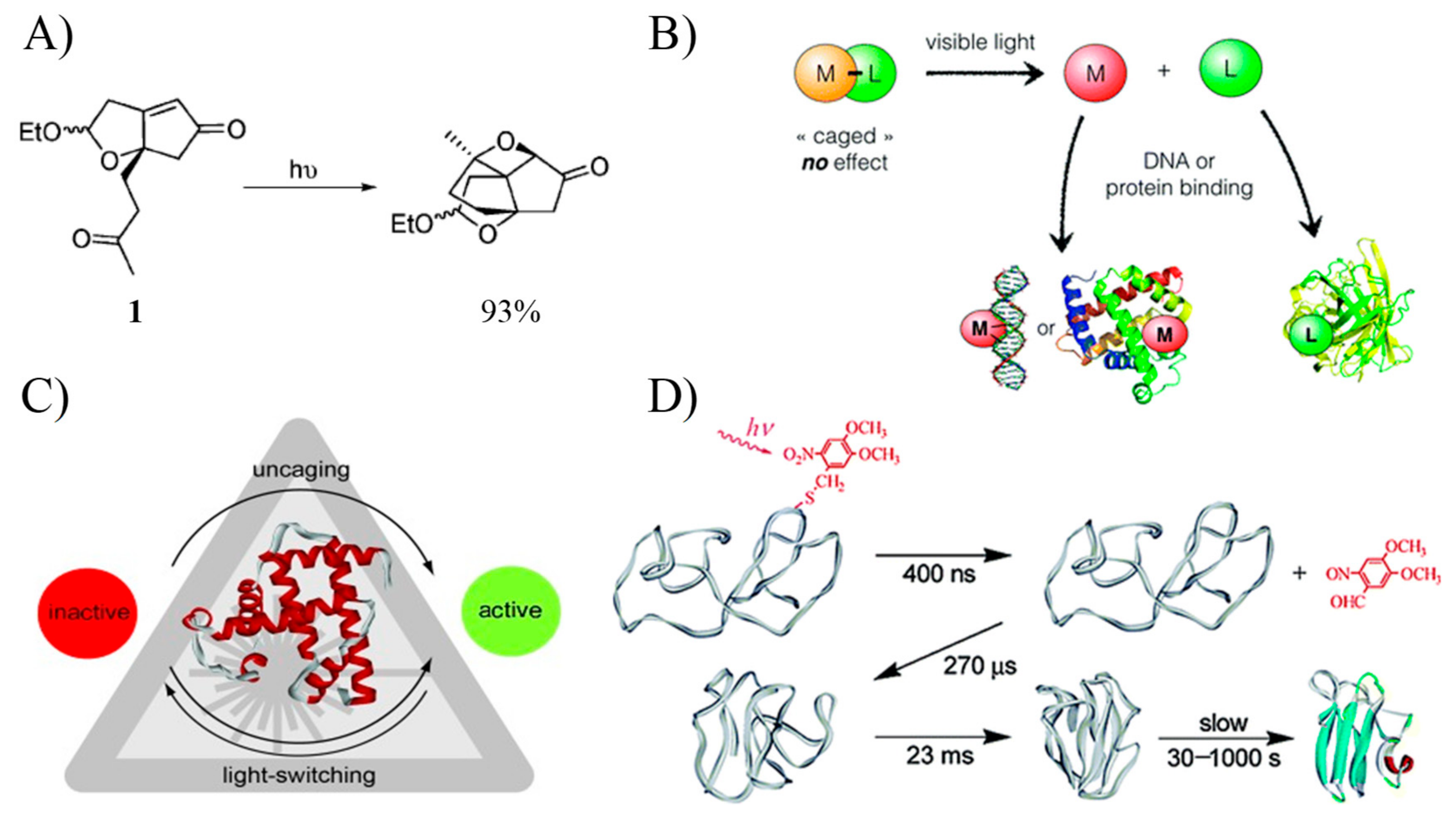
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, M.T. Synthetic applications of intramolecular enone-olefin photocycloadditions. Chem. Rev. 1988, 88, 1453–1473. [Google Scholar] [CrossRef]
- Iriondo-Alberdi, J.; Perea-Buceta, J.E.; Greaney, M.F. A Paternò-Büchi Approach to the Synthesis of Merrilactone, A. Org. Lett. 2005, 7, 3969–3971. [Google Scholar] [CrossRef]
- Engels, J.; Schlaeger, E.J. Synthesis, structure, and reactivity of adenosine cyclic 3′,5′-phosphate-benzyltriesters. Inorg. Chem. 1977, 20, 907–911. [Google Scholar] [CrossRef]
- Kaplan, J.H.; Forbush, B.; Hoffman, J.F. Rapid photolytic release of adenosine 5′-triphosphate from a protected analog: Utilization by the sodium:potassium pump of human red blood cell ghosts. Biochemistry 1978, 17, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, R.E.; Bina, M.; Deibe, R.; Luebke, K.; Morrison, H. Photochemically induced binding of Rh(phen)2Cl2+ to DNA. Photochem. Photobiol. 1989, 49, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S. Why develop photoactivated chemotherapy? Dalton Trans. 2018, 47, 10330–10343. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Heckel, A. Biologically active molecules with a “light switch”. Angew. Chem. Int. Ed. 2006, 45, 4900–4921. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; Fujimoto, Y.; Choi, J.; Baden, N.; Katagiri, N.; Akiyama, M.; Hulsker, R.; Ubbink, M.; Okajima, T.; Takabe, T.; et al. Conformational changes during apoplastocyanin folding observed by photocleavable modification and transient grating. J. Am. Chem. Soc. 2006, 128, 7551–7558. [Google Scholar] [CrossRef] [PubMed]
- Junkers, T. [2+2] Photo-cycloadditions for polymer modification and surface decoration. Eur. Polym. J. 2015, 62, 273–280. [Google Scholar] [CrossRef]
- Pomposo, J. (Ed.) Single-Chain Polymer Nanoparticles: Synthesis, Characterization, Simulations and Applications; Wiley-VCH: Weinheim, Germany, 2017; pp. 341–388. [Google Scholar]
- Tao, J.; Liu, G. Polystyrene-block-poly(2-cinnamoylethyl methacrylate) Tadpole Molecules. Macromolecules 1997, 30, 2408–2411. [Google Scholar] [CrossRef]
- Njikang, G.; Liu, G.; Curda, S.A. Tadpoles from the Intramolecular Photo-Cross-Linking of Diblock Copolymers. Macromolecules 2008, 41, 5697–5702. [Google Scholar] [CrossRef]
- Njikang, G.; Liu, G.; Hong, L. Chiral Imprinting of Diblock Copolymer Single-Chain Particles. Langmuir 2011, 27, 7176–7184. [Google Scholar] [CrossRef]
- Thanneeru, S.; Li, W.; He, J. Controllable Self-Assembly of Amphiphilic Tadpole-Shaped Polymer Single-Chain Nanoparticles Prepared through Intrachain Photo-cross-linking. Langmuir 2019, 35, 2619–2629. [Google Scholar] [CrossRef]
- He, J.; Tremblay, L.; Lacelle, S.; Zhao, Y. Preparation of polymer single chain nanoparticles using intramolecular photodimerization of coumarin. Soft Matter 2011, 7, 2380–2386. [Google Scholar] [CrossRef]
- Fan, W.; Tong, X.; Farnia, F.; Yu, B.; Zhao, Y. CO2-Responsive Polymer Single-Chain Nanoparticles and Self-Assembly for Gas-Tunable Nanoreactors. Chem. Mater. 2017, 29, 5693–5701. [Google Scholar] [CrossRef]
- Fan, W.; Tong, X.; Li, G.; Zhao, Y. Photoresponsive liquid crystalline polymer single-chain nanoparticles. Polym. Chem. 2017, 8, 3523–3529. [Google Scholar] [CrossRef]
- Frank, P.G.; Tuten, B.T.; Prasher, A.; Chao, D.; Berda, E.B. Intra-Chain Photodimerization of Pendant Anthracene Units as an Efficient Route to Single-Chain Nanoparticle Fabrication. Macromol. Rapid Commun. 2014, 35, 249–253. [Google Scholar] [CrossRef]
- Rodriguez, K.J.; Hanlon, A.M.; Lyon, C.K.; Cole, J.P.; Tuten, B.T.; Tooley, C.A.; Berda, E.B.; Pazicni, S. Porphyrin-Cored Polymer Nanoparticles: Macromolecular Models for Heme Iron Coordination. Inorg. Chem. 2016, 55, 9493–9496. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Y.; Zhao, H. Amphiphilic Janus Twin Single-Chain Nanoparticles. Chem. Eur. J. 2018, 24, 3005–3012. [Google Scholar] [CrossRef]
- Liu, Q.; Ju, Y.; Zhao, H. Bioassemblies Fabricated by Coassembly of Protein Molecules and Monotethered Single-Chain Polymeric Nanoparticles. Langmuir 2018, 34, 13705–13712. [Google Scholar] [CrossRef]
- Bilgi, M.; Balta, D.K.; Temel, B.A.; Temel, G. Single-Chain Folding Nanoparticles as Carbon Nanotube Catchers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2709–2714. [Google Scholar] [CrossRef]
- Chidanguro, T.; Blank, D.R.; Garrett, A.; Reese, C.M.; Schekman, J.M.; Yu, X.; Patton, D.L.; Ayres, N.; Simon, Y.C. Fabrication of single-chain nanoparticles through the dimerization of pendant anthracene groups via photochemical upconversion. Dalton Trans. 2018, 47, 8663–8669. [Google Scholar] [CrossRef]
- Frisch, H.; Bloesser, F.R.; Barner-Kowollik, C. Controlling Chain Coupling and Single-Chain Ligation by Two Colours of Visible Light. Angew. Chem. Int. Ed. 2019, 58, 3604–3609. [Google Scholar] [CrossRef]
- Frisch, H.; Menzel, J.P.; Bloesser, F.R.; Marschner, D.E.; Mundsinger, K.; Barner-Kowollik, C. Photochemistry in Confined Environments for Single-Chain Nanoparticle Design. J. Am. Chem. Soc. 2018, 140, 9551–9557. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Huang, T.; Guan, S.; Zhao, Y.; Chen, A. Self-Assembly of Single Chain Janus Nanoparticles with Tunable Liquid Crystalline Properties from Stilbene-Containing Block Copolymers. Macromolecules 2019, 52, 2956–2964. [Google Scholar] [CrossRef]
- Dashan, I.; Balta, D.K.; Temel, B.A.; Temel, G. Preparation of single chain nanoparticles via photoinduced radical coupling process. Eur. Polym. J. 2019, 113, 183–191. [Google Scholar] [CrossRef]
- Offenloch, J.T.; Blasco, E.; Bastian, S.; Barner-Kowollik, C.; Mutlu, H. Self-reporting visible light-induced polymer chain collapse. Polym. Chem. 2019, 10, 4513–4518. [Google Scholar] [CrossRef] [Green Version]
- Heiler, C.; Bastian, S.; Lederhose, P.; Blinco, J.P.; Blasco, E.; Barner-Kowollik, C. Folding polymer chains with visible light. Chem. Commun. 2018, 54, 3476–3479. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Qian, G.; Xiao, Y.; Deng, S.; Wang, M.; Hu, A. A Convergence of Photo-Bergman Cyclization and Intramolecular Chain Collapse Towards Polymeric Nanoparticles. J. Polym. Sci. Part A: Polym. Chem. 2011, 49, 5330–5338. [Google Scholar] [CrossRef]
- Greb, L.; Mutlu, H.; Barner-Kowollik, C.; Lehn, J.-M. Photo- and Metallo-responsive N-Alkyl α-Bisimines as Orthogonally Addressable Main-Chain Functional Groups in Metathesis Polymers. J. Am. Chem. Soc. 2016, 138, 1142–1145. [Google Scholar] [CrossRef]
- Li, G.; Tao, F.; Wang, L.; Li, Y.; Bai, R. A facile strategy for preparation of single-chain polymeric nanoparticles by intramolecular photo-crosslinking of azide polymers. Polymer 2014, 55, 3696–3702. [Google Scholar] [CrossRef]
- Rubio-Cervilla, J.; Malo de Molina, P.; Robles-Hernández, B.; Arbe, A.; Moreno, A.J.; Alegría, A.; Colmenero, J.; Pomposo, J.A. Facile Access to Completely Deuterated Single-Chain Nanoparticles Enabled by Intramolecular Azide Photodecomposition. Macromol. Rapid Commun. 2019, 40, 1900046. [Google Scholar] [CrossRef]
- De-La-Cuesta, J.; Pomposo, J.A. Photoactivation of Aggregation-Induced Emission Molecules for Fast and Efficient Synthesis of Highly Fluorescent Single-Chain Nanoparticles. ACS Omega 2018, 3, 15193–15199. [Google Scholar] [CrossRef] [Green Version]
- Foster, E.J.; Berda, E.B.; Meijer, E.W. Metastable Supramolecular Polymer Nanoparticles via Intramolecular Collapse of Single Polymer Chains. J. Am. Chem. Soc. 2009, 131, 6964–6966. [Google Scholar] [CrossRef] [PubMed]
- Ter Huurne, G.M.; Palmans, A.R.A.; Meijer, E.W. Supramolecular Single-Chain Polymeric Nanoparticles. CCS Chem. 2019, 1, 64–82. [Google Scholar] [CrossRef] [Green Version]
- Mes, T.; van der Weegen, R.; Palmans, A.R.A.; Meijer, E.W. Single-Chain Polymeric Nanoparticles by Stepwise Folding. Angew. Chem. Int. Ed. 2011, 50, 5085–5089. [Google Scholar] [CrossRef] [PubMed]
- Altintas, O.; Willenbacher, J.; Wuest, K.N.R.; Oehlenschlaeger, K.K.; Krolla-Sidenstein, P.; Gliemann, H.; Barner-Kowollik, C. A Mild and Efficient Approach to Functional Single-Chain Polymeric Nanoparticles via Photoinduced Diels−Alder Ligation. Macromolecules 2013, 46, 8092–8101. [Google Scholar] [CrossRef]
- Blasco, E.; Yameen, B.; Quick, A.S.; Krolla-Sidenstein, P.; Welle, A.; Wegener, M.; Barner-Kowollik, C. Designing π-Conjugated Polymeric Nano- and Microstructures via Light Induced Chemistry. Macromolecules 2015, 48, 8718–8728. [Google Scholar] [CrossRef]
- Willenbacher, J.; Wuest, K.N.R.; Mueller, J.O.; Kaupp, M.; Wagenknecht, H.-A.; Barner-Kowollik, C. Photochemical Design of Functional Fluorescent Single-Chain Nanoparticles. ACS Macro Lett. 2014, 3, 574–579. [Google Scholar] [CrossRef]
- Geiselhart, C.M.; Offenloch, J.T.; Mutlu, H.; Barner-Kowollik, C. Polybutadiene Functionalization via an Efficient Avenue. ACS Macro Lett. 2016, 5, 1146–1151. [Google Scholar] [CrossRef]
- Wuest, K.N.R.; Lu, H.; Thomas, D.S.; Goldmann, A.S.; Stenzel, M.H.; Barner-Kowollik, C. Fluorescent Glyco Single-Chain Nanoparticle-Decorated Nanodiamonds. ACS Macro Lett. 2017, 6, 1168–1174. [Google Scholar] [CrossRef]
- Heiler, C.; Offenloch, J.T.; Blasco, E.; Barner-Kowollik, C. Photochemically Induced Folding of Single Chain Polymer Nanoparticles in Water. ACS Macro Lett. 2017, 6, 56–61. [Google Scholar] [CrossRef]
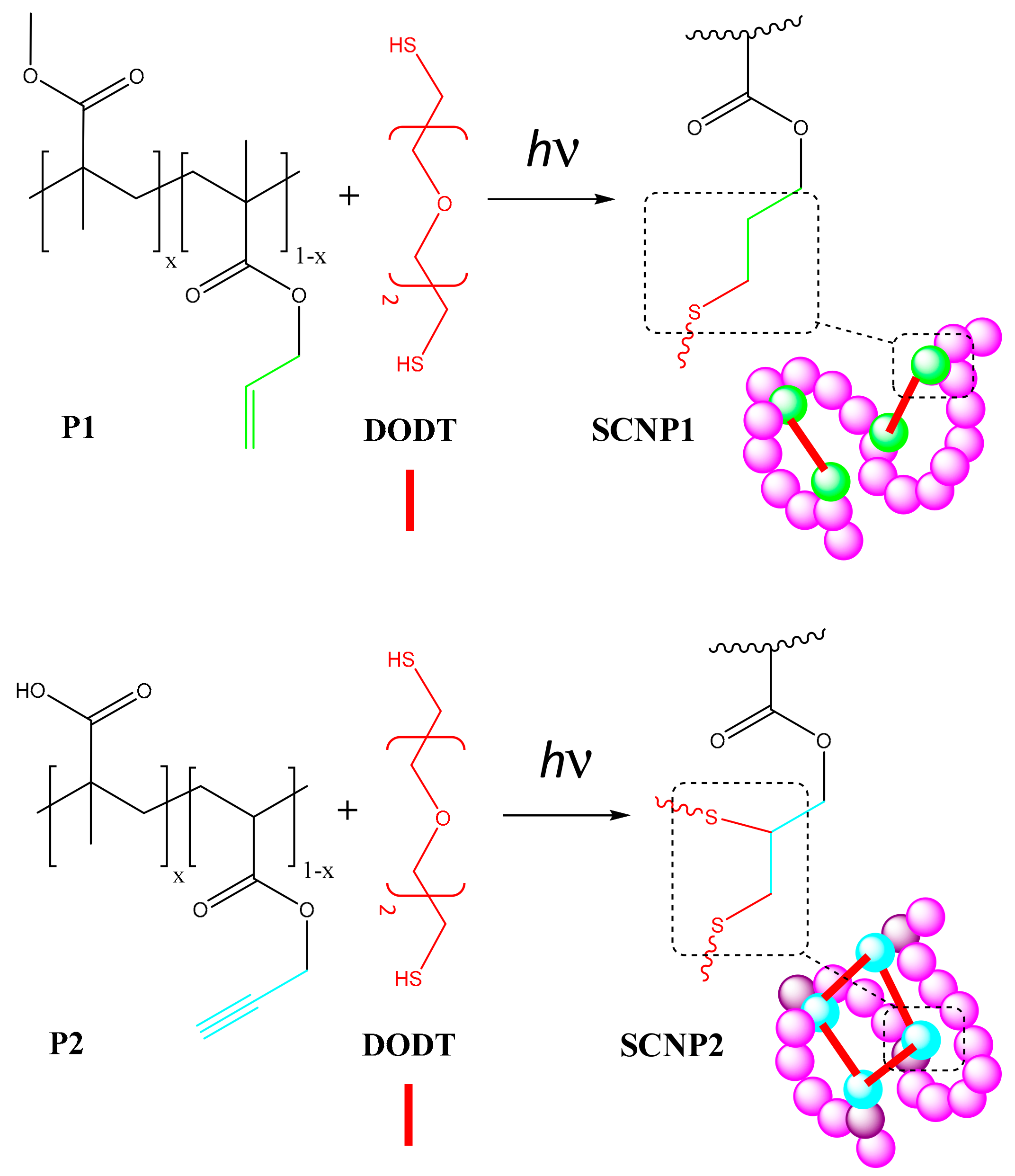
- Perez-Baena, I.; Asenjo-Sanz, I.; Arbe, A.; Moreno, A.J.; Lo Verso, F.; Colmenero, J.; Pomposo, J.A. Efficient Route to Compact Single-Chain Nanoparticles: Photoactivated Synthesis via Thiol−Yne Coupling Reaction. Macromolecules 2014, 47, 8270–8280. [Google Scholar] [CrossRef]
- Rubio-Cervilla, J.; Barroso-Bujans, F.; Pomposo, J.A. Merging of Zwitterionic ROP and Photoactivated Thiol-Yne Coupling for the Synthesis of Polyether Single-Chain Nanoparticles. Macromolecules 2016, 49, 90–97. [Google Scholar] [CrossRef]
- Fischer, T.S.; Spann, S.; An, Q.; Luy, B.; Tsotsalas, M.; Blinco, J.P.; Mutlu, H.; Barner-Kowollik, C. Self-reporting and refoldable profluorescent single-chain nanoparticles. Chem. Sci. 2018, 9, 4696–4702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashan, I.; Balta, D.K.; Temel, B.A.; Temel, G. Preparation of Single Chain Nanoparticles via Photoinduced Double Collapse Process. Macromol. Chem. Phys. 2019, 220, 1900116. [Google Scholar] [CrossRef]
- Frisch, H.; Kodura, D.; Bloesser, F.R.; Michalek, L.; Barner-Kowollik, C. Wavelength-Selective Folding of Single Polymer Chains with Different Colors of Visible Light. Macromol. Rapid Commun. 2019, 1900414. [Google Scholar] [CrossRef] [Green Version]
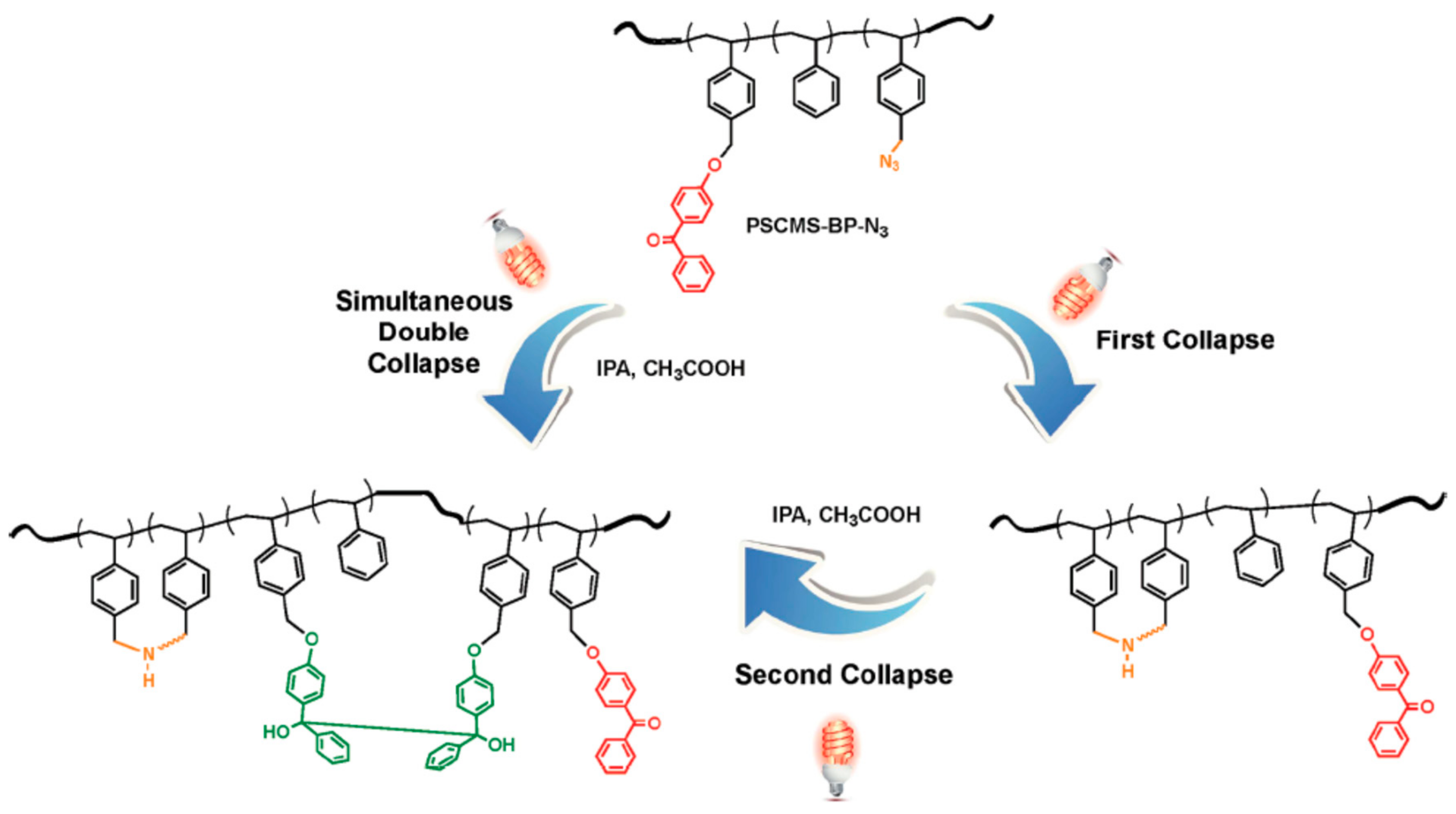
- Claus, T.K.; Zhang, J.; Martin, L.; Hartlieb, M.; Mutlu, H.; Perrier, S.; Delaittre, G.; Barner-Kowollik, C. Stepwise Light-Induced Dual Compaction of Single-Chain Nanoparticles. Macromol. Rapid Commun. 2017, 38, 1700264. [Google Scholar] [CrossRef]
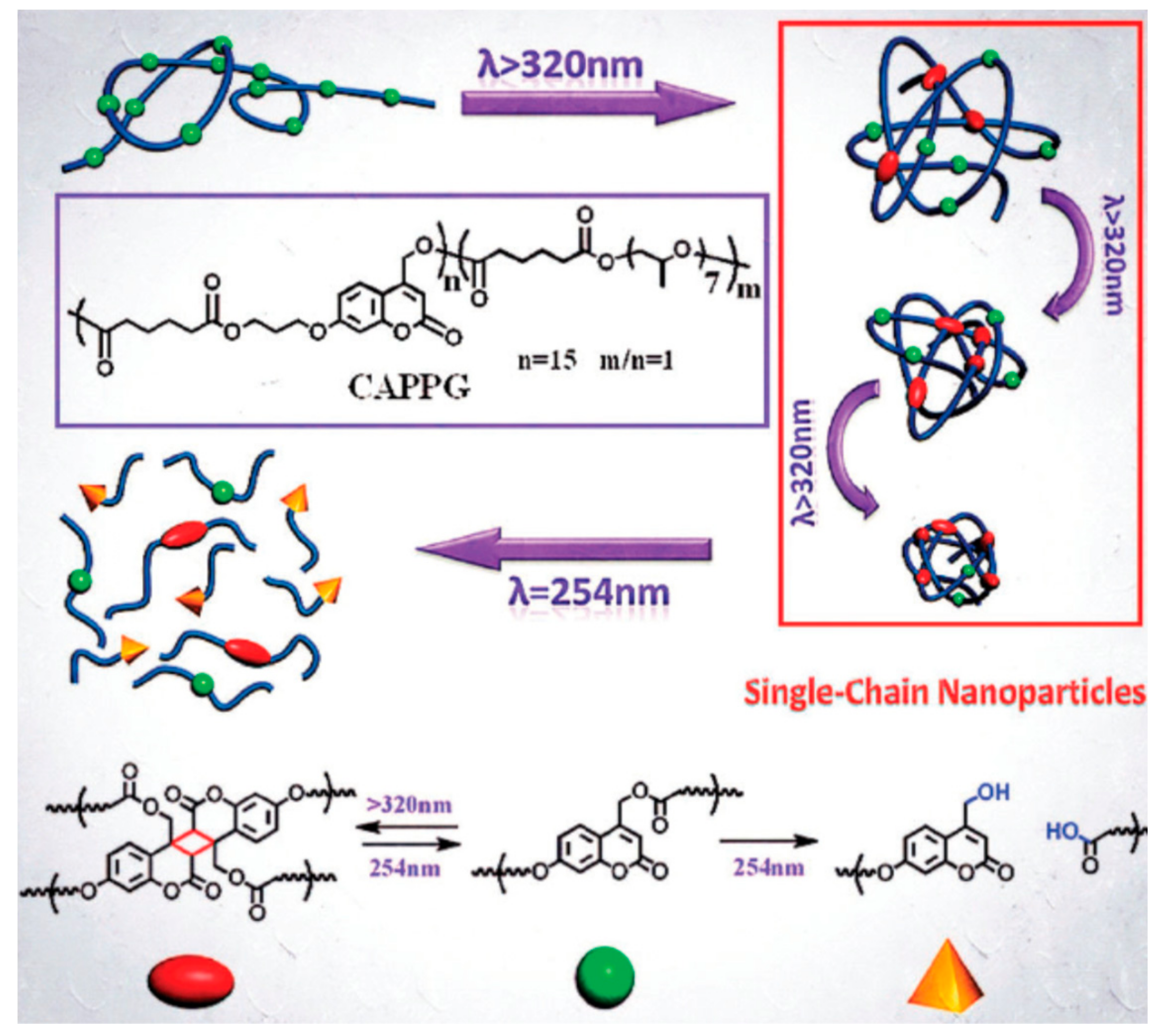
- Fan, W.; Tong, X.; Yan, Q.; Fu, S.; Zhao, Y. Photodegradable and size-tunable single-chain nanoparticles prepared from a single main-chain coumarin-containing polymer precursor. Chem. Commun. 2014, 50, 13492–13494. [Google Scholar] [CrossRef]
- Babaoglu, S.; Balta, D.K.; Temel, G. Synthesis of Photoactive Single-Chain Folded Polymeric Nanoparticles via Combination of Radical Polymerization Techniques and Menschutkin Click Chemistry. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1998–2003. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verde-Sesto, E.; Blázquez-Martín, A.; Pomposo, J.A. Advances in the Phototriggered Synthesis of Single-Chain Polymer Nanoparticles. Polymers 2019, 11, 1903. https://doi.org/10.3390/polym11111903
Verde-Sesto E, Blázquez-Martín A, Pomposo JA. Advances in the Phototriggered Synthesis of Single-Chain Polymer Nanoparticles. Polymers. 2019; 11(11):1903. https://doi.org/10.3390/polym11111903
Chicago/Turabian StyleVerde-Sesto, Ester, Agustín Blázquez-Martín, and José A. Pomposo. 2019. "Advances in the Phototriggered Synthesis of Single-Chain Polymer Nanoparticles" Polymers 11, no. 11: 1903. https://doi.org/10.3390/polym11111903